Abstract
Background & Aims
We previously demonstrated that short-term oral administration of the probiotic Lactobacillus reuteri 6475 enhanced bone density in male but not female mice. We also established that L. reuteri 6475 enhanced bone health and prevented bone loss in estrogen-deficient female mice. In this study, we tested whether a mild inflammatory state and/or a long-term treatment with the probiotic was required to promote a positive bone effect in estrogen-sufficient female mice.
Methods
A mild inflammatory state was induced in female mice by dorsal surgical incision (DSI). Following DSI animals were orally supplemented with L. reuteri or vehicle control for a period of 8 weeks. Gene expression was measured in the intestine and bone marrow by qPCR. Distal femoral bone density and architecture was analyzed by micro-CT.
Results
We report that 8 weeks after DSI there is a significant increase in the weight of spleen, thymus and visceral (retroperitoneal) fat pads. Expression of intestinal cytokines and tight junction proteins are also altered 8 weeks post-DSI. Interestingly, L. reuteri treatment was found to display both intestinal region- and inflammation-dependent effects. Unexpectedly we identified that 1) L. reuteri treatment increased bone density in females but only in those that underwent DSI and 2) DSI benefited cortical bone parameters. In the bone marrow, dorsal surgery induced CD4+ T cell numbers, a response that was unaffected by L. reuteri treatment, whereas expression of RANKL, OPG and IL-10 were significantly affected by L. reuteri treatment.
Conclusion
Our data reveals a previously unappreciated effect of a mild surgical procedure causing a long-lasting effect on inflammatory gene expression in the gut and the bone. Additionally, we demonstrate that in intact female mice, the beneficial effect of L. reuteri on bone requires an elevated inflammatory status.
Introduction
Throughout life the adult human skeleton is continuously remodeled with approximately 5–10% of the existing bone replaced every year [1]. Remodeling is accomplished through the coupled activities of osteoclasts, cells responsible for the degradation of bone, and osteoblasts, cells that produce organic bone matrix and promote its mineralization [2,3]. The balanced process of bone remodeling is negatively influenced by numerous factors. Up-regulated expression of pro-inflammatory and pro-osteoclastogenic cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-1β, interferon gamma (IFNγ) and receptor activator of nuclear factor-kappa B ligand (RANKL), have been shown to disrupt bone remodeling and lead to bone loss [4–7]. In contrast, increased levels of the anti-osteoclastogenic cytokine osteoprotegerin (OPG) or the anti-inflammatory cytokine IL-10 increase bone density by directly inhibiting osteoclast formation or inhibiting production of pro-inflammatory cytokines [8–10]. Consistent with a role for local inflammation in bone density regulation, rheumatoid arthritis (RA), periodontitis, loosened joint prostheses and tooth implants, all increase inflammation in close proximity to bone and can cause local increases in RANKL and pro-inflammatory cytokines, leading to bone loss [11]. In addition, chronic diseases associated with systemic inflammation (marked by elevated serum cytokine levels) can also promote bone loss that is distant from the site of initiation of inflammation as seen in periodontitis; in autoimmune diseases such as psoriasis, type 1 diabetes, RA and inflammatory bowel disease (IBD) [11–14]; as well as in aging [15].
In recent years, the intestinal microbiota has emerged as a potentially important regulator of systemic health. Dysbiosis of the intestinal microbiota is linked to the pathogenesis of a number of diseases including diabetes, obesity, IBD, RA and liver disease which are also associated with adverse bone pathology [16–22]. Studies have demonstrated that modulation of the microbiota with probiotic bacteria is able to reduce disease processes and a few studies, including studies from our lab, further establish that probiotics can prevent bone pathology complications [23–25]. Additionally, our studies have also highlighted the beneficial effect of probiotic supplementation on general bone health under non-diseased conditions [26]. In these studies, we found that the effect of L. reuteri on bone is gender-dependent. In particular, we demonstrated that 4-week supplementation with L. reuteri 6475 increased bone density in male but not female mice [26]. We later demonstrated that estrogen deficient female mice (ovariectomized model) respond to oral L. reuteri 6475 administration and display bone health benefits. Specifically, treatment with probiotic bacteria protected against estrogen deficiency-induced trabecular and cortical bone loss [23,24]. Given that estrogen is known to be an important immunomodulator, it was not entirely surprising that some aspects of immune components were altered in the ovariectomized mice. Immune responses have previously been shown to differ between males and females. For example, in models of sepsis females with H. hepaticus-induced colitis and lipopolysaccharide (LPS)-induced airway inflammation display reduced immune response and/or increased survival compared to males [27–29]. Taken together, this suggests that estrogen can lower baseline inflammatory status and modulate immune responses to insult.
The studies noted above made us question if a minor surgical procedure could induce inflammation and affect responses to L. reuteri treatment, especially under estrogen sufficient conditions in females. We utilized dorsal surgical incision (DSI), a procedure commonly used in ovariectomy and sham mouse surgery studies that represents a precise, reproducible cut in the skin. Remarkably, we report that DSI in female mice induces several inflammatory components observed as long as 8 weeks after the procedure. Interestingly, while L. reuteri has no effect on intact female mice (consistent with previous observations [26]), it increased femoral trabecular bone density in those that had undergone DSI. Our findings suggest that in the presence of estrogen, a mild inflammatory status is required for L. reuteri to exert a beneficial effect on the bone.
Materials and Methods
Animals and Experimental Design
Female Balb/c mice 11 weeks of age were obtained from The Jackson Laboratory (Bar Harbour, Maine). Mice were allowed to acclimate to animal facility for 1 week prior to start of experiment. After 1 week mice were randomly split into four groups (9–18 per group): control (+/- probiotic) or dorsal surgical incision (+/- probiotic). DSI entailed placing mice under isofluorane anesthesia for < 5 minutes and making a 2 cm lower-mid dorsal incision extending through the skin and muscle layer and then using surgical staples to close the site. Two days after surgery mice were treated by gavaging with 300 μl (1x109 cfu/ml) L. reuteri 6475 or MRS broth (vehicle control), three times per week for 4 or 8 weeks. L. reuteri was additionally added to the drinking water (of L. reuteri treated groups only) at a concentration of 3.3x108 cfu/ml. Mice were given Teklad 2019 chow (Madison, WI) and water ad libitum and were maintained on a 12 h light/dark cycle. All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee and conformed to NIH guidelines.
Bacterial Culture Conditions
L. reuteri ATCC PTA 6475 was initially cultured on deMan, Rogosa, Sharpe media (MRS, Difco)—agar plates and kept under anaerobic conditions at 37°C for a maximum of 1 week. For gavaging of all mice, one colony of L. reuteri was picked and anaerobically cultured in 10ml of MRS broth for 16-18h at 37°C. MRS broth was additionally used as a negative control. For drinking water, L. reuteri was anaerobically cultured in 10 ml of MRS broth for 16-18h at 37°C. The following day, the overnight culture was sub-cultured into fresh MRS and grown until log phase (OD600 = 0.4). L. reuteri was spun down, re-suspended in sterile PBS, cfu/ml calculated and stored at -80°C until use. L. reuteri was re-suspended in drinking water at a final concentration of 3.3x108 cfu/ml.
μCT Bone Imaging
Fixed femurs were scanned using a GE Explore Locus microcomputed tomography (μCT) system at a voxel resolution of 20μm obtained from 720 views. Beam angle of increment was 0.5, and beam strength was set at 80 peak kV and 450 uA. Each run consisted of control (non-surgery and DSI) and L. reuteri- treated mouse bones, and a calibration phantom to standardize grayscale values and maintain consistency. Bone measurements were performed blind. Femoral bone analyses were performed in a region of trabecular bone defined at 1% of the total length proximal to the growth plate and extending 2 mm toward the diaphysis excluding the outer cortical bone. Trabecular bone mineral content, bone volume fraction, thickness, spacing, and number values were computed by a GE Healthcare MicroView software application for visualization and analysis of volumetric image data. Cortical measurements were performed in a 2x2x2 mm cube centered midway down the length of the bone.
Femoral Dynamic Measures
For dynamic histomorphometric measures of bone formation, mice were injected intraperitoneally with 200 μl of 10 mg/ml calcein (Sigma, St. Louis, MO) dissolved in sterile saline at 7 and 2 days prior to harvest. Femora (n = 8–12 per group) were embedded in paraffin blocks sectioned, viewed under a fluorescent microscope and digital images obtained. The distance between the calcein lines (mineral apposition rate, MAR) and their length along the bone surface were measured and used to calculate bone formation rate (BFR).
Flow Cytometric Analysis
Following euthanasia femora were cleaned of muscle and bone marrow (BM) cells isolated by either flushing or spinning (n = 10–18 per group). 1x106 cells were incubated with Fc block (BD Pharmingen, CA, USA) for 15 min. Cells were stained with anti-mouse CD3-AlexaFluor 700 (500A2, eBioscience), anti-mouse CD4-FITC (RM 4–5, eBioscience) and anti-mouse CD8a-PE-Cyanine5.5 (5–6.7, eBioscience) for 30 minutes before fixing in formaldehyde. Data were acquired on a BD LSRII (Becton Dickinson, Franklin Lakes, NJ) and analyzed with FlowJo (Version 10; FlowJo, LLC, Ashland OR).
Bone Marrow and Intestine RNA Analysis
Bone marrow was obtained as described above. Immediately following euthanasia, intestines were cleaned of connective tissue and luminal contents, snap frozen in liquid nitrogen and stored at -80°C. Frozen intestines were crushed under liquid nitrogen conditions with a Bessman Tissue Pulverizer (Spectrum Laboratories, Rancho Dominguez, CA). RNA was isolated from frozen samples (n = 6–10 per group) using TriReagent (Molecular Research Center, Cincinnati, OH) and integrity assessed by formaldehyde-agarose gel electrophoresis. cDNA was synthesized by reverse transcription using Superscript II Reverse Transcriptase Kit and oligo dT(12–18) primers (Invitrogen, Carlsbad, CA). cDNA was amplified by quantitative PCR (qPCR) with iQ SYBR Green Supermix (BioRad, Hercules, CA), and gene specific primers (synthesized by Integrated DNA Technologies, Coralville, IA; Table 1). Hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA levels were used as an internal control.
Table 1. qPCR Primers.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| HPRT | AAGCCTAAGATG AGCGCAAG | TTACTAGGCAGATGGCCACA |
| OPG | TGGAGATCGAATTCTGCTTG | TCAAGTGCTTGAGGGCATAC |
| RANKL | TTTGCAGGACTCGACTCTGGAG | TCCCTCCTTTCATCAGGTTATGAG |
| IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT |
| TGFβ1 | GCAACAATTCCTGGCGTTACC | CCCTGTATTCCGTCTCCTTGGT |
| TNF | AAGGGAGAGTGGTCAGGTTGCC | CCTCAGGGAAGAGTCTGGAAAGG |
| IFNγ | GGCTGTCCCTGAAAGAAAGC | GAGCGAGTTATTTGTCATTCGG |
| IL-1β | TCCCCGTCCCTATCGACAAAC | GCGGTGATGTGGCATTTTCTG |
| Occludin | GCTCAGGGAATATCCACCTAT | CACAAAGTTTTAACTTCCCAGACG |
Real time PCR was carried out for 40 cycles using the iCycler (Bio-Rad) and data evaluated using the iCycler software. Each cycle consisted of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. Negative controls included primers without cDNA.
Serum Measurements
Blood was collected at the time of harvest, allowed to clot at room temperature for 5 min, then centrifuged at 5000g for 10 min. Serum was removed, aliquoted and frozen in liquid nitrogen, and stored at -80°C. Serum tartrate resistant acid phosphatase 5b (TRAP5b) and Osteocalcin (OC) were measured using Mouse TRAP (SB-TR103, Immunodiagnostic Systems Inc., Fountain Hills, AZ) and OC assay kits (BT-470, Biomedical Technologies Inc., Stoughton, MA), respectively, according to the manufacturer’s protocol (n = 7–18 per group).
Statistical Analysis
All measurements are presented as the mean ± SEM. Significant outliers were removed using the Grubb’s test for outliers. 1-way ANOVA was performed using GraphPad Prism software version 6 (GraphPad, San Diego, CA, USA). A p-value ≤0.05 was considered significant.
Results
Effect of Dorsal Surgical Incision (DSI) and L. reuteri on General Body Parameters
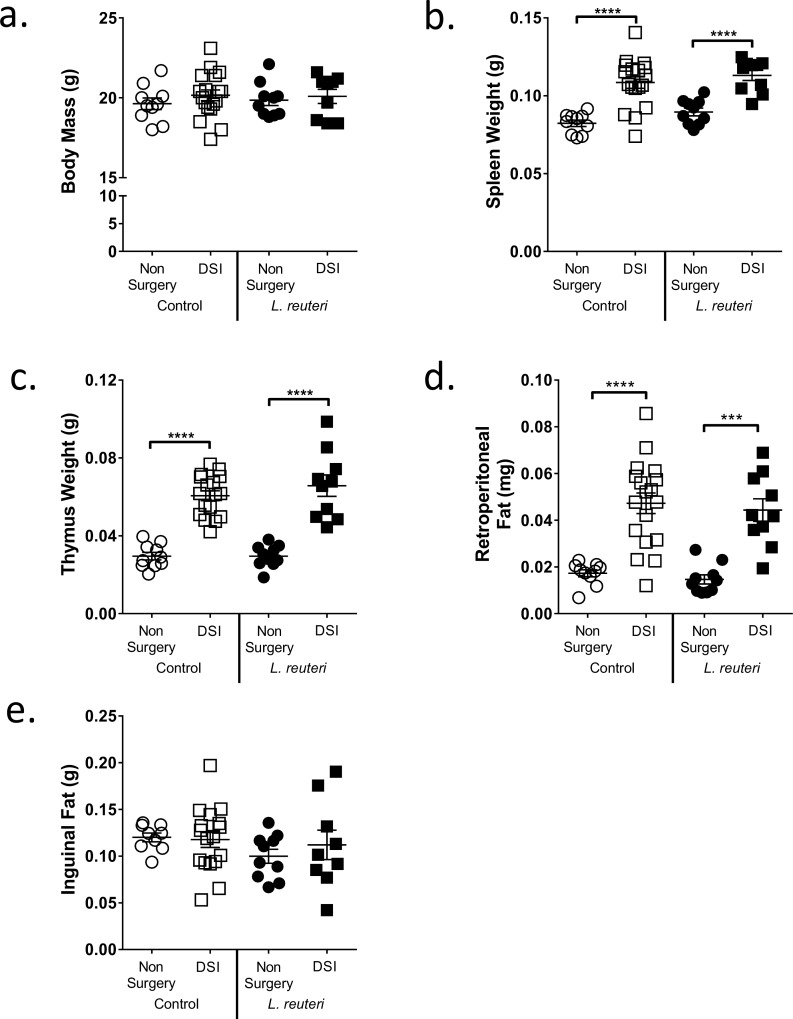
Previous studies demonstrated that supplementation with L. reuteri for 4 weeks significantly increased femoral bone density in specific-pathogen free male mice and prevented ovariectomy-induced bone loss in female mice. However, 4 weeks of L. reuteri treatment failed to have a significant effect on bone density in intact female mice [23,26]. Therefore, we investigated whether long-term (8 weeks) L. reuteri supplementation could modulate bone density in intact female mice and also tested whether an underlying inflammatory condition was required to obtain a beneficial bone response. Intact mice and mice that underwent DSI were given either L. reuteri 6475 or MRS broth (vehicle) orally by drinking water and by gavage 3x a week for the duration of the study. Mice were sacrificed at the end of the study and general body parameters measured (Fig 1). Interestingly, DSI mice (± L. reuteri) displayed significantly increased weight of spleen (p<0.001), thymus (p<0.001) and retroperitoneal fat (p<0.01) when compared to non-surgery mice 8 weeks post-surgery. DSI and L. reuteri had no significant effects on body mass and inguinal fat mass compared to the respective controls.
Fig 1. DSI Increases General Markers of Inflammation.
a) Body Mass, b) spleen c) thymus d) retroperitoneal fat and e) inguinal fat were weighed after an 8 week period in non-surgery and DSI mice ± L. reuteri. DSI significantly increased spleen weight (p<0.0001), thymus weight (p<0.0001) and retroperitoneal fat (p<0.001) but had no significant effect on body mass or inguinal fat. n = 9–18 per group. Data is mean ± SEM. Statistical analysis performed by 1-way ANOVA with Fisher’s LSD post-test.
Assessing the Impact of DSI and L. reuteri on Trabecular Bone Volume Fraction and Cortical Bone
To assess whether long-term L. reuteri supplementation benefits bone health, we examined the distal femur metaphyseal trabecular region by microcomputed tomography 8 weeks after the start of treatment (Table 2). Remarkably, we identified a significant increase (nearly 50%) in bone volume fraction (BVF; Fig 2) in the DSI + L. reuteri cohort compared to the DSI + broth group (p<0.05) and non-surgery control (p<0.05). Consistent with this finding, trabecular number (Tb. N, p<0.05) increased and trabecular spacing (Tb. Sp.) decreased (p<0.05) in the L. reuteri treated DSI cohort compared to the non-surgery control (Table 2). In contrast, we did not observe any change in BVF in the non-surgery + L. reuteri mice compared to the non-surgery controls, consistent with previous observations [26]. Analysis of femoral diaphysis cortical parameters (Table 2) revealed that DSI had a major effect on the cortical bone structure and strength. Specifically, DSI significantly increased inner perimeter (p<0.01), outer perimeter (p<0.01), marrow area (p<0.05), cortical area (p<0.05), total area (p<0.0001), bone mineral content (p<0.05) and moment of inertia (p<0.01) when compared to the non-surgery control. L. reuteri had no effect on cortical parameters in either the non-surgery or DSI treated cohorts.
Table 2. Femoral and Cortical Bone Parameters.
| Femur Trabecular Bone Parameters | Non-Surgery | DSI | Non-Surgery+ L. reuteri | DSI+ L. reuteri |
| % BVF | 22.4±2.1 | 24.1±1.7 | 20.9±2.8 | 31.4±2.3* |
| Tb. N. (1/mm) | 4.44±0.31 | 5.18±0.23 | 4.26±.030 | 5.63±0.37* |
| Tb. Th. (μm) | 48±2 | 45±2 | 47±3 | 50±2 |
| Tb. Sp. (mm) | 0.19±0.01 | 0.15±0.01 | 0.20±0.02 | 0.13±0.01* |
| BFR(μm) | 1.80±0.31 | 2.82±0.36 | 2.03±0.50 | 2.95±0.44 |
| MAR (μm/day) | 0.26±0.02 | 0.50±0.09* | 0.39±0.10 | 0.62±0.16* |
| Femoral Cortical Bone Parameters | Non-Surgery | DSI | Non-Surgery+ L. reuteri | DSI+ L. reuteri |
| Inner Perimeter (mm) | 2.65±0.04 | 2.81±0.04** | 2.71±0.02 | 2.83±0.04** |
| Outer Perimeter (mm) | 4.22±0.05 | 4.46±0.04** | 4.29±0.03 | 4.54±0.05** |
| Marrow Area (mm2) | 0.48±0.01 | 0.55±0.02** | 0.51±0.01 | 0.56±0.02 |
| Cortical Area (mm2) | 0.84±0.02 | 0.93±0.02** | 0.87±0.01 | 0.98±0.03** |
| Total Area (mm2) | 1.32±0.03 | 1.48±0.02** | 1.38±0.02 | 1.54±0.03** |
| BMD (mg/cc) | 857±17 | 905±32 | 844±10 | 821±32 |
| BMC (μg) | 14.5±0.5 | 16.9±0.5** | 14.7±0.3 | 16.2±0.8* |
| MOI (mm4) | 0.24±0.01 | 0.30±0.01** | 0.26±0.01 | 0.33±0.01** |
Femur distal trabecular bone parameters. % BVF, bone volume fraction; Tb. N., trabecular number; Tb. Th., trabecular thickness; Tb. Sp., trabecular spacing; MAR, mineral apposition rate; BFR, bone formation rate; MOI, the cross sectional moment of inertia at the z-axis. Values are averages ± SEM. N = 7–18 per group. * Significant compared to non-surgery control
* p<0.05
** p<0.01. Statistical analysis performed with 1 Way ANOVA followed by or Fisher’s LSD post-test.
Fig 2. L. reuteri Significantly Increases Bone Volume in Female Mice 8 Weeks after DSI.
Non-surgery and DSI mice were treated ± L. reuteri for 8 weeks and trabecular bone density analyzed by μCT. i) L. reuteri significantly increased %BVF in the DSI mice compared to DSI control (p<0.05) and non-surgery control (p<0.05). No significant difference was observed between the non-surgery and DSI controls. L. reuteri had no effect in the non-surgery cohort. Representative μCT isosurface images of ii) DSI and iii) DSI + L. reuteri. n = 8–18 per group. Statistical analysis performed by 1-way ANOVA with Fisher’s LSD post-test.
Long-term Effects of DSI and L. reuteri on Intestinal Cytokine Gene Expression
Knowing that DSI has systemic effects on bone, we wondered if DSI also affected the GI tract, contributing to the bone changes induced by surgery and L. reuteri supplementation. To test this hypothesis, we examined genes associated with gut-bone signaling pathways: jejunal and colonic gene expression of markers of inflammation (pro- and anti-inflammatory cytokines) and genes involved in gut barrier function (occludin) (Fig 3) which have been suggested to be involved in bone density regulation [30].
Fig 3. DSI and L. reuteri Significantly Alter Regional Intestinal Gene Expression.
Non-surgery and DSI mice were treated ± L. reuteri for 8 weeks and gene expression analyzed in the jejunum and colon by qPCR. In the jejunum DSI significantly increased expression of TNFα (p<0.001), IL-1β (p<0.01), IFNγ (p<0.05) and TGFβ (p<0.001). Significant decreases in IL-10 (p<0.01) and occludin (p<0.01) were observed. In the non-surgery cohort L. reuteri significantly reduced IL-10 (p<0.01) expression. In the DSI cohort significant reductions were observed for TNFα (p<0.0001), IFNγ (p<0.001) and TGFβ (p<0.0001). In the colon, DSI resulted in a significant increase in TNFα (p<0.05) and a decrease in occludin expression (p<0.05). L. reuteri significantly reduced expression of occludin (p<0.05), IL-1β (p<0.05) and TGFβ (p<0.01) in the non-surgery cohort. In the DSI cohort, L. reuteri significantly increased expression of TNFα (p<0.01) and IL-1β (p<0.01) while increasing expression of IL-10. n = 8–10 per group. * = significant to non-surgery control, ^ = significant to DSI control. Statistical analysis performed by 1-way ANOVA with Fisher’s LSD post-test.
In the jejunum, DSI promoted inflammation as indicated by significant increases in gene expression of TNFα (p<0.001), IL-1β (p<0.01), TGFβ (p<0.001), IFNγ (p<0.05) and significantly decreased expression of IL-10 (p<0.01) and occludin (p<0.01). This was consistent with our hypothesis of DSI altering gut homeostasis, even 8 weeks after surgery. Even more interesting, some of these changes induced by DSI were reversed by L. reuteri treatment: expression of TNFα (p<0.0001), IFNγ (p<0.001) and TGFβ (p<0.0001) were decreased while occludin expression was increased in L. reuteri treated DSI group. Supplementation of the non-surgery cohort with L. reuteri significantly decreased expression of IL-10 (p<0.01). This was interesting even though intact females do not exhibit any bone effect following L. reuteri supplementation.
Compared to the jejunum, colonic TNFα significantly increased (p<0.05) while IL-10 trended upwards 8 weeks post-DSI. As in the jejunum, expression of occludin (p<0.01), and TGFβ (p<0.05) were significantly decreased in DSI mice. Unexpectedly, L. reuteri supplementation of the DSI group caused significant increases in expression of TNFα (p<0.01, 4-fold), IL-1β (p<0.01, 4-fold) and increased IL-10 (3-fold) compared to treated non-surgery mice. These increases were much greater than what was seen in the jejunum. In contrast, and more consistent with previous studies, L. reuteri treatment of intact female mice led to a significant decrease in occludin (p<0.01), IL-1β (p<0.05) and TGFβ (p<0.01) expression in the non-surgery cohort. Together, these results suggest that L. reuteri differentially affects intestinal gene expression depending on the intestinal segment and on presence or absence of a distant surgical stress.
Modulation of the Bone Marrow CD4+ T Cell Population by DSI and L. reuteri
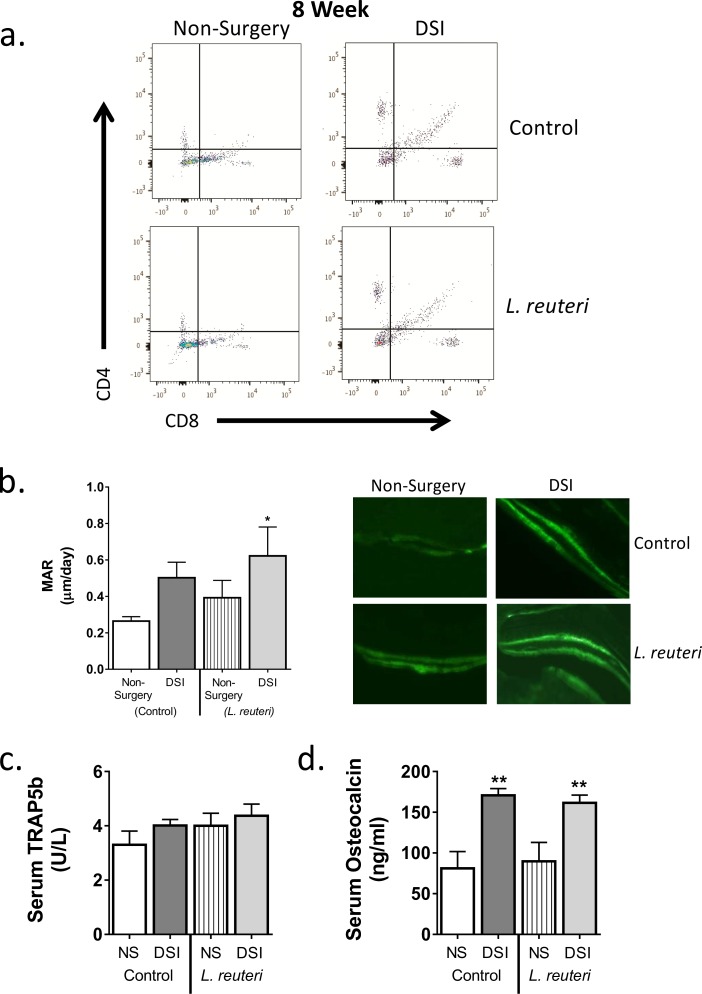
To determine whether DSI and/or oral L. reuteri administration could lead to a bone marrow (BM) cellular composition that is associated with changes in bone density, we analyzed BM CD4+ T cell numbers by flow cytometry at 8 weeks (Fig 4). BM CD4+ T cells have been shown to be important regulators of bone health [31]. Eight weeks following DSI, we identified a significant increase in the percentage of BM CD4+ T cells in the DSI group (17.7±1.1%) compared to the non-surgery cohort (6.8±0.7%; p<0.0001). L. reuteri treatment had no effect on CD4+ T cell numbers. No significant difference was observed in CD8+ T cell numbers between any of the conditions. These results suggest that the L. reuteri effect on bone density in the DSI group is likely independent of CD4+ T cell changes in the BM.
Fig 4. DSI Increases CD4+ T Cell Numbers in Bone Marrow and Significantly Increases Markers of Osteoblast Bone Formation after 8 Weeks.
Non-surgery and DSI mice were treated ± L. reuteri for 8 weeks. Bone marrow was isolated and CD4+ T cell numbers analyzed by flow cytometry and osteoclast and osteoblast bone remodeling markers analyzed. a) Representative flow cytometry scatter plots. CD4+ T cells numbers were significantly increased in DSI mice (p<0.0001) compared to the non-surgery cohort. L. reuteri had no significant effect on CD4+ T cell numbers. b) MAR was measured by calcein incorporation, DSI + L. reuteri significantly increased MAR compared to non-surgery controls (p<0.05). Representative fluorescent images of calcein incorporation are included on the right. c, d) DSI had no effect on serum TRAP5b (c) but significantly increased levels of serum osteocalcin (d) compared to the non-surgery control (p<0.01). L. reuteri had no effect on TRAP5b or osteocalcin levels. n = 7–18 per group. * = significant to non-surgery control, ^ = significant to DSI control. Data is mean ± SEM. Statistical analysis performed by 1-way ANOVA with Tukey post-test or Fisher’s LSD test.
Analysis of Osteoblast/Osteoclast Bone Remodeling Markers
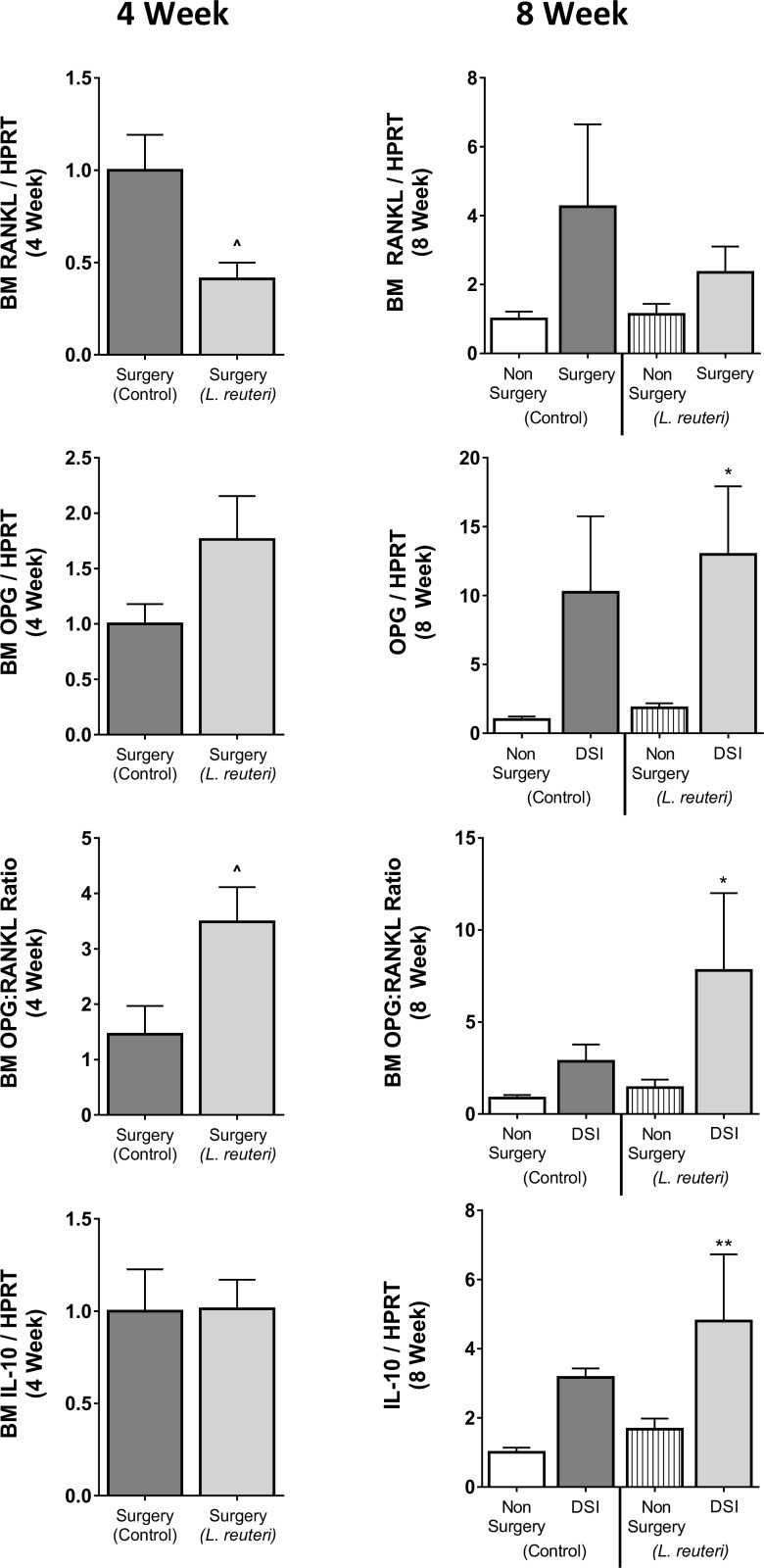
To determine whether L. reuteri supplementation decreased catabolic and/or increased anabolic bone responses, markers of osteoclast and osteoblast activity were measured in bone, BM and serum at 8 weeks. Serum levels of osteocalcin were significantly increased in the DSI cohort (2.1-fold-170.7 ± 8.5 ng/ml, p<0.01) compared to the non-surgery controls (81.1 ± 20.6 ng/ml; Fig 5). TRAP5b levels, a specific marker of osteoclast activity, were modestly increased in the DSI group (4.0 ± 0.2 U/L) compared to non-surgery controls (3.3 ± 0.5 U/L). L. reuteri treatment had no significant effect on serum TRAP5b and osteocalcin levels in either the non-surgery or DSI groups. Consistent with the serum osteocalcin levels, analysis of calcein incorporation, a dynamic measure of bone formation, identified an increase in mineral apposition rate in the DSI mice (0.50 ± 0.09μm/day) and DSI + L. reuteri mice (0.62 ± 0.16μm/day; p<0.05) compared to the non-surgery controls (0.26 ± 0.02 μm/day) (Fig 4). Consistent with the changes in MAR, BFR trended to be higher in the DSI cohorts compared to the non-surgery controls, though this increase was not significant (Table 2). To further assess the effect of DSI and/or L. reuteri on BM, we examined the expression of the osteoclastogenic cytokine RANKL, the anti-osteoclastogenic cytokine OPG and the anti-inflammatory cytokine IL-10 (Fig 5). Eight weeks post-surgery, expression of RANKL and OPG were increased in the DSI group compared to the non-surgery group (4.3- and 10.2-fold respectively). This resulted in an increase in the OPG:RANKL ratio in the DSI control group compared to the non-surgery controls. In addition, IL-10 expression was enhanced in the DSI controls compared to the non-surgery controls. None of these parameters were affected significantly by L. reuteri treatment in the DSI group. Because of the observed bone effects at 8-weeks post-surgery/treatment, we reasoned that gene expression changes may have occurred earlier in the process. Therefore, we examined expression of the above-described genes in the BM from mice that underwent DSI ± L. reuteri 4 weeks after surgery. As posited, at 4 weeks, L. reuteri caused a significant reduction (2.4-fold) in RANKL gene expression (p<0.05) and 1.8 fold increase in OPG expression. Analysis of the OPG:RANKL ratio revealed a significant elevation (2.4-fold) in the DSI + L. reuteri cohort when compared to the DSI mice (p<0.05). L. reuteri had no effect on BM IL-10 expression at 4 weeks. Together, these studies suggest that L. reuteri likely modulates genes responsible for bone remodeling in the BM. Whether these effects are consequences of intestinal changes or direct effect of L. reuteri secretory products will be the focus of future studies.
Fig 5. DSI and L. reuteri Alter Bone Marrow Gene Expression at 4 and 8 Weeks.
Bone marrow was isolated from 4 and 8 week Non-surgery and DSI mice ± L. reuteri and gene expression analyzed by qPCR. At 4 weeks L. reuteri significantly reduced RANKL expression in the DSI cohort (p<0.05). L. reuteri additionally increased OPG expression resulting in a significant increase in the OPG:RANKL ratio (p<0.05) compared to the DSI control. At 8 weeks RANKL and OPG expression trended higher in the DSI control compared to the non-surgery control, resulting in an increased OPG:RANKL ratio. IL-10 expression was elevated in the DSI control over the non-surgery control. In the non-surgery cohort L. reuteri increased OPG and IL-10 expression. In the DSI cohort, L. reuteri decreased RANKL while increasing OPG expression resulting in a trend towards an increased OPG:RANKL ratio. n = 6–10 per group. * = significant to non-surgery control, ^ = significant to DSI control. Statistical analysis performed by 1-way ANOVA with Fisher’s LSD test.
Discussion
Recent studies have revealed a positive effect of probiotic supplementation on bone health in intact male mice and in estrogen-deficient female mice [23,24,26]. In contrast however, supplementation in intact female mice displayed no bone effect [26]. In the present study we reveal that dorsal skin incision (DSI) has long-lasting systemic effects on inflammatory status, and that treatment with the probiotic bacterium L. reuteri 6475 exerts a beneficial bone effect in female DSI mice in addition to modulating the expression of pro-inflammatory and pro-osteoclastogenic cytokines in the intestine and bone marrow.
Under normal physiological conditions inflammation is a controlled adaptive response by the body consequent to injury or infection, returning it to homeostasis. Dysregulation of this inflammatory process can lead to adverse pathology such as that seen in IBD, RA, estrogen deficiency and obesity [16–19,32]. Inflammatory cytokines can promote osteoclast bone resorption and bone loss. Previous studies investigating the effect of L. reuteri on bone health utilized intact male mice and the OVX model of estrogen deficiency; both models displaying significantly elevated markers of inflammation compared to intact female mice. In the present study, a model of non-pathological inflammation was required for L. reuteri to exhibit a bone effect in female mice.
Wound healing (surgery) induces a coordinated and controlled acute inflammatory response and was chosen over other models of inflammation such as low dose LPS- or DSS- induced inflammation, as these are closely related to pathological models of obesity and IBD respectively [33–36]. While inflammation associated with wound healing is generally considered localized to the site of insult, the present study revealed that at 8 weeks post-dorsal skin incision, general markers of systemic inflammation were still elevated: spleen, thymus and visceral fat weights were significantly increased. In addition, expression of pro-inflammatory genes, TNFα, IL-1β and IFNγ were elevated in the intestine. These markers indicated that the DSI mice, while visibly healed and showing no overt signs of illness or distress, were experiencing a low-grade systemic inflammation compared to the non-surgery controls.
In pathological states, such as RA or IBD, high levels of inflammation have an adverse effect on bone health. Pro- inflammatory and–osteoclastogenic cytokines such as, TNFα, IL-1β, IFNγ and RANKL, are increased while expression of the anti-osteoclastogenic cytokine OPG is decreased. This results in enhanced osteoclast formation and activity and suppressed osteoblast bone formation, leading to net bone loss [28,37–39]. The effect of low-grade inflammation on bone health however, is not as well defined. In adverse conditions such as obesity and diabetes, which are associated with a dysregulated chronic low grade inflammation, a lower BV/TV has been reported due to increased osteoclastogenesis and decreased osteoblast differentiation [20,25,40–42]. In the present study, no difference in trabecular BVF was observed between the non-surgery and the DSI mice. However, we did observe that DSI increased cortical bone parameters, including thickness, mineral content and calculated strength. MAR and serum osteocalcin levels were also elevated in the DSI mice, suggesting in this model that inflammation increased bone remodeling and that bone outcomes are site dependent. Specifically, surgery-induced changes were targeted to cortical bone where resorption and formation are not in balance and lead to structural changes. Osteocytes are known to play a critical role in the maintenance of homeostatic bone remodeling, through the production of RANKL [43,44] and could be involved in this process and contribute to site specific differences. We specifically examined bone marrow changes to assess the role of immune cells in DSI and L. reuteri responses. We identified that RANKL, OPG and IL-10 gene expression in DSI BM significantly increased along with the number of CD4+ T cells, another known producer of RANKL [1]. The results of these analyses support the notion that DSI increases bone remodeling that is in balance in trabecular bone but not in cortical bone. This effect is in contrast to what has been observed in obesity and diabetes, where RANKL is elevated and OPG and IL-10 expression reduced [42,45]. These observations suggest that the context of inflammation, controlled versus pathologic, has an important role in the outcome on bone health. Having identified that DSI induced a controlled systemic inflammatory response in female mice that had no detrimental effect on bone health, we demonstrate that supplementation with L. reuteri could produce the beneficial bone effect observed in male mice and OVX female mice.
In-line with previous reports for intact and diabetic male mice, L. reuteri had no effect on general body mass in either the non-surgery or DSI cohorts [25,26]. Consistent with previously published bone data supporting a requirement for inflammation in the response to probiotics, L. reuteri had an anabolic effect on bone only in the DSI cohort [23,26]. Also in line with this, 2-way ANOVA, used to examine if L. reuteri and surgery effects on mineral apposition rate were dependent or independent, determined that L. reuteri had no significant effect on MAR on its own. The exact mechanism through which L. reuteri increases bone density is currently not fully understood. However, in the present study L. reuteri modulated expression of BM RANKL, OPG and IL-10 gene expression, shifting the balance towards an anti-osteoclastogenic environment. Interestingly, the level of modulation was dependent on the presence of inflammation with the greatest changes observed in the DSI cohort, potentially due to the increased numbers of CD4+ T cells present in the BM. Studies have identified that probiotic bacteria can modulate T cell differentiation, up-regulating CD4+Foxp3+ Tregs while down-regulating Th1 and Th17 cytokine expression [46–48]. In the present study the numbers of CD4+Foxp3+ Tregs were not measured though the increase in expression of IL-10 and decreased RANKL gene expression suggests that L. reuteri is potentially modulating CD4+ T cell lineage selection. What remains to be determined however, is whether L. reuteri is modulating lineage selection within or before migration to the BM. While in the present study changes in BM gene expression were observed in the surgery cohort at 4 weeks a previous study from our laboratory reported no changes in bone density in sham operated mice at this time point [23]. This suggests that in female mice, under non-pathological low-grade inflammation, long-term supplementation with L. reuteri is required to increase bone density. The exact mechanisms of how intestinal microbiota affects bone health is still under investigation, though modulation of intestinal pro-inflammatory cytokine expression has been suggested to have an important role [26,49].
In the present study L. reuteri had regional and inflammation-dependent effects on intestinal cytokine gene expression. In the DSI cohort jejunal pro-inflammatory cytokine expression was reduced, supporting the observations in male mice by McCabe, Britton et al [26]. Interestingly however, an increase in colonic pro-inflammatory cytokine expression, notably TNF, was observed. Why L. reuteri would have region specific effects on cytokine expression in the intestine is not clear from this study, though a similar effect with commensal bacteria had been observed in gnotobiotic pigs [50]. A possible explanation for the regional difference in cytokine response is the difference in cellular make-up of the sections of the intestine; higher numbers and density of Peyer’s patches are found in the small intestine while equivalent M-cell containing macroscopic structures are found in the large intestine. Additionally 10- to 20-fold more intraepithelial lymphocytes can be isolated from the small intestine compared to the colon [51]. This suggests L. reuteri has differential effects and highlights a complex interplay between microbiota and host.
The L. reuteri-induced increase in intestinal pro-inflammatory cytokine expression is not without precedent. In a study by Pagnini et al [52] the probiotic cocktail VSL#3 stimulated ileal epithelial production of TNF. This increase in TNF was thought to protect against the onset of intestinal inflammation in the SAMP mouse model of Crohn’s disease-ileitis via local stimulation of the epithelial innate immune system, restoring epithelial barrier function. The effect of L. reuteri on epithelial barrier function in the current study cannot be conclusively determined. As with cytokine expression, region- and inflammation-dependent effects were apparent in epithelial tight junction protein mRNA expression. In the jejunum, under an inflammatory setting, L. reuteri increased mRNA expression of the tight junction protein occludin. In contrast, L. reuteri led to decreased occludin expression in the colon under non-inflammatory conditions. Whether these changes translate to changes in intestinal permeability however, remains to be investigated.
Comparison of the present study to the effects of L. reuteri on male mice and the estrogen-deficient OVX female mice raises a number of intriguing queries. Normal intact female mice display significantly lower intestinal inflammation compared to their male counterparts (unpublished data from our laboratory), while following estrogen deficiency inflammatory levels increase [32]. This suggests a minimal level of inflammation is required for L. reuteri to exert a beneficial bone effect. What this activating factor is however remains to be determined; whether it’s the presence of pro-inflammatory cytokines, certain activated immune cells in or around the intestine or some other hitherto unknown factor.
In summary, this study provides the first evidence that the probiotic bacteria L. reuteri requires a mild inflammatory status in vivo to provide a beneficial bone effect in female mice. We show that surgical dorsal skin incision induces a controlled systemic low -grade chronic inflammation that increases expression of pro-inflammatory cytokines in the intestine and the BM. We reveal that L. reuteri modulates expression of intestinal pro-inflammatory cytokines and tight junction protein expression in a regional- and inflammation-dependent manner and that L. reuteri can regulate BM gene expression of RANKL, OPG and IL-10. These data reveal the complex interaction between host and microbiota and the gut–bone axis.
Acknowledgments
The authors thank the Investigative Histology Laboratory in the Department of Physiology, Division of Human Pathology and the Biomedical Imaging Center at Michigan State University for their assistance with histology and imaging, respectively. The authors are also grateful to the staff of Campus Animal Resources for the excellent care of our animals. These studies were supported by funding from the National Institute of Health, grants RO1 DK101050 and RO1 AT007695.
Data Availability
All relevant data are within the paper.
Funding Statement
Study funded by National Center for Complementary and Integrative Health (https://nccih.nih.gov/), U.S National Institutes of Health (Grant Code: 1R01AT007695-01) awarded to LRM, NP and RAB and by National Institute of Diabetes and Digestive and Kidney Diseases (www.niddk.nih.gov/), U.S National Institutes of Health (Grant code: R01DK101050) awarded to LRM and NP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5: 667–76. 10.1038/nrrheum.2009.217 [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25: 629–48. 10.1146/annurev.cellbio.042308.113308 [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170: 427–35. 10.2353/ajpath.2007.060834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275: 4858–64. Available: http://www.ncbi.nlm.nih.gov/pubmed/10671521 [DOI] [PubMed] [Google Scholar]
- 5.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. 2005;115 10.1172/JCI200523394.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117: 122–132. 10.1172/JCI30074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair HC, Athanasou NA. Recent advances in osteoclast biology and pathological bone resorption. Histol Histopathol. 2004;19: 189–99. Available: http://www.ncbi.nlm.nih.gov/pubmed/14702187 [DOI] [PubMed] [Google Scholar]
- 8.Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007;8: 4 10.1186/1471-2121-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Yao S, Wise GE. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur J Oral Sci. 2006;114: 42–49. 10.1111/j.1600-0722.2006.00283.x [DOI] [PubMed] [Google Scholar]
- 10.Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141: 3478–3484. 10.1210/en.141.9.3478 [DOI] [PubMed] [Google Scholar]
- 11.Souza PPC, Lerner UH. The role of cytokines in inflammatory bone loss. Immunol Invest. 2013;42: 555–622. 10.3109/08820139.2013.822766 [DOI] [PubMed] [Google Scholar]
- 12.Amarasekara DS, Yu J, Rho J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J Immunol Res. Hindawi Publishing Corporation; 2015;2015: 1–12. 10.1155/2015/832127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe L, Zhang J, Raehtz S. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2011;21: 187–206. 10.1615/CritRevEukarGeneExpr.v21.i2.70 [DOI] [PubMed] [Google Scholar]
- 14.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. Nature Publishing Group; 2014;14: 329–42. 10.1038/nri3661 [DOI] [PubMed] [Google Scholar]
- 15.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908: 244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 16.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. Nature Publishing Group; 2011;7: 569–578. 10.1038/nrrheum.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunne JL, Triplett EW, Gevers D, Xavier R, Insel R, Danska J, et al. The intestinal microbiome in type 1 diabetes. Clin Exp Immunol. 2014;177: 30–37. 10.1111/cei.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology. Elsevier, Inc; 2014;146: 1489–1499. 10.1053/j.gastro.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdam FJ, Fuentes S, De Jonge C, Zoetendal EG, Erbil R, Greve JW, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21: 607–615. 10.1002/oby.20466 [DOI] [PubMed] [Google Scholar]
- 20.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192: 292–297. 10.1111/j.1749-6632.2009.05252.x [DOI] [PubMed] [Google Scholar]
- 21.Schnabl B, Brenner D a. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. Elsevier, Inc; 2014;146: 1513–1524. 10.1053/j.gastro.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakchbandi I a, van der Merwe SW. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol. Nature Publishing Group; 2009;6: 660–670. 10.1038/nrgastro.2009.166 [DOI] [PubMed] [Google Scholar]
- 23.Britton R a, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229: 1822–30. 10.1002/jcp.24636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9: e92368 10.1371/journal.pone.0092368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Motyl KJ, Irwin R, MacDougald O a., Britton R a., McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic L. reuteri. Endocrinology. 2015; EN20151308. 10.1210/EN.2015-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCabe LR, Irwin R, Schaefer L, Britton R a. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228: 1793–8. 10.1002/jcp.24340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diodato MD, Knöferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14: 162–169. 10.1006/cyto.2001.0861 [DOI] [PubMed] [Google Scholar]
- 28.Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis. 2013;19: 1586–97. 10.1097/MIB.0b013e318289e17b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Card JW, Carey M a., Bradbury J a., DeGraff LM, Morgan DL, Moorman MP, et al. Gender Differences in Murine Airway Responsiveness and Lipopolysaccharide-Induced Inflammation. J Immunol. 2006;177: 621–630. 10.4049/jimmunol.177.1.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010;191: 7–13. 10.1083/jcb.201006123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. The Canadian Society of Clinical Chemists; 2012;45: 863–873. 10.1016/j.clinbiochem.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 32.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116: 1186–1194. 10.1172/JCI28550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo S, Dipietro L a. Factors affecting wound healing. J Dent Res. 2010;89: 219–229. 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eming S a, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127: 514–525. 10.1038/sj.jid.5700701 [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56: 1761–1772. 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 36.Irwin R, Raehtz S, McCabe LR. Intestinal inflammation without weight loss decreases bone density and growth. Am J Physiol Gastrointest Liver Physiol. 2015;Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McInnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine. 2011. pp. 2205–2219. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 38.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296: G1020–G1029. 10.1152/ajpgi.90696.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu S, Wang Y, Lu J, Xu J. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol Int. 2012;32: 3397–3403. 10.1007/s00296-011-2175-5 [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil GS. Inflammation and metabolic disorders 1. Nature. 2006;444: 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 41.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. Elsevier B.V.; 2009;44: 1097–1104. 10.1016/j.bone.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 42.Kyung T-W, Lee J-E, Phan T Van, Yu R, Choi H-S. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. J Nutr. 2009;139: 502–506. 10.3945/jn.108.100032 [DOI] [PubMed] [Google Scholar]
- 43.O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. Elsevier B.V.; 2013;54: 258–263. 10.1016/j.bone.2012.08.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. Nature Publishing Group; 2011;17: 1231–1234. 10.1038/nm.2452 [DOI] [PubMed] [Google Scholar]
- 45.Halade G V., El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. Elsevier Inc.; 2011;46: 43–52. 10.1016/j.exger.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon H-K, Lee C-G, So J-S, Chae C-S, Hwang J-S, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010;107: 2159–64. 10.1073/pnas.0904055107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smits HH, Engering A, Van Der Kleij D, De Jong EC, Schipper K, Van Capel TMM, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115: 1260–1267. 10.1016/j.jaci.2005.03.036 [DOI] [PubMed] [Google Scholar]
- 48.Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5 10.1371/journal.pone.0009009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh D V, Hu W, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7: e31951 10.1371/journal.pone.0031951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirkey TW, Siggers RH, Goldade BG, Marshall JK, Drew MD, Laarveld B, et al. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med (Maywood). 2006;231: 1333–1345. [DOI] [PubMed] [Google Scholar]
- 51.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014; 10.1038/nri3738 [DOI] [PubMed] [Google Scholar]
- 52.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107: 454–459. 10.1073/pnas.0910307107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.