Abstract
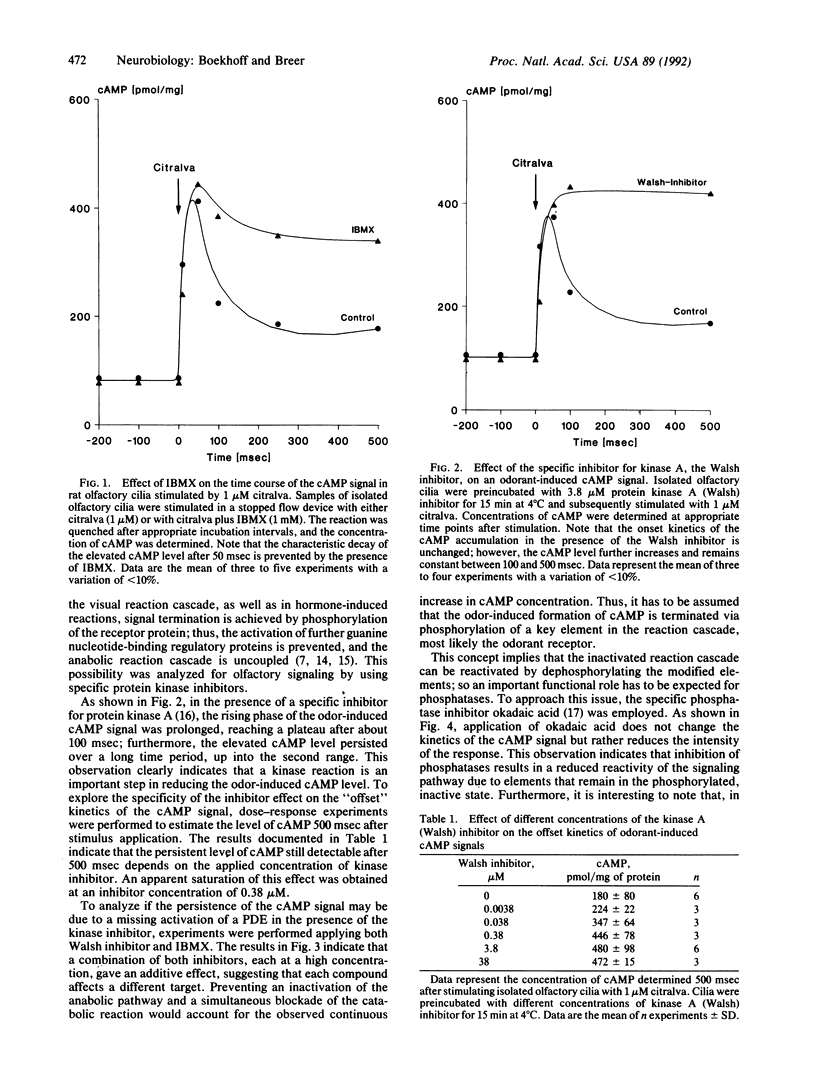
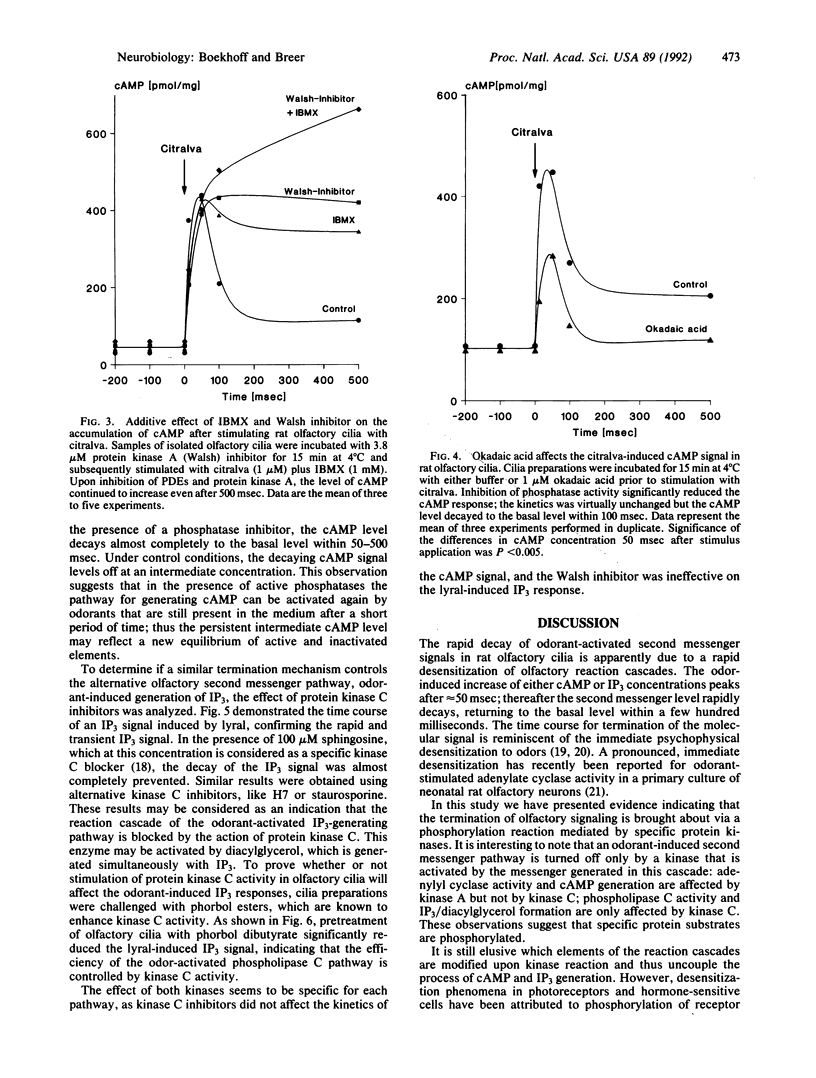
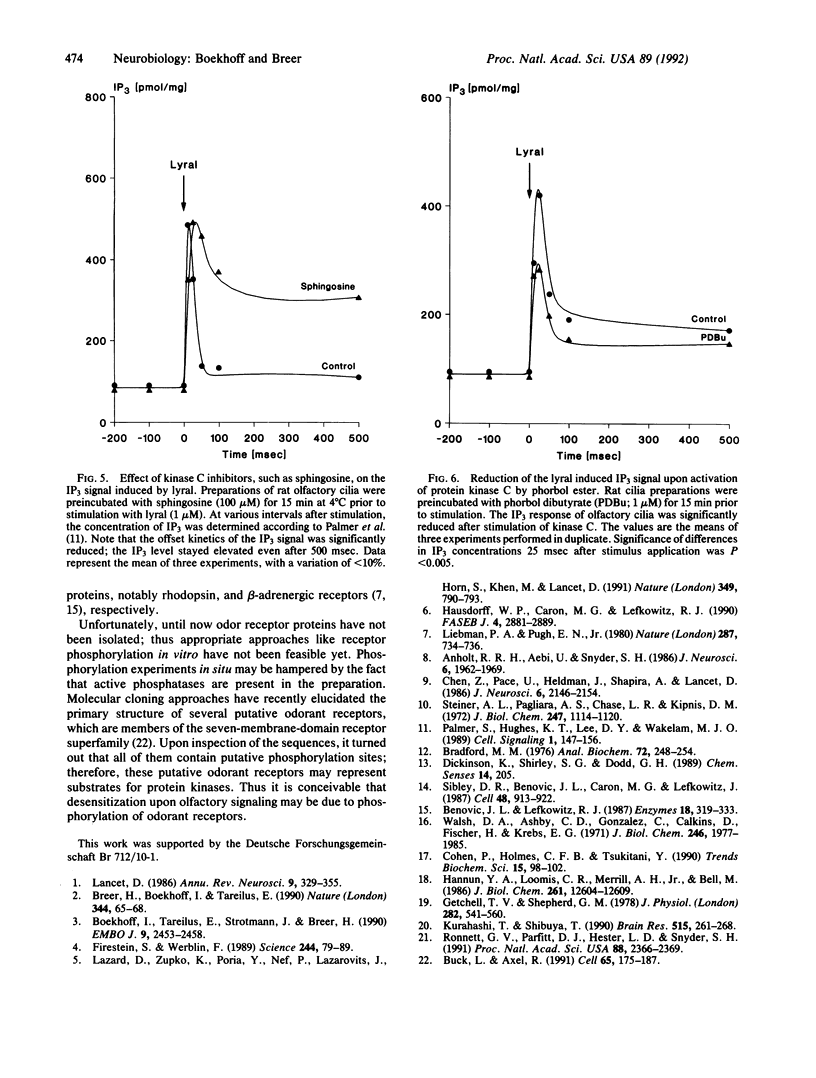
By using isolated rat olfactory cilia and a fast kinetics methodology, it has been demonstrated that odorant-induced second messenger signaling in the millisecond time range is terminated via phosphorylation reactions catalyzed by specific protein kinases. The cyclic adenosine nucleotide pathway is turned off by kinase A activity, whereas the inositol trisphosphate cascade is terminated by kinase C. The data support the concept that desensitization of odorant responses involves phosphorylation of key elements in the transduction cascade.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anholt R. R., Aebi U., Snyder S. H. A partially purified preparation of isolated chemosensory cilia from the olfactory epithelium of the bullfrog, Rana catesbeiana. J Neurosci. 1986 Jul;6(7):1962–1969. doi: 10.1523/JNEUROSCI.06-07-01962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I., Tareilus E., Strotmann J., Breer H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990 Aug;9(8):2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breer H., Boekhoff I., Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990 May 3;345(6270):65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Pace U., Heldman J., Shapira A., Lancet D. Isolated frog olfactory cilia: a preparation of dendritic membranes from chemosensory neurons. J Neurosci. 1986 Aug;6(8):2146–2154. doi: 10.1523/JNEUROSCI.06-08-02146.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Firestein S., Werblin F. Odor-induced membrane currents in vertebrate-olfactory receptor neurons. Science. 1989 Apr 7;244(4900):79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. J Physiol. 1978 Sep;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Kurahashi T., Shibuya T. Ca2(+)-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990 May 7;515(1-2):261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Lancet D. Vertebrate olfactory reception. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- Lazard D., Zupko K., Poria Y., Nef P., Lazarovits J., Horn S., Khen M., Lancet D. Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature. 1991 Feb 28;349(6312):790–793. doi: 10.1038/349790a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Parfitt D. J., Hester L. D., Snyder S. H. Odorant-sensitive adenylate cyclase: rapid, potent activation and desensitization in primary olfactory neuronal cultures. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2366–2369. doi: 10.1073/pnas.88.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]



