Liu, J., Yang, H., Lu, Q., Wen, X., Chen, F., Peng, L., Zhang, L., and Lu, C. (2012). PSBP-DOMAIN PROTEIN1, a nuclear-encoded thylakoid lumenal protein, is essential for photosystem I assembly in Arabidopsis. Plant Cell 24: 4992–5006.
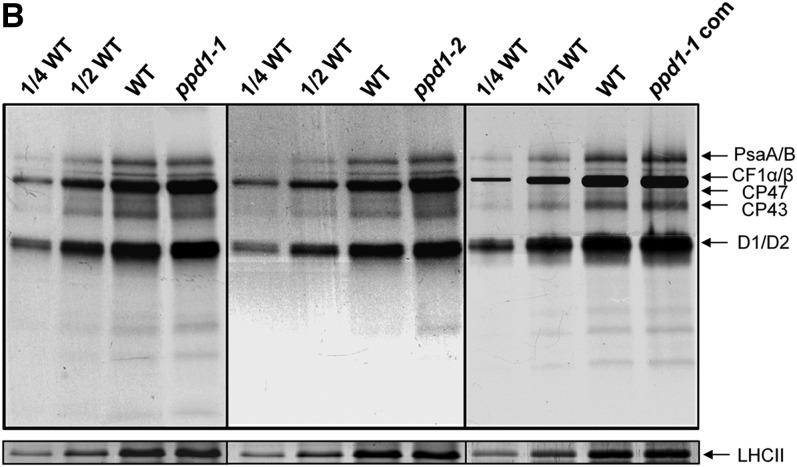
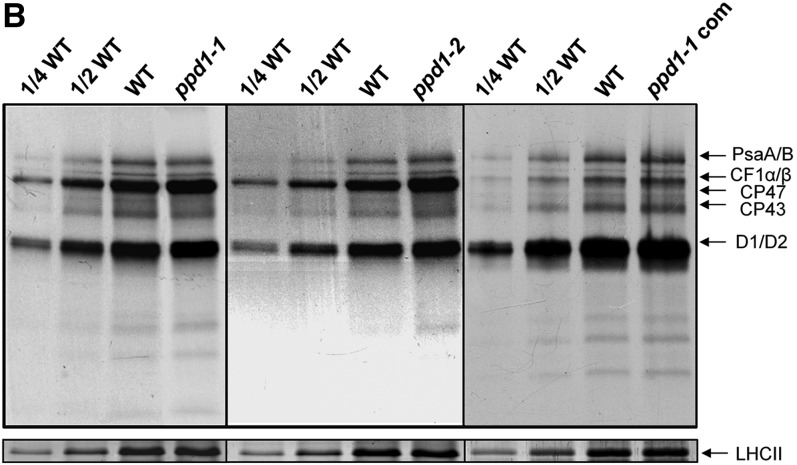
Figure 4B has been corrected. In the original version, there was inappropriate manipulation by the first author, Jun Liu, of the β-subunit of CF1 (CF1β) band to increase the apparent intensity of this band. The original unadjusted image of in vivo pulse labeling of thylakoid membrane proteins in ppd1-1 complemented plants (ppd1-com) is used in the corrected figure. The figure legend and conclusions of the manuscript are not changed by the correction.
Figure 4.
Original: PPD1 Acts on PSI Accumulation at the Posttranslational Level.
(B) In vivo pulse labeling analysis of thylakoid membrane proteins of the wild-type, ppd1-1, ppd1-2, and the complemented mutant. After a 20-min pulse labeling of Arabidopsis young seedlings in the presence of cycloheximide for 30 min to inhibit synthesis of nuclear-encoded proteins, thylakoid membrane proteins were isolated and fractionated by SDS-PAGE and visualized autoradiographically. Nonlabeled LHCII visualized by staining with CBB served as loading control. Four independent pulse-labeling experiments were performed and the same results were obtained each, and a representative one is shown.
Figure 4.
Corrected: PPD1 Acts on PSI Accumulation at the Posttranslational Level.
(B) In vivo pulse labeling analysis of thylakoid membrane proteins of the wild-type, ppd1-1, ppd1-2, and the complemented mutant. After a 20-min pulse labeling of Arabidopsis young seedlings in the presence of cycloheximide for 30 min to inhibit synthesis of nuclear-encoded proteins, thylakoid membrane proteins were isolated and fractionated by SDS-PAGE and visualized autoradiographically. Nonlabeled LHCII visualized by staining with CBB served as loading control. Four independent pulse-labeling experiments were performed and the same results were obtained each, and a representative one is shown.
Footnotes
Editor’s note: the corrected figure and accompanying text were reviewed by members of The Plant Cell editorial board.