Abstract
Strong laboratory services and systems are critical for delivering timely and quality health services that are vital to reduce patient attrition in the HIV treatment and prevention cascade. However, challenges exist in ensuring effective laboratory health systems strengthening and linkages. In particular, linkages and referrals between laboratory testing and other services need to be considered in the context of an integrated health system that includes prevention, treatment, and strategic information. Key components of laboratory health systems that are essential for effective linkages include an adequate workforce, appropriate point-of-care (POC) technology, available financing, supply chain management systems, and quality systems improvement, including accreditation. In this review, we highlight weaknesses of and gaps between laboratory testing and other program services. We propose a model for strengthening these systems to ensure effective linkages of laboratory services for improved access and retention in care of HIV/AIDS patients, particularly in low- and middle-income countries.
Introduction
Over the past decade, low- and middle-income countries, in collaboration with international partners, have made tremendous progress towards improving access to diagnosis, treatment, and prevention of HIV/AIDS. New HIV infections, morbidity, and mortality have decreased, and the proportions of people who know their HIV status and are enrolled into antiretroviral treatment (ART) programs have increased significantly.1–3 Sustaining and maximizing these gains is critical but there are many challenges. While ART efforts are being scaled up globally, patient attrition remains high between HIV diagnosis, enrollment, and retention in care.4–6
Laboratory testing plays a central role in this cascade of HIV treatment and prevention services, defined as series of stages from HIV diagnosis, linkage to, and retention in care, commencement of antiretroviral treatment (ART), adherence, and viral load suppression that translates to reduced risk of death and HIV transmission.7,8 Testing often stands at the thresholds of different stages of the cascade: diagnosing infection, determining eligibility for ART and/or prophylaxis, determining the efficacy and safety of treatment, and facilitating clinical decision-making (Fig. 1). However, it is often at these thresholds that patients are lost to follow-up. For example, a recent systemic review of the proportion of adult patient retention between any two points within the HIV treatment cascade showed that 59%, 46%, and 68% of patients were retained from HIV testing to receipt of CD4 count results, staging to ART eligibility, and from ART eligibility to ART initiation, respectively.5 Weaknesses in linkages between testing and follow-up clinical care are a major cause for low retention, compromise treatment, and prevention efforts. Strategies are needed to address these weaknesses and to ensure effective linkages and referrals to other services after laboratory testing within the cascade pathway. Models that improve linkages of services within an integrated health system are successful.9 In this article we review current gaps, discuss ways to address them, and highlight the central role of laboratory services and systems and their linkages and referrals to other components of the HIV treatment and preventio cascade.
FIG. 1.
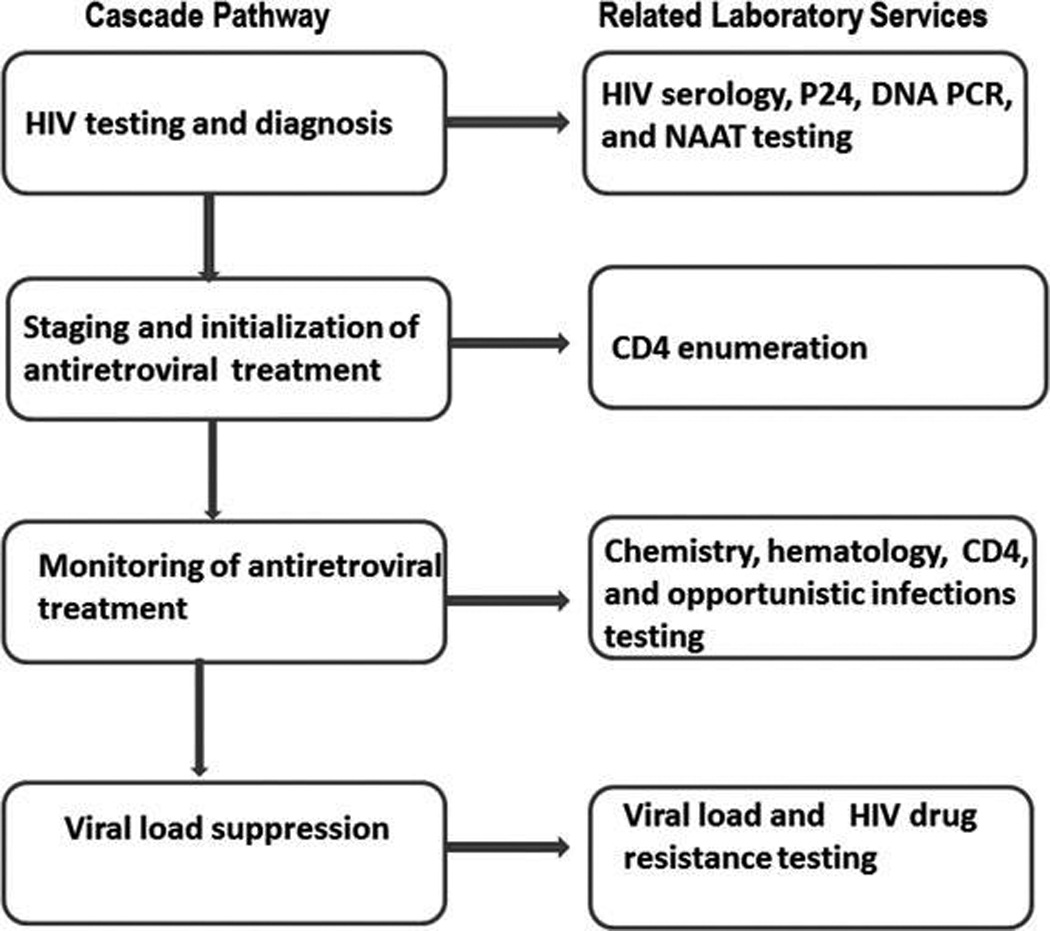
Different stages of the HIV Treatment and Prevention Cascade. It starts from HIV diagnosis to CD4 measurement for clinical staging and determination of eligibility for ART. This is followed by monitoring for efficacy and safety of treatment through measurement of chemistry, hematology, and CD4 parameters, and opportunistic infections testing. The final stage involves measurement of viral load to determine outcome of treatment and follow up HIV drug resistance testing for patients failing therapy.
Methodological Approach
Electronic sources that included PubMed, Cochrane library, MEDLINE, AIDSONLINE, and conference abstracts were searched to obtain current information on this subject and to ensure that similar reviews have not been published elsewhere. The search themes include laboratory health systems strengthening in resource-limited settings, linkages, and referrals between the laboratory and HIV treatment and prevention cascades, and attrition and loss to follow up. The initial approach involved reviewing abstracts and titles of articles. Articles with information relevant to the search themes were selected for detailed review. Full text of pertinent references that are cited in the original articles were also searched and reviewed to confirm validity and consistency of the information stated. Some of the information and references are also included in the current review article. Full text and abstract with bias or doubtful methodologies were excluded.
Results and Discussions
The write-up of the article was structured to ensure consistency of information flow and relevance to the title, starting with a proposed model to show the centrality of the laboratory in HIV treatment and prevention cascade and then a review of the literature and discussions to emphasize the need to strengthen linkages between the laboratory prevention, treatment, strategic information, and other health systems strengthening attributes that are associated with laboratory services and systems as outlined below. Detailed results and discussions are presented as follows:
Need for Laboratory Services and Systems Integration, Linkages, and Referrals to Other Services
The World Health Organization (WHO) recommends strengthening integrated health systems as a way to leverage resources and to ensure access to affordable healthcare. Some examples are the integration of HIV/AIDS, tuberculosis, malaria, maternal and child health (MCH), and noncommunicable diseases (NCDs)10 and the integration of prevention-of-mother-to-child-transmission (PMTCT) services into MCH systems.11 Successful integration of services requires effective linkage of one service system to another for an uninterrupted continuum of care. Referral processes within an integrated HIV/AIDS care continuum depend heavily on successful laboratory linkages.8 This is because the laboratory is centrally located or has a cross-cutting relationship with other services such as prevention, treatment, and surveillance, which are key attributes of the cascade. Hence, efforts should be put in place to ensure effective integration and strengthening of these linkages and referral services between the laboratory and other health services and systems within a treatment and prevention cascade.
Laboratory prevention linkages
HIV prevention is designed for intervention before exposure, at exposure, immediately after exposure, and for longer periods after exposure. Timely diagnosis of individuals and referral to treatment at any of these stages are dependent, at least in part, on the laboratory. The absence of HIV testing and counseling (HTC) services with linkages to laboratory capacity to support a diagnosis has been responsible for low rates of testing, low knowledge of HIV status, and poor design and implementation of HIV prevention strategies in certain settings. For example, earlier data from more than 10 sub-Saharan African countries indicate that more than 60% of people living with HIV (PLWH) do not know their HIV status.12 The most recent UNAIDS report2 indicates that more than 50% of people living with HIV worldwide do not know their HIV status. Additionally, studies from Uganda and Kenya have reported that more than 80–90% of PLWH do not know their partner’s HIV status.13,14 This could be due to several factors, including the fact that in some resource-limited settings the majority of HIV-infected persons either live in rural communities or they are unable to access HIV diagnostic services at the central laboratory level due to logistical challenges, stigma, and discrimination.15 To overcome this, strategies to encourage the uptake of point-of-care (POC) HIV rapid testing and its effective integration and linkages to HTC services are necessary to ensure scale-up diagnostic and referral support services and systems within these countries.
Although studies have shown concordance between HIV testing conducted at a central laboratory compared to peripheral and community levels, including testing performed by trained nonlaboratory personnel, some HTC programs still choose to send positive HIV samples from combination community HIV rapid testing to the central laboratory for retesting and confirmation. This may impact program uptake and contribute to attrition and loss to follow-up care documented in some settings because some of the people tested do not return for their test results.1,5 Delays in filling such programmatic gaps will impact current efforts as timely knowledge of HIV serostatus through testing is critical for effective HIV prevention and treatment support services. As these programs are established in the communities and rural settings, policy changes are needed. These include supporting task shifting that will allow trained nonlaboratory persons, including counselors, outreach workers, and nurses to be engaged in HIV rapid testing since the limited qualified laboratory staff will not be able to support the increased demand for more qualified testers.16 Ensuring the accuracy of HIV rapid test results is critical. All testers should be well trained with emphasis on practical hands-on and then certified for competency. Testers and sites should participate in external quality assurance or proficiency testing programs and the use of standard documentation to ensure quality of rapid testing. Community-based HIV rapid testing and immediate release of test results is necessary to decrease the number of patient loss to follow-up. This can only be achieved where there is improved laboratory-prevention linkage. There is evidence of reduction in HIV transmission behaviors among individuals and couples in settings where laboratory-prevention linkages have been strengthened.17
Another challenge encountered by the interface between laboratory services and prevention strategies is the lack of capacity for more sophisticated testing that is needed for the diagnosis of early or acute HIV infection. In HIV infection, viral load is usually very high during the acute or early phase of infection.18 Transmission rates during acute or early HIV infection are higher than in chronic infection.19 The treatment as prevention paradigm is based on the premise that high viral loads lead to increased likelihood of HIV transmission.20 Hence, strategies to identify infected persons early and to get them into treatment to reduce viral loads and prevent transmission will be very helpful. Unfortunately, routine serological assays for HIV diagnosis are limited by their sensitivity to detect HIV infection during the acute or early infection period because of the window period, or the time it takes for serological antibodies to develop.21 This may result in a missed diagnosis and lead to an increased risk of further transmission. To fill this gap, more sensitive diagnostic assays, including pooled HIV RNA screening, and other antigen tests should be used as follow-up tests, particularly for discordant serological samples and for samples from people at high risk for HIV infection whose results are negative with serological assays. Early HIV diagnosis among infants to determine their status and to institute strategies to prevent infection among those diagnosed HIV negative is an important prevention approach. Serological testing is not accurate in diagnosing HIV infection among newborn infants since passive transfer of maternal antibodies can persist for as long as 18 months.22 Molecular testing such as DNA PCR would be required for early infant diagnosis (EID) to support PMTCT programs. However, advanced molecular testing for EID and early or acute adult HIV infection, including pooled HIV RNA, may not be available at the peripheral or community levels of the national health systems in most developing countries.23 To improve access to high technology and more sensitive diagnostic assays to support HIV prevention among infants and adults, it is recommended that laboratory referral services and laboratory networks for these diagnostic platforms should be strengthened and linked to HIV prevention services. Ultimately, POC tests are urgently needed to expand the capacity for accurate diagnostics in a decentralized manner, but until that time reference laboratories will continue to have a critical role.
Laboratory treatment and care linkages
The 2011 landmark HIV Prevention Trials Network 052 study showed conclusively that treatment reduced HIV viral load and decreases the risk of HIV transmission.20 Several recommendations, including the WHO clinical staging guidelines12 and various health economics cost-benefit theories,24 have highlighted the benefits of different treatment options to include initiation of ART at CD4 cell counts > 350 and > 500 cells/mm3, as well as Option B+ strategies25 that does not require measurement of CD4 cells to initiate treatment.26 These recommendations show that all newly diagnosed patients should be placed on ART as soon as possible to obtain optimal benefits. A study from South Africa showed that early HIV diagnosis and early ART reduced early infant mortality by 76% and HIV progression by 75%.27 The laboratory has a central role in HIV treatment monitoring (e.g., staging and enrollment into ART, effectiveness of treatment, toxicity, and drug resistance testing). Despite this, poor access to laboratory services is still negatively impacting HIV treatment program uptake. For example, in Malawi, data from more than 8000 mother–infant pairs showed that infected infants have at least a five to eight times higher rate of death compared to uninfected children because of lack of a laboratory facility for HIV DNA PCR testing that is needed to identify infected children who could benefit from ARV treatment.28 A systemic review article on patient retention in an ART program in sub-Saharan Africa showed that less than 59% of patients either received CD4 results or were staged after diagnosis; and 75% of these patients were retained in care 1 year after initiation of ART.29 Also, a joint WHO/UNAIDS/UNICEF30 publication stated that only 24% of HIV-positive pregnant women in low- and middle-income countries received CD4 test results. Finally, increased access to HIV viral load testing has also become a critical and limiting issue in low- and middle-income countries due to the expansion of ART.1 Weak or poor access to laboratory testing services to support HIV treatment undoubtedly contributes to these challenges. These services need to be restructured and strengthened to improve treatment program uptake and to reduce the high patient attrition rate. For example, in Mozambique, after introducing point of careCD4 for same day results, the proportion of patients lost to follow-up before completion of CD4 staging dropped from 57% to 21%.3 A holistic approach to improving access to various laboratory services to support HIV treatment is necessary.
Laboratory strategic information linkages
Capturing patient data to monitor and ensure retention in care is an important component of the HIV treatment and prevention cascade. Key aspects of the cascade pathway depend on generation, tracking, analysis, and use of laboratory data. Some studies have reported substantial loss of patients at several steps in the cascade due to poor capturing and tracking of laboratory data.6,32 The lack of functional health information systems (HIS) and laboratory information systems (LIS) to track patients between different services has partially contributed to this challenge. LIS are needed to capture and report all testing data to the appropriate sections of the cascade and to the national central system by interfacing with HIS for wider access and coverage for policy decision making. Attrition at different steps of the continuum of care due to poor data capturing tools has been responsible for overall low levels of viral load suppression in a large population of PLWH.7 There have also been documented cases of mismatched HIV test results or the inability of testing services to correctly track and deliver HIV test results because of poor or weak laboratory data collection systems.5 Bridging gaps and establishing linkages between the laboratory services and strategic information systems will be necessary to attain and sustain a positive HIV prevention and treatment impact. For example, in Uganda, incorporation of electronic records into clinical care led to a 30% reduction in missed visits compared to when these records were not incorporated.33 Setting up surveillance systems to capture laboratory data for analysis and decision making are necessary to refine and implement new strategies to improve access and utilization of HIV treatment and prevention services.
Health system strengthening attributes to improve laboratory services
Health systems strengthening (HSS) include attributes that ensure and guide a country’s ability to deliver quality health for the well-being of its population.34 There are key laboratory attributes that should be strengthened as components of an integrated health system to ensure that they are effectively linked to other services for maximum program impact.
Laboratory institutions and capacity strengthening
A lack of an adequately trained workforce has partly been responsible for some of the laboratory health systems challenges in developing countries.35 Part of this has been attributed to the lack of training institutions with a curriculum tailored to the needs of the laboratory and to a high attrition rate of already trained staff.36 Institutional capacity and training curriculum need to be reviewed, strengthened, and coordinated with strategies to retain staff. Also, strengthening laboratory services and systems, and developing and utilizing evidence-based user-friendly tools to improve quality systems, plus accreditation of laboratories as benchmarks for laboratory competence, are important. This will ensure a cadre of qualified laboratory staff and improved quality systems necessary to release test results in support of HIV treatment and prevention programs. Furthermore, appropriate human resources for health should be addressed. While task shifting has been used to fill certain gaps in health workforce cadre, efforts at seeking proportional or balance workforce to workload should be pursued.
Health systems financing
Health systems financing, negotiating reduced cost of laboratory commodities including procurement of equipment (servicing and maintenance contracts) and reagents, and building robust linkages through public—private partnerships with suppliers should also be targeted as an approach toward ensuring access to affordable testing.37 Data presented in this review highlight weak or poor access to laboratory services, partly associated with high cost or nonavailability of laboratory testing services. This negatively impacts treatment and prevention efforts. Cost reduction for laboratory equipment and reagents through health systems financing will ultimately reduce the cost per test and will improve access to HIV diagnostic services, enrollment into care and treatment, and reduction in patient attrition within the cascade. Furthermore, availability of funds through health systems financing will improve the supply chain management system and ensure rapid procurement and delivery of laboratory commodities. This will reduce turnaround time for testing and delivery of results, resulting in improved endpoint service delivery within the HIV treatment and prevention cascade.
Point of care technology
Difficulties in accessing laboratory services for CD4 cell counts, HIV viral load, and DNA PCR in resource poor settings have hampered the effectiveness of PMTCT programs and other adult HIV diagnostic and treatment services.1,30 This has partly been attributed to the sophistication and cost of these technologies since they are not affordable in resource-constrained countries. The impact of simple, cheap, and affordable POC technologies in program expansion has been demonstrated in certain settings. For example, where there was prompt access to POC HIV rapid testing and CD4 cell counts, there was a tremendous improvement in the proportion of patients who were diagnosed, staged, and referred into care.1,31,38 Despite the benefits of POC technologies, they are not widely available for use in most countries because some of the technologies are still in the developmental stages. Moreover, in instances where POC technology is available, regulatory and bureaucratic issues have delayed their uptake. Developing appropriate policies and guidelines for POC technologies in sub-Saharan Africa will ensure that these beneficial technologies are rapidly rolled out for increased access. As POC technologies become available, they should be integrated and linked to treatment and prevention services to reduce gaps that lead to attrition of patients within the cascade pathway.
Operational Research Monitoring and Evaluation
As the entire health system is revisited to address new opportunities and challenges, monitoring and evaluation of any proposed models or structures is needed. As programs are expanded, both process and impact evaluations should be conducted to identify and correct any implementation gaps. Two possible indicators for measuring positive impact of HIV prevention and treatment cascade are reductions in HIV incidence and a corresponding increase in the number of people placed on ART. Suitable laboratory incidence assays are needed to measure HIV incidence. The treatment cascade contains different laboratory endpoints such as number of people diagnosed and linked to care, and the number of people retained in care measured through evidence of early infant and adult HIV diagnosis, CD4, and viral load test results. Targets should be set and indicators for these variables developed and measured to determine successes and challenges at various levels or stages of the treatment cascade. Implementing scientific research toward achieving these targets is highly encouraged. Using existing laboratory data for program impact evaluation as a quick means for developing policies to improve performance is recommended as a good start.
Conclusions
Collaborations between international and local partners and governments in low- and middle-income countries through evidence-based scientific research and directly funded interventions have led to tremendous improvements in HIV prevention and treatment efforts. The laboratory has a central role in all stages of the HIV treatment and prevention cascade pathway. Despite this, laboratory health systems remain weak and are poorly linked with other services within the continuum of care in low- and middle-income countries. Because of these gaps, it is difficult to realize HIV prevention and treatment benefits at the individual and population level within these countries. To overcome this, laboratory services and systems need to be strengthened to improve HIV treatment and prevention program uptake. Ensuring linkages and referrals between the laboratory services and other services within an integrated health system is also needed to improve access to diagnosis, clinical monitoring, and to reduce attrition and lost to follow-up of HIV/AIDS patients on ART.
Acknowledgments
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC).The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC/Agency for Toxic Substances and Disease Registry.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO HIV/AIDS. Fact sheet No 360. [Accessed August 26, 2013]; Available at: http://www.who.int/mediacentre/factsheets/fs360/en/index.html.
- 2.UNAIDS. World AIDS day report 2012: results. [Accessed June 24, 2013]; Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf. [Google Scholar]
- 3.Marum E, Taegtmeyer M, Parekh B, et al. “What took you so long?” The impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. J Acquir Immune Defic Syndr. 2012;60:S63–S69. doi: 10.1097/QAI.0b013e31825f313b. [DOI] [PubMed] [Google Scholar]
- 4.Nglazi MD, Kaplan R, Wood R, Bekker LG, Lawn SD. Identification of losses to follow-up in a community-based antiretroviral therapy clinic in South Africa using a computerized pharmacy tracking system. BMC Infect Dis. 2010;10:329. doi: 10.1186/1471-2334-10-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: A systematic review. PLoS Med. 2011;8:1–16. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somi G, Keogh SC, Todd J, et al. Low mortality risk but high loss to follow-up among patients in the Tanzanian national HIV care and treatment programme. Trop Med Int Health. 2012;17:497–506. doi: 10.1111/j.1365-3156.2011.02952.x. [DOI] [PubMed] [Google Scholar]
- 7.Kilmarx PH, Mutasa-Apollo T. Patching a leaky pipe: The cascade of HIV care. Curr Opin HIV AIDS. 2013;8:59–64. doi: 10.1097/COH.0b013e32835b806e. [DOI] [PubMed] [Google Scholar]
- 8.HIV Health Services San Francisco. [Accessed May 21, 2013];Linkages from HIV testing to HIV care. Available at: http://www.sfhivcare.com/PDFs/linkages_from_testing_to_care_best_practices.pdf. [Google Scholar]
- 9.Mutalemwa PP, Kisinza W, Urassa JAE, et al. Integrating reproductive and child health and HIV services in Tanzania: Implication to policy, systems and services. Tanzan J Health Res. 2013;15:1–10. doi: 10.4314/thrb.v15i2.8. [DOI] [PubMed] [Google Scholar]
- 10.Gounder CR, Chaisson RE. A diagonal approach to building primary healthcare systems in resource-limited settings: Women-centred integration of HIV/AIDS, tuberculosis, malaria, MCH and NCD initiatives. Trop Med Int Health. 2012;17:1426–1431. doi: 10.1111/j.1365-3156.2012.03100.x. [DOI] [PubMed] [Google Scholar]
- 11.Ginsburg AS, Hoblitzelle CW, Sripipatana TL, Wilfert CM. Provision of care following prevention of mother-to-child HIV transmission services in resource-limited settings. AIDS. 2007;21:2529–2532. doi: 10.1097/QAD.0b013e3282f155f4. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Geneva: World Health Organization; 2010. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach: 2010 revision. [PubMed] [Google Scholar]
- 13.Bunnell R, Cherutich P. Universal HIV testing and counselling in Africa. Lancet. 2010;371:214. doi: 10.1016/S0140-6736(08)60929-0. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser R, Bunnell R, Hightower A, et al. Factors associated with HIV infection in married or cohabitating couples in Kenya: Results from a nationally representative study. PLoS One. 2011;6:e17842. doi: 10.1371/journal.pone.0017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odimegwu C, Adedini SA, Ononokpono DN. HIV/AIDS stigma and utilization of voluntary counselling and testing in Nigeria. BMC Public Health. 2013;13:465. doi: 10.1186/1471-2458-13-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zachariah R, Ford N, Philips M, et al. Task shifting in HIV/AIDS: Opportunities, challenges and proposed actions for sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2009;103:549–558. doi: 10.1016/j.trstmh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Sherr L, Lopman B, Kakowa M, et al. Voluntary counselling and testing: Uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. AIDS. 2007;21:851–860. doi: 10.1097/QAD.0b013e32805e8711. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Lodi S, Fox Z, et al. Rate of CD4 decline and HIV-RNA change following HIV seroconversion in men who have sex with men: A comparison between the Beijing PRIMO and CASCADE cohorts. J Acquir Immune Defic Syndr. 2012;62:441–446. doi: 10.1097/QAI.0b013e31827f5c9a. [DOI] [PubMed] [Google Scholar]
- 19.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faraoni S, Rocchetti A, Gotta F, et al. Evaluation of a rapid antigen and antibody combination test in acute HIV infection. J Clin Virol. 2013;57:84–87. doi: 10.1016/j.jcv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Jain KK, Mahajan RK, Shevkani M, Kumar P. Early infant diagnosis: A new tool of HIV diagnosis in children. Indian J Med Res. 2011;36:139–142. doi: 10.4103/0970-0218.84134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemnji G, Nkengasong J, Parekh BS. HIV testing in developing countries: What is required? Indian J Med Res. 2011;134:8. doi: 10.4103/0971-5916.92625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nosyk B, Montaner JSG. The evolving landscape of the economics of HIV treatment and prevention. PLoS Med. 2012;9:1–2. doi: 10.1371/journal.pmed.1001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chimbwandira F, Mhango E, Makombe S, et al. Impact of an innovative approach to prevent mother-to-child transmission of HIV—Malawi, July 2011–September 2012. MMWR. 2013;62:148–149. [PMC free article] [PubMed] [Google Scholar]
- 26.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 27.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dadabhai S, Kumwenda NI, Sun J, Kumwenda J, Rahman MH, Taha T. Child health outcomes in Blantyre, Malawi: 20-years of data from multiple longitudinal HIV cohorts. [Abstract MOPE 131]. Poster presented at 19th International AIDS Conference; July 22–27, 2012; Washington DC. [Google Scholar]
- 29.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: A systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO/UNICEF/UNAIDS. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector: progress report 2009. [Accessed April 23, 2013]; Available at: http://www.who.int/hiv/pub/tuapr_2009_en.pdf.
- 31.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: An observational cohort study. Lancet. 2011;378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 32.Kumwenda M, Tom S, Chan AK, et al. Reasons for accepting or refusing HIV services among tuberculosis patients at a TB-HIV integration clinic in Malawi. Int J Tuberc Lung Dis. 2011;15:1663–1669. doi: 10.5588/ijtld.10.0741. [DOI] [PubMed] [Google Scholar]
- 33.Alamo ST, Wagner GJ, Sunday P, et al. Electronic medical records and same day patient tracing improves clinic efficiency and adherence to appointments in a community based HIV/AIDS care program, in Uganda. AIDS Behav. 2012;16:368–374. doi: 10.1007/s10461-011-9996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Everybody’s business: Strengthening health systems to improve health outcomes: WHO’s framework for action. [Accessed August 26, 2013]; Available at: http://www.who.int/healthsystems/strategy/everybodys_business.pdf.
- 35.Abimiku AG. Building laboratory infrastructure to support scale-up of HIV/AIDS treatment, care and prevention. Am J Clin Path. 2009;131:875–886. doi: 10.1309/AJCPELMG6GX6RQSM. [DOI] [PubMed] [Google Scholar]
- 36.Olmsted SS, Moore M, Meili RC, et al. Strengthening laboratory systems in resource-limited settings. Am J Clin Pathol. 2010:374–380. doi: 10.1309/AJCPDQOSB7QR5GLR. [DOI] [PubMed] [Google Scholar]
- 37.Evans DB, Etienne C. Bulletin of the World Health Organization. Vol. 88. Geneva: WHO; 2010. [Accessed July 18, 2013]. Health systems financing and the path to universal coverage; p. 402. Available at: http://www.who.int/bulletin/volumes/88/6/10-078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guenter D, Greer J, Barbara A, Robinson G, Roberts J, Browne G. Rapid point-of-care HIV testing in community based anonymous testing program: A valuable alternative to conventional testing. AIDS Patient Care STDs. 2008;22:195–204. doi: 10.1089/apc.2007.0137. [DOI] [PubMed] [Google Scholar]


