Abstract
Objective
To assess if the presence of intra-articular Gd-DTPA2- during clinical MR arthrography significantly alters the T2* relaxation times of hip articular cartilage in patients with femoroacetabular impingement (FAI).
Materials and Methods
The institutional review board (IRB) approved this study, and informed consents were obtained in this HIPAA compliant study. T2* mapping of ten patient volunteers (F/M: 7/3, age range 14-49, mean±SD = 33.0 ± 12.2 years) with symptomatic FAI was performed before and after intra-articular Gd-DTPA2- administration. Overall 323 regions of interest (ROIs) were defined in acetabular and femoral cartilage each, pre- and post-Gd injection. Agreement of the relaxation times between pre- and post-Gd was assessed using Krippendorff's alpha coefficient and linear regression through the origin.
Results
Pre-Gd and post-Gd T2* relaxation times in both acetabular and femoral cartilage were found to agree strongly. Specifically, estimated Krippendorff's alphas were greater than 0.8 for both acetabular and femoral cartilage, and linear regressions through the origin yielded estimated slopes very close to 1 and R2 values greater than 0.98.
Conclusions
These results indicate that intra-articular injection of Gd-DTPA2-, along the protocol described in this study, has little effect on T2* values of femoral and acetabular cartilage. The results suggest that T2* mapping can be safely performed as an addition to standard clinical hip imaging protocol utilizing Gd-DTPA2-.
Keywords: T2*, hip FAI, gadolinium, acetabular cartilage, MRI
Introduction
Direct MR arthrography utilizing intra-articular gadolinium contrast agent and non-contrast MR imaging of the hip are the two most common cross-sectional imaging methods used to evaluate patients with femoroacetabular impingement (FAI) [1]. FAI is a cause of hip pain due to abnormal periarticular morphology that results in pathologic abutment between the head-neck junction and the acetabular rim leading to labral as well as chondral lesions and subsequent osteoarthritis [2, 3]. Usually young patients seek medical attention when symptoms of FAI occur, including hip pain, primarily in the groin, that interferes with activities of daily living. Only patients with relatively well-preserved acetabular cartilage will benefit from arthroscopic repair procedures. Despite the fact that in the majority of cases only minimal degenerative changes are noted on hip radiographs with the joint space being preserved, varying degrees of cartilage damage are detected arthroscopically [4]. To identify patients who would benefit from arthroscopic intervention, more accurate cartilage assessment is desirable [5].
Preliminary results using T2* mapping in FAI indicate that one can distinguish normal from abnormal hip articular cartilage [6-10], while standard MR imaging lacks diagnostic accuracy for the evaluation of cartilage lesions [11]. The advantages of T2* relaxation time mapping in hip joint cartilage include wide availability on clinical scanners, shorter imaging time, independent characterization of acetabular and femoral cartilage, high image resolution, and high signal-to-noise ratio (SNR) as compared to other quantitative methods, such as T1 or T2 mapping [9]. T2 as well as T2* are sensitive to the collagen matrix and water content in the articular cartilage. The main difference betweenT2* and T2 measurements is the lack of a 180-degree refocusing pulse, which makes T2* more sensitive to microscopic as well as macroscopic changes in the magnetic field [12, 13]. The inherently higher SNR of T2* is crucial when assessing the thin articular cartilage of the hip joint. Furthermore, the lower SAR of the T2* measurements compared to T2 measurements makes T2* appealing, especially at higher field strengths. The purpose of this study was to evaluate the influence of Gd-DTPA2- on T2* mapping values, an important step in validating clinical T2* mapping as a clinical marker of pre-radiologic cartilage damage in FAI.
Clinical MRI protocols for FAI typically utilize direct MR arthrography and include the intra-articular injection of the Gadolinium-based contrast agent, gadopentetate dimeglumine (Gd-DTPA2-). The influence of intra-articular contrast on concurrent T2* mapping sequences is unknown. While Gd-DTPA2- has a strong T1- shortening effect, it also influences T2 (and T2*) relaxation times [14]. Since the Gd-DTPA2- contrast agent distributes into cartilage in inverse relation to the proteoglycan (PG) content (i.e. the fixed charge density) of cartilage [15], the presence of Gd-DTPA2- could theoretically lead to an unexpected and complex pattern of T2 (and T2*) shortening.
To our knowledge, investigation of T2* relaxation time of acetabular cartilage with and without Gd-DTPA2- has only been reported in our previous study and at that in a limited number of cases [10]. A systematic evaluation of the effect of Gd-DTPA2- on T2* relaxation in cartilage is currently lacking. The purpose of this study was to evaluate the potential effect of intra-articular Gd-DTPA2- injection, as typically used in clinical hip FAI imaging protocol, on the T2* relaxation time values in acetabular and femoral cartilage in symptomatic FAI patients at 3.0 Tesla and to determine whether T2* mapping may be performed as an add-on to a clinical FAI MR protocol regardless of use of contrast agent.
Materials and Methods
Study population
The institutional review board of the University of Minnesota approved this HIPAA compliant prospective study (0911M74227). The study population consisted of ten consecutive patients with symptomatic FAI who presented to the orthopaedics clinic at the University of Minnesota without preexisting outside MR imaging between July 2013 and March 2014 (3 males, age range 42-48, mean 44 years; 7 females, age range 14-49, mean 28.3 years) and volunteered to participate in the study. Two of the ten patient volunteers were included in our previous initial investigation, which utilized different regions of interest (ROIs) and a different statistical analysis approach [10].
Inclusion criteria were all patients diagnosed with symptomatic FAI as defined by standard radiographic parameters who presented with symptoms consistent with and positive physical examination for FAI [16, 17]. Exclusion criteria were radiographic evidence of degenerative joint disease as defined by Tönnis grade >1 and prior hip surgery [18].
Subjects were referred for routine clinical hip MR arthrography. Informed consent was obtained from each patient volunteer for an additional T2* mapping sequence conducted immediately prior to intra-articular injection of Gd-DTPA2- and as the last sequence of the MR arthrogram.
MR Imaging
The MRI protocol consisted of T2* mapping in the sagittal plane before (pre-Gd scan) and at the end of a standard clinical MR arthrogram protocol (post-Gd scan) to allow evaluation of the effect of Gd-DTPA2- on T2*. Anatomical landmarks were utilized for identical positioning of the sagittal pre- and post-Gd T2* mapping slices. All the scans were performed on a clinical 3.0 Tesla scanner (Trio; Siemens Medical Solutions, Erlangen, Germany) using a body matrix phased array coil. The scanning protocol, including the sequences and parameters used is detailed in table 1.
Table 1.
Scanning protocol used in the study. The protocol started with T2* acquisition (pre-Gd scan) in sagittal plane, followed by intra-articular injection of Gd-DTPA2-, clinical imaging protocol and finally post-Gd T2* acquisition in the same slices.
| Sequence | Plane | TR / TE (ms) | Slices and thickness | Resolution (mm) | Scan time |
|---|---|---|---|---|---|
| T2* GRE pre-Gd | SAG | 1330 / 4.4, 11.9, 19.4, 27, 34.5, 42.1, 49.6, 57.1 | 15-24, 3 mm | 0.42 × 0.42 | 7 min |
|
| |||||
| Intra-articular Gd-DTPA2- injection | |||||
|
| |||||
| T1w TSE FS | SAG, COR | 650-820 / 12-13 | 25-35, 3 mm | 0.45 × 0.6 | 3-4 min |
| T2w TSE FS | COR, SAG, | 4000-5700 / 57-87 | 24, 3-4 mm | 0.45 × 0.6 | 3-4 min |
| PDw TSE | COR, Obl. AX | 3000 / 30-35 | 24, 3-4 mm | 0.45 × 0.6 | 3-4 min |
| DESS | 3-D | 12.3 / 4.9 | - | 0.78 × 0.78 × 0.78 | 6 min |
| T2* GRE post-Gd | SAG | 1330 / 4.4, 11.9, 19.4, 27, 34.5, 42.1, 49.6, 57.1 | 15-24, 3 mm | 0.42 × 0.42 | 7 min |
GRE = gradient recalled echo, TSE = turbo spin echo, FS = fat saturated, AX = axial, SAG = sagittal, COR = coronal, Obl. = oblique
Direct MR arthrography
After local skin anesthesia (1 mL of %1 lidocaine), intra-articular needle placement was confirmed with a small amount of 180 mg/mL concentration of Iohexol (Omnipaque; GE Healthcare, Milwaukee, WI, USA) under fluoroscopic guidance. For the MR arthrogram, 10 – 20 ml of a standard dilute gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA) solution at a concentration of 2.5 mmol/L (1:200 dilution, prepared by mixing 0.1 mL of 500 mmol/L Gd with 15 mL of saline and 5 mL of 1% lidocaine) was injected to full capacity of the joint until resistance was met. The interval between contrast agent injection and T2* mapping was about 30 - 45 minutes. No specific exercise or joint movement maneuvers were applied in the procedure.
Data analysis
In order to minimize the impact of variations in positioning of the hips within the magnet, internal anatomic landmarks were utilized. For slice positioning in the sagittal plane the anterior inferior iliac spine (AIIS) was used as osseous landmark. To allow comparison of the T2* relaxation time values pre- and post-Gd injection, regions of interest (ROIs) were defined as follows: the transverse ligament was located in the most medial sagittal slice where the fovea capitis was still seen. A central line (CL) was defined as a line orthogonal to the transverse ligament, passing though the center of the femoral head. The CL was used as the initial anatomic landmark-based guide in every slice. Starting from the CL, the acetabular and femoral cartilage was divided into sectors of 10°, towards both anterior and posterior chondro-labral junctions (CLJs). The ROIs were finally defined using the angular guides by manually drawing ROIs in both acetabular and femoral cartilage, excluding the last sector immediately adjacent to CLJ that included less than 10° of cartilage (Figure 1). The ROIs were drawn on 3 to 4 consecutive slices positioned in the weight-bearing region of the hip joint, where the through-plane curvature of the articular surface was always less than 30 degrees to avoid partial volume effects. The ROIs were defined using the first or second echo images of the T2* series. By carefully defining the ROIs as described above in the same or closest matching slices between pre- and post-Gd scans, the mismatch between the ROI locations between pre- and post-Gd scans was minimized. This landmark-based ROI definition also avoided any mismatch between pre- and post-Gd scans due to potential in-plane rotations. ROIs were defined using Osirix image viewing and analysis software (http://www.osirix-viewer.com)[19] and exported in tabulated format for further analysis. One operator defined all the ROIs to minimize the mismatch and discrepancy. ROIs that were clearly affected by imaging artifacts, such as flow or pulsation artifacts were excluded from the final analysis. The final number of ROIs was 323 in acetabular cartilage and 323 in femoral cartilage, both in pre-Gd and post-Gd datasets.
Figure 1.
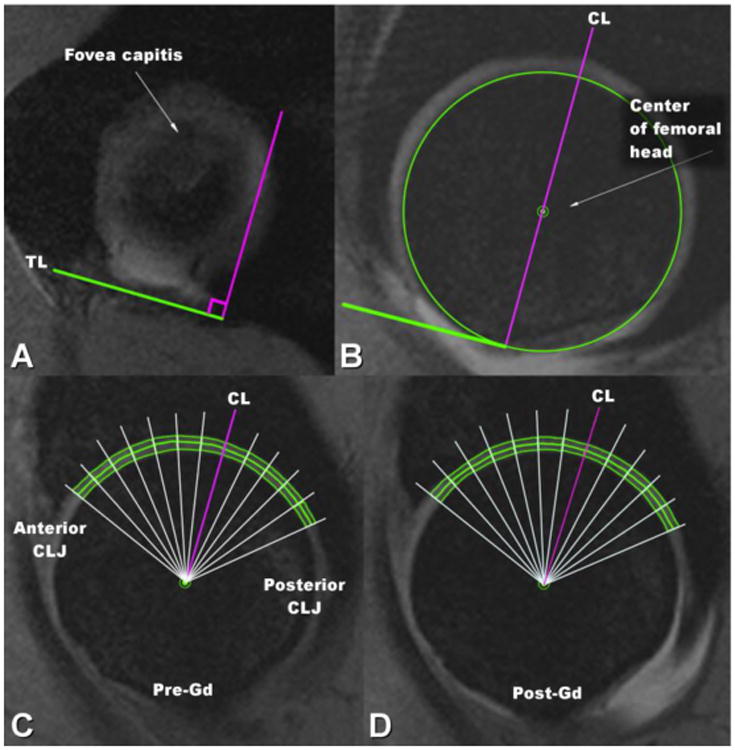
Selection of the ROIs in acetabular and femoral cartilage for statistical analysis of T2* values. A) As seen in the most medial sagittal slice of hip where fovea capitis is present, the transverse ligament (TL) was used as an anatomic landmark. B) Central line (CL) was defined as a line perpendicular to the TL, passing through the center of the femoral head. C) and D) the cartilage was divided into sectors of 10°, starting from CL towards both anterior and posterior chondro-labral junctions (CLJs) in pre-Gd image (C) and in post-Gd image (D), with the ROIs manually drawn in both acetabular and femoral cartilage utilizing the angular guides.
Statistical analysis
T2* relaxation times before and after Gd injection were evaluated separately for the cartilage of the femoral head and of the acetabulum. We considered two natural measures of agreement: Krippendorff's alpha coefficient [20] and the estimated slope from linear regression through the origin.
Krippendorff's alpha coefficient is defined as
where Do denotes observed disagreement and De denotes expected disagreement. The coefficient varies between 0 and 1, with 0 indicating no agreement and 1 indicating perfect agreement; a value greater than 0.8 indicates strong agreement.
Krippendorff's alpha is applicable to a number of different types of data. In estimating alpha, one should choose a measure of discrepancy that is appropriate for the data at hand. For data of the type considered here, the usual measure of discrepancy is the squared difference. However, statistics based on squared differences are not robust [21], and so we elected to use absolute differences instead. We achieved this by altering function kripp.alpha from the irr package [22] for R. We computed a 95% confidence interval for alpha using a bootstrap procedure (as implemented in R package kripp.boot [23, 24]).
We conducted a second analysis based on linear regression through the origin. Specifically, we regressed the post-Gd values on the pre-Gd values. If the two samples agree perfectly, all points in a scatter plot of the data will fall on the line of equality. In a statistical setting, i.e., when we should not expect perfect agreement, an estimated slope close to 1 indicates strong agreement.
All statistical analyses were conducted using R version 3.0.2 [25].
Results
The estimated Krippendorff's alpha for the acetabular data was 0.869, and the 95% bootstrap confidence interval was (0.838, 0.895). For the femoral data, the estimate was 0.836, the confidence interval (0.801, 0.867). These results are strong evidence of strong agreement between pre- and post-Gd T2* measurements.
The no-intercept linear regression for the acetabular data yielded an estimated slope of 0.999 (Figure 2). The value of R2 was 0.984, which implies that over 98% of the variation in post-Gd T2* is explained by pre-Gd T2*. The estimated slope for the femoral data was 0.982, and the value of R2 was 0.994. These results again indicate strong agreement (Figure 2). (We note that the femoral data required a variance-stabilizing transformation. Specifically, we transformed both the pre- and post-Gd T2* values by raising them to the 2 / 3 power. We arrived at this exponent using the boxcox [26] function of the MASS package [27].)
Figure 2.
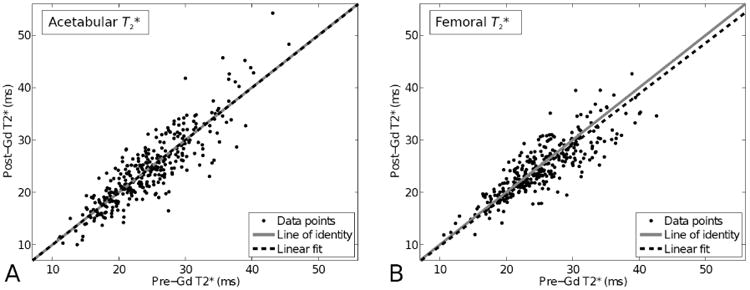
Scatterplots of the T2* relaxation times post-Gd vs. pre-Gd. A) Acetabular cartilage and B) femoral cartilage. Dots (•) denote the data points, solid gray line is the line of identity and dashed lines show the linear fits between pre-Gd and post-Gd T2* values.
The group averages of the T2* relaxation times demonstrated minimal differences, with the pre-Gd T2* values slightly larger on both the femoral and acetabular side of the joint (Table 2). Visually there was a striking similarity between pre-Gd and post-Gd T2* maps of the hip cartilage, as demonstrated in one slice of one FAI patient (Figure 3).
Table 2.
Group means for T2* values in acetabular and femoral cartilage before and after Gd-injection.
| Group | Mean ± SD T2* (ms) | |
|---|---|---|
| Acetabular cartilage | Pre-Gd (n = 323) | 24.3 ± 6.0 |
| Post-Gd (n = 323) | 24.3 ± 6.7 | |
| Femoral cartilage | Pre-Gd (n = 323) | 25.2 ± 5.5 |
| Post-Gd (n = 323) | 24.6 ± 5.1 | |
Figure 3.
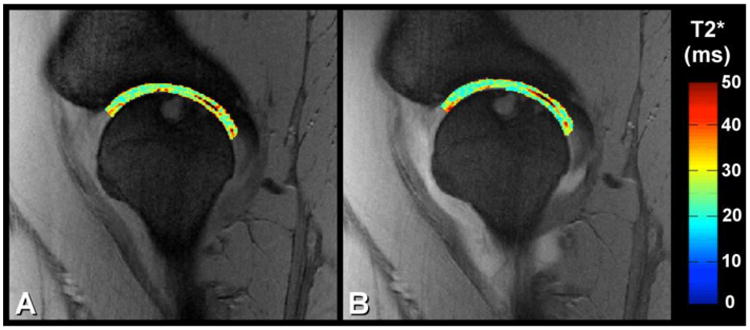
Examples of T2* relaxation time maps in a selected slice in one patient volunteer. A) Pre-Gd T2* relaxation time map in acetabular and femoral cartilage and B) post-Gd T2* relaxation time map in acetabular and femoral cartilage. The relaxation time maps were overlaid on top of the shortest-echo time source image used in calculation of the T2* maps.
Discussion
In this study we evaluated if intra-articular Gd-DTPA2- utilized in direct MR arthrography significantly changes T2* relaxation times of acetabular and femoral cartilage. While the feasibility of T2* measurement in the hip has been established, we questioned if the practicality of using T2* on a routine basis might be limited due to the routine use of intra-articular Gd-DTPA2- in clinical hip MRI. The results of this study, however, indicate strong agreement between T2* measurements with and without Gd-DTPA2-, for femoral and acetabular cartilage.
The strong agreement between T2* relaxation times measured with and without contrast may not be generalizable to other quantitative cartilage modalities. In a previous study describing the measurement of T2 relaxation time in the distal knee femoral cartilage with a dGEMRIC (delayed Gadolinium Enhanced MRI of Cartilage) protocol [14] a slight overall shortening of T2 relaxation time was observed in normal volunteers at 1.5 T as a result of intra-venous “double-dose” injection of Gd-DTPA2-. In another recent study, significantly shorter T2 values were reported in the femoral cartilage of the knee after similar injection in osteoarthritis patients, highlighting the possibility of increased contrast agent accumulation in damaged cartilage [28]. In our study, the use of T2* instead T2 and the use of an intra-articular instead of intravascular contrast appears to have avoided this issue. Indirect MR arthrography using intravenous administration of Gadolinium contrast agent generally utilizes significantly higher concentration than the intra-articular injection; the temporal dynamics as well as the contrast diffusion pathways via the synovium are likely very different between the systemic intravascular and local intra-articular injections as well.
The post-Gd T2* relaxation time of hip articular cartilage may be affected in several ways. At increased grades of cartilage degeneration, T2* may be increased due to the breakdown of the collagen fibril network and the introduction of more free water in the tissue [29-31]. Alternately, shortening of T2* due to increased calcium deposits and formation of fibrocartilage may also be seen [10, 32, 33]. Accumulation of the contrast agent in articular cartilage may also be dependent on other tissue properties such as reduction in proteoglycan content [14, 15, 28]. Mechanical unloading of the joint cartilage after lying on the scanner table for given time before the scan has also been implicated as a source of change in the relaxation time [6]. These findings have not, however, been universal. Apprich et al reported T2* values (acquired at 3.0 T) of acetabular cartilage in FAI patients to be 21.51 ms and 21.09 ms in early and late unloading phase, respectively [6]. They reported no significant change in T2* over unloading time for both healthy and damaged cartilage in these patients [6], corresponding to what was expected in the present patient population. Besides mechanical unloading, physiological wash-in and wash-out processes will affect the observed relaxation times. Fairly stable conditions, applicable to the present study for up to approximately 40 minutes may be inferred from a study by Andreisek et al, investigating contrast-to-noise ratios after intra-articular contrast injection [34]. Furthermore, in our study, the ROI analysis was done to enable accurate comparison of the relaxation times before and after Gd-injection, not to investigate differences between femoral / acetabular or regions generally thought to be affected by FAI. The range of the relaxation times in the present study spanned 10 – 54.2 ms (see also Figure 2), covering what would be deemed normal or degenerated, either in pre-Gd or post-Gd measurements based on thresholds established in previous publications. Good correspondence between pre-Gd and post-Gd measurements was observed for the whole range, indicating the results should hold for a wide range from normal to degenerated hip cartilage. However, along the aims of this study, changes related to degeneration were not evaluated.
There are a number of limitations in this study. Firstly, inherent in the comparison of slice-based MR findings after subject re-positioning, identical ROIs cannot be guaranteed. However, we have estimated that in the worst case the discrepancy (non-overlap of the slices) may have been up to 65%, with only 1.05 mm of the actual imaging slices overlapping (there was a 0.9 mm slice gap). This estimation excludes variations in the slice orientation, which would result in slight variation of the position mismatch within a slice. However, the slice orientation was carefully prescribed using the anatomical landmarks and would not significantly increase the worst-case discrepancy. Secondly, in few slices the differentiation between acetabular and femoral cartilage was not well defined, potentially resulting in inclusion of both joint compartments, increasing the scatter in the analysis. In addition, we evaluated results for a relatively low-dose intra-articular Gd-DTPA2- injection as described in this study; for different contrast agents and different concentrations, separate investigations are necessary and these results may only serve as general guidelines. While the dilute Gd-DTPA2- solution of our study was found not to induce significant changes, a larger dose, or systemic application may result in significantly different results. Further investigations on this topic would be warranted. Finally, potential regional differences in T2* value due to the magic angle effect were not investigated; however, potential effects would disappear in the paired nature of the analysis.
Conclusions
In conclusion, our results showed a strong agreement between T2* values before and after intra-articular contrast in acetabular and femoral cartilage and suggest that measurement of T2* relaxation time may be conducted independent of the presence of dilute intra-articular Gd-DTPA2-. This should allow for multi-center trials to be conducted in the broader context of validating T2* mapping as a non-invasive marker for cartilage pathology in FAI
Acknowledgments
The authors gratefully acknowledge support from the NIH grant P41 EB015894.
Footnotes
The authors have nothing to disclose
Contributor Information
Shabnam Mortazavi, Email: shabnam64447@gmail.com.
John Hughes, Email: hughesj@umn.edu.
Patrick Morgan, Email: morga050@umn.edu.
Jutta Ellermann, Email: eller001@umn.edu.
References
- 1.Bredella MA, Ulbrich EJ, Stoller DW, Anderson SE. Femoroacetabular impingement. Magnetic resonance imaging clinics of North America. 2013;21:45–64. doi: 10.1016/j.mric.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 3.Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467:616–622. doi: 10.1007/s11999-008-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379–1388. doi: 10.1016/j.arthro.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Mankin HJ. Articular Cartilage, Part II: Degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am. 1997;79:612–632. [Google Scholar]
- 6.Apprich S, Mamisch TC, Welsch GH, et al. Evaluation of articular cartilage in patients with femoroacetabular impingement (FAI) using T2* mapping at different time points at 3.0 Tesla MRI: a feasibility study. Skeletal Radiol. 2012;41:987–995. doi: 10.1007/s00256-011-1313-1. [DOI] [PubMed] [Google Scholar]
- 7.Bittersohl B, Hosalkar HS, Hughes T, et al. Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5T using a three-dimensional (3D), gradient-echo (GRE) sequence: a prospective study. Magn Reson Med. 2009;62:896–901. doi: 10.1002/mrm.22096. [DOI] [PubMed] [Google Scholar]
- 8.Bittersohl B, Miese FR, Hosalkar HS, et al. T2* mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthritis Cartilage. 2012;20:653–660. doi: 10.1016/j.joca.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Bittersohl B, Miese FR, Hosalkar HS, et al. T2* mapping of acetabular and femoral hip joint cartilage at 3 T: a prospective controlled study. Invest Radiol. 2012;47:392–397. doi: 10.1097/RLI.0b013e3182518d57. [DOI] [PubMed] [Google Scholar]
- 10.Ellermann J, Ziegler C, Nissi MJ, et al. Acetabular cartilage assessment in patients with femoroacetabular impingement by using T2* mapping with arthroscopic verification. Radiology. 2014;271:512–523. doi: 10.1148/radiol.13131837. [DOI] [PubMed] [Google Scholar]
- 11.Mamisch TC, Bittersohl B, Hughes T, et al. Magnetic resonance imaging of the hip at 3 Tesla: clinical value in femoroacetabular impingement of the hip and current concepts. Semin Musculoskelet Radiol. 2008;12:212–222. doi: 10.1055/s-0028-1083105. [DOI] [PubMed] [Google Scholar]
- 12.Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissi MJ, Toth F, Carlson CS, E J. Proc Intl Soc Mag Reson Med. Milan, Italy: 2014. Improved visualization of cartilage canals using semi-quantitative susceptibility mapping; p. 3986. [Google Scholar]
- 14.Nieminen MT, Menezes NM, Williams A, Burstein D. T2 of articular cartilage in the presence of Gd-DTPA2. Magn Reson Med. 2004;51:1147–1152. doi: 10.1002/mrm.20083. [DOI] [PubMed] [Google Scholar]
- 15.Bashir A, Gray ML, Burstein D. Gd-DTPA(2-) as a measure of cartilage degradation. Magnet Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 16.Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467:638–644. doi: 10.1007/s11999-008-0680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188:1540–1552. doi: 10.2214/AJR.06.0921. [DOI] [PubMed] [Google Scholar]
- 18.Tonnis D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clinical orthopaedics and related research. 1976:39–47. [PubMed] [Google Scholar]
- 19.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krippendorff K. Content analysis: An introduction to its methodology. Third. Thousand Oaks, CA: Sage; 2013. [Google Scholar]
- 21.Mielke PW, Berry KJ. Permutation methods: A distance function approach. New York: Springer; 2007. [Google Scholar]
- 22.Gamer M, Lemon J, Singh IFP. irr: Various Coefficients of Interrater Reliability and Agreement. 2012 [Google Scholar]
- 23.Gruszczynski M. kripp boot: R wrapper for bootstrap resampling on intercoder reliability data. 2013 [Google Scholar]
- 24.Efron B, Tibshirani R. Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statistical Science. 1986;1:54, 75. [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 26.Box GEP, Cox DR. An Analysis of Transformations. J Roy Stat Soc B. 1964;26:211–252. [Google Scholar]
- 27.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth. New York: Springer; 2002. [Google Scholar]
- 28.Yoon HJ, Yoon YC, Choe BK. T2 values of femoral cartilage of the knee joint: comparison between pre-contrast and post-contrast images. Korean journal of radiology : official journal of the Korean Radiological Society. 2014;15:123–129. doi: 10.3348/kjr.2014.15.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieminen MT, Rieppo J, Toyras J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- 30.Lammentausta E, Kiviranta P, Nissi MJ, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: Relationships with tissue mechanical properties. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24:366–374. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- 31.Nissi MJ, Töyräs J, Laasanen MS, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22:557–564. doi: 10.1016/j.orthres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Kohl S, Hosalkar HS, Mainil-Varlet P, Krueger A, Buechler L, Siebenrock K. Histology of damaged acetabular cartilage in symptomatic femoroacetabular impingement: an observational analysis. Hip Int. 2011;21:154–162. doi: 10.5301/HIP.2011.6515. [DOI] [PubMed] [Google Scholar]
- 33.Sampatchalit S, Chen L, Haghighi P, Trudell D, Resnick DL. Changes in the acetabular fossa of the hip: MR arthrographic findings correlated with anatomic and histologic analysis using cadaveric specimens. AJR Am J Roentgenol. 2009;193:W127–133. doi: 10.2214/AJR.08.1913. [DOI] [PubMed] [Google Scholar]
- 34.Andreisek G, Duc SR, Froehlich JM, Hodler J, Weishaupt D. MR arthrography of the shoulder, hip, and wrist: evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. AJR American journal of roentgenology. 2007;188:1081–1088. doi: 10.2214/AJR.06.0719. [DOI] [PubMed] [Google Scholar]


