Abstract
Hypomethylating agents (HMA), such as 5-azacitidine or decitabine, are currently used to treat patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) until treatment failure. However, the outcomes for patients who discontinue therapy after achieving partial response (PR) or complete remission (CR) but before treatment failure have not been reported. We present a series of 16 patients with higher-risk MDS (n=5; 31%) or AML (n=11; 69%) who achieved PR (n=1) or CR (n=15) and stopped HMA therapy while in response in the context of clinical trials. They received a median of 12 courses (range 1–24) and achieved response after a median of 1 course of therapy (1–4). Loss of response after discontinuation of therapy was rapid, with a median progression-free survival of 4 months (95% CI: 2–6). Median overall survival (OS) from the time of therapy discontinuation was 15 months (95% CI: 6–24). Patients who received 12 cycles of therapy or more had significantly better OS (median: 20 months [95% CI: 12–27]) than those who received fewer than 12 cycles (median: 4 months [95% CI: 1–8]) (p= 0.043). Poor-risk cytogenetics were also associated with lower 1-year OS (33% versus 69%; p= 0.046). According to these results and considering the poor prognosis after HMA failure, HMA interruption should be avoided once a sustained response has been achieved.
Keywords: Myelodysplastic syndromes, hypomethylating agents, treatment discontinuation
1. INTRODUCTION
Hypomethylating agents (HMA), including 5-aza-2′-deoxycytidine (decitabine) or 5-azacitidine, are first-line treatment in higher-risk myelodysplastic syndromes (MDS) and are also commonly used in elderly acute myeloid leukemia (AML) patients [1–6]. In contrast to conventional induction-consolidation chemotherapy in AML, for which the role of maintenance therapy is not yet accepted [7], HMA therapy in MDS and AML is currently considered chronic treatment and is used until relapse or progression of the primary disease [8]. This approach is based on the design of the AZA-001 trial [3] and clinical experience indicating that early interruption of these agents is associated with treatment failure. Because of these data, therapy is generally prolonged as long as possible because relapse in the context of HMA failure is associated with very poor prognosis [9]. However, prolonged HMA therapy can often become a burden on quality of life and financial status because these drugs must be administered in a clinical setting. Despite these barriers to prolonged therapy administration, the natural history of patients with MDS or AML who electively cease HMA therapy while in response has not been systematically studied and could inform future management strategies for these patients.
To address this issue, here we report on the outcomes of a group of patients with MDS and AML treated with HMA in whom therapy was interrupted while having partial or complete response. To do this, we included patients treated on 3 early clinical trials [10–12] of HMA conducted at The University of Texas MD Anderson Cancer Center between 2004 and 2006, in which therapy was planned for a maximum of only 12 or 24 months because the optimal schedule of therapy had not yet been established. Our primary aim was to calculate progression-free survival (PFS) and overall survival (OS) after stopping therapy, and as a secondary objective, we sought to explore clinical variables associated with outcome.
2. PATIENTS AND METHODS
Between 2004 and 2006, 2 phase I/II clinical trials with 5-azacitidine or decitabine treatment for patients with MDS or AML in combination with valproic acid (VPA) [10, 11] and a randomized study of 3 dose schedules of decitabine [12] were conducted at MD Anderson. A total of 173 patients were treated: 53 with 5-azacitidine-VPA-all-trans retinoic acid (5azaVPA) [10], 54 with decitabine-VPA (DecVPA) [11], and 64 with 3 different schedules of decitabine (DAC3) [12]. VPA protocols included refractory or relapsed AML, higher-risk MDS, or AML/MDS patients who refused or were ineligible for upfront chemotherapy. Decitabine dose in the DecVPA study was 15 mg/m2 i.v. daily for 10 consecutive days, and azacitidine dose in the 5azaVPA study was 75 mg/m2 i.v. daily for 7 consecutive days. The DAC3 study included MDS patients who had not received prior high-dose chemotherapy. In those protocols, a maximum of 12 or 24 courses of therapy were planned. The 3 schedules of decitabine for the DAC3 study were: 10mg/m2 i.v. daily for 10 consecutive days, 20mg/m2 i.v. daily for 5 consecutive days, and 20 mg/m2 s.c. daily for 5 consecutive days.
In this analysis, we have specifically analyzed the outcomes of patients who achieved response and who stopped treatment for any cause while in response. We also excluded any patients who received hematopoietic stem cell transplants, which could impact survival analyses. Response and disease progression were assessed according to IWG criteria [13]. PFS was defined as time from discontinuation of treatment until progression or death from any cause. OS was defined as time from discontinuation of treatment to death from any cause.
Statistical analysis was performed with SPSS v.21 (IBM, Endicott, NY). The Chi-square test was used to compare categorical variables. Continuous variables were compared by Student’s t or Mann-Whitney U tests depending on whether or not the sample was normally distributed; the Kolmogorov-Smirnov test was used to assess for normal distribution. Estimated PFS and OS curves were calculated using the Kaplan-Meier method, and the log-rank test was used to test the variables with influence on survival data.
3. RESULTS
3.1. Patient characteristics
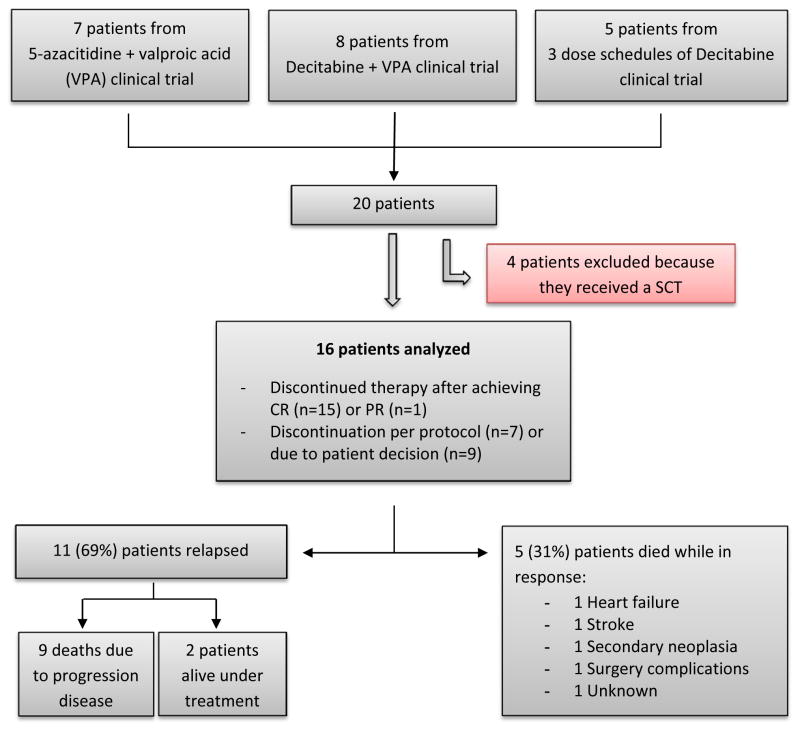
Of the total of 173 patients, we identified 20 patients (12%) who achieved response and stopped therapy electively (Figure 1). Seven patients were treated in the 5azaVPA trial, 8 in DecVPA, and 5 in DAC3. We excluded patients who underwent an allogeneic stem cell transplant (n=4) and had a final cohort of 16 patients for analysis. Patient characteristics are summarized in Table 1. Eleven patients (69%) had AML. Fifteen out of 16 patients (94%) were in complete remission (CR) when treatment ended. The median number of cycles administered was 12 (range: 1–24). In 7 patients (44%), therapy was stopped after receiving the maximum courses of treatment scheduled on their protocol, which was either 12 or 24 courses, depending on the specific clinical trial. The remaining 9 patients (56%) discontinued therapy of their own volition due to personal causes or financial issues. There were no treatment discontinuations due to side effects.
Figure 1.
Study schema
Table 1.
Patient characteristics
| CHARACTERISTIC | N = 16 |
|---|---|
| Median age (range) | 68 (51–80) |
| Diagnosis; n (%) | |
| Untreated MDS | 6 (38%) |
| Untreated AML | 5 (31%) |
| Relapsed/refractory AML | 3 (19%) |
| Previous lines of treatment | 1 |
| MDS evolving AML (previously untreated) | 2 (12%) |
| Cytogenetic risk (IPSS); n (%) | |
| Good prognosis | 9 (56%) |
| Intermediate risk | 4 (25%) |
| Poor prognosis | 3 (19%) |
| Reason for stopping treatment; n (%) | |
| Patient decision | 9 (56%) |
| Per protocol schedule | 7 (44%) |
| Median no. of courses received until response; n (range) | 1 (1–4) |
| Median no. of courses received; (range) | 12 (1–24) |
| More than 12 courses n (%) | 10 (63%) |
| Less than 12 courses n (%) | 6 (37%) |
| Response at treatment stop; n (%) | |
| CR | 15 (94%) |
| PR | 1 (6%) |
| Cytogenetic response at treatment stop; n (%) | N=7 |
| CCyR | 6/7 (86%) |
| Lack of cytogenetic response | 1/7 (14%) |
MDS, myelodysplastic syndromes; AML, acute myeloid leukemia; IPSS, International Prognostic Scoring System; CR, complete response; PR, partial response; CCyR, complete cytogenetic response.
3.2. Survival
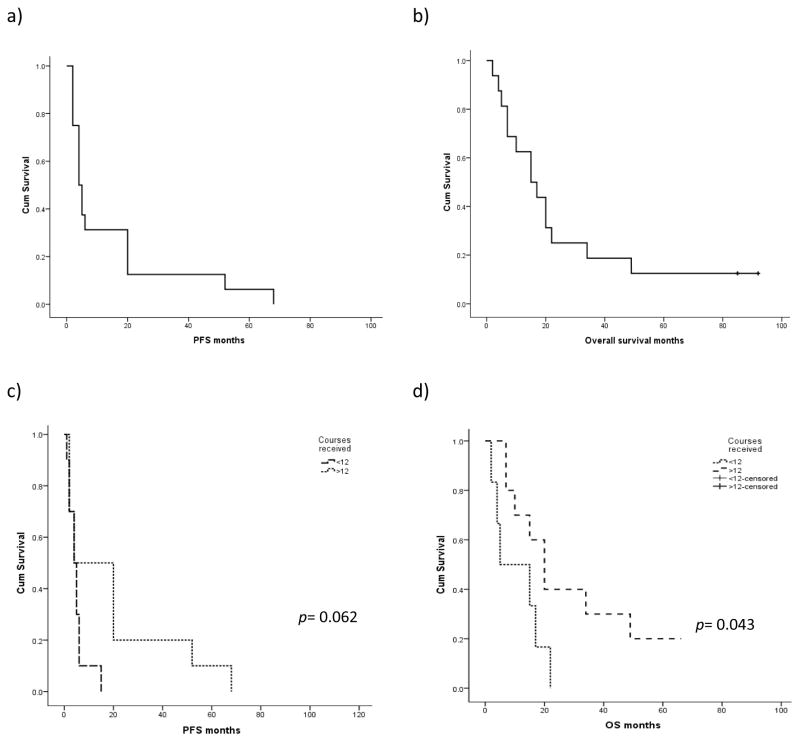
The total estimated median OS from time of diagnosis was 24 months (95% CI: 20–28). For the whole series, the estimated median OS and PFS from the time of therapy discontinuation were 15 months (95% CI: 6–24) and 4 months (95% CI: 2–6), respectively (Fig 2).
Figure 2.
Estimated PFS (a) and OS (b) for the whole series (n=16). PFS and OS according to number of courses received (c,d).
The main variable with impact on both OS and PFS in the univariate analysis (Table 2) was total number of courses of therapy. Those patients who received more than 12 cycles of HMA showed significantly better OS, with a median OS of 20 months (95% CI: 12–27) versus 4 months (95% CI: 1–18) for patients who received fewer than 12 courses (p=0.043). Patients receiving more than 12 courses also tended to have longer PFS. Although the median PFS for patients was 4 months regardless of whether or not they completed 12 courses of therapy, the PFS rate at 12 months was 50% for patients who completed 12 cycles versus 17% for those who did not (p = 0.062) (Fig 2). Cytogenetics at the time of diagnosis also had an influence on OS; those patients with higher-risk cytogenetics at diagnosis (including complex karyotypes or chromosome 7 alterations) had a poorer OS, with an estimated OS at 12 months of 33% versus 69% for those with lower-risk cytogenetics (p = 0.06). There were no differences between diagnosis, age, or whether or not the patient had received any previous treatment.
Table 2.
Univariate analyses for OS and PFS
| VARIABLE | OS [median, 95% IC] | p value | PFS [median, 95% IC] | p value |
|---|---|---|---|---|
| Diagnosis | ||||
| MDS | 20 (1–42) | 0.19 | 4 (2–20) | 0.21 |
| AML | 7 (1–17) | 4 (1–7) | ||
| Cytogenetics | ||||
| Poor prognosis | 14 (4–10) | 0.06 | 2 (2–5) | 0.07 |
| Interm/good | 20 (14–25) | 5 (1–7) | ||
| Previous treatment | ||||
| Untreated | 15 (6–24) | 0.29 | 4 (3–6) | 0.26 |
| Pretreated | 20 (15–25) | 20 (1–42) | ||
| Courses until response | ||||
| =1 | 15 (1–30) | 0.82 | 4 (2–6) | 0.56 |
| > 1 | 15 (3–27) | 4 (0.4–7) | ||
| Total courses received | ||||
| ≥ 12 | 20 (12–27) | 0.043 | 4 (1–16) | 0.06 |
| < 12 | 5 (1–18) | 4 (2–6) | ||
| Reason to stop HMA | ||||
| Per protocol | 34 (1–79) | 0.012 | 20 (1–38) | 0.1 |
| Own decision | 10 (1–19) | 4 (2–6) | ||
OS, overall survival; PFS, progression-free survival; MDS, myelodysplastic syndromes; AML, acute myeloid leukemia; HMA, hypomethylating agents.
Fourteen patients (88%) died, and progressive disease was the most frequent cause of death (9 out of 14, 64%); in 4 patients, death occurred due to heart failure, stroke, secondary neoplasia, or surgical complications while they maintained complete response. Cause of death was unknown in 1 patient due to loss of follow-up.
3.3. Outcomes by cause of treatment discontinuation
Seven patients received the whole treatment scheduled by their protocol, (12 courses in 4 patients and 24 courses in 3 patients). All 7 achieved CR after a median of 1 course of therapy (range: 1–3) and stopped receiving treatments while still in CR. After the discontinuation of treatment, 6 out of 7 patients relapsed after a median of 12 months (range: 2–68). This group of patients who completed their therapy protocols received significantly more courses of therapy than the other 9 patients (12 courses versus 6 courses for those who voluntarily ceased treatment; p=0.001) and generally had longer time to progression or relapse from their treatment stop date, with a median of 24 months for those who completed the scheduled treatment versus 6 months for those who voluntarily ceased treatment. However, this trend was not statistically significant (p=0.08). Among these patients who received their entire scheduled regimen, median OS was significantly higher (34 months [range: 1–69] versus 10 months [range: 1–18]; p= 0.012).
In 9 cases, the patients voluntarily ceased their treatment because of personal or financial issues. They received a median of 1 course before achieving response (range: 1–2), with a median of 6 total cycles (range: 1–14). Eight of the 9 patients were in CR when they stopped treatment. Afterward, 6 out of 8 patients in CR relapsed or progressed at a median of 4 months (range: 4–6) and later died because of either progressive disease or disease-related issues.
3.4. Outcomes, patterns of failure, and salvage therapy
During the follow-up, 11 patients (69%) relapsed after treatment discontinuation, with a median of 4 months (range: 2–68) from last course of therapy on-protocol. Of them, at last follow-up, 2 patients (13%) are still alive and receiving active treatment, with a median follow-up of 89 months (85–92). The remaining 5 out of 16 patients died while in response.
There were 5 patients who experienced disease-free survival of over 18 months; all of them received more than 12 courses of treatment, and none of them had high-risk cytogenetics. However, even in these cases, all of them had further relapse of their primary disease at a median of 20 months (range: 18–68).
In the group of 11 patients (69%) who relapsed or progressed after discontinuing treatment, 5 patients had cytopenias and became transfusion-dependent after their initial response, 4 patients experienced disease relapse as AML, and 2 patients experienced relapse as a high-risk MDS that further progressed to AML. All of the patients that previously had any cytogenetic alteration and who achieved complete cytogenetic response showed the same alterations at the time of relapse.
Among these 11 relapsing patients, 7 of them received treatment for MDS/AML after relapsing. HMA were reintroduced in 6 patients (decitabine in 2 and azacitidine in 4); the HMA used was the same as that used previously in 2 cases, and it was changed from azacitidine to decitabine or vice-versa in 4 cases. The two patients currently alive remained sensitive to HMA therapy after first relapse; one of them restarted decitabine and achieved a second CR that was maintained for 17 months at last follow-up, and the other one achieved a bone marrow CR with persistent cytopenias after treatment with SGI-110, a new HMA,[14] and was alive after 3 months of treatment at last follow-up. Cytarabine and clofarabine were tested in 2 patients after HMA discontinuation, and investigational therapy was given to 2 patients without any response.
4. DISCUSSION
In the present report, we evaluated 16 patients who received HMA-based therapy on various clinical trials and who later discontinued therapy after achieving a response. According to our data, loss of response was rapidly observed in most of patients, with a median PFS of 4 months (range: 2–68) from the end of treatment. This PFS is similar to the median OS of patients who failed to respond to HMA in the parent trials.[10, 11] Additionally, although the number of patients was limited, we have identified two variables that could be associated with outcome: number of therapy courses administered before stopping therapy and cytogenetics at time of diagnosis. Those patients who received more than 12 courses of HMA therapy and who did not have high-risk cytogenetics had significantly long OS and tended to also have longer PFS.
In clinical practice, some patients choose to stop treatment when they achieve any response for a variety of reasons. This is especially true of treatments such as HMA, which are usually considered chronic therapy and are administered monthly in a clinical setting. These requirements can result in financial issues caused by the cost of these medications and the need to travel to a clinic to receive them. Furthermore, some patients experience a decrease in their quality of life as a result of chronic HMA treatment. Despite these difficulties, our data support the continuation of HMA therapy until lack of response because of the quick progression of the disease and poor prognosis after HMA failure. According to our data, only 2 out of 7 patients (29%) who received therapy after relapse responded to further treatment. It is remarkable that both patients achieved any response with HMA, which means they remained sensitive to these agents after the cessation of therapy; one of them remained sensitive to decitabine after first relapse, and the other one showed a response to the new HMA SGI-110 [14]. However, the quality of the response to the second HMA is not as favorable as that of the first treatment. Previous reports of HMA retreatment have shown poorer duration and quality of response than initial treatment [15], which is in accordance with our findings here. In the majority of patients, response to a second HMA is not achieved after first failure. However, the fact that 2 patients retained sensitivity to this group of drugs after first relapse implies that the mechanism involved in cross-resistance to HMA should be further investigated.
The proposed mechanism for loss of response to HMA is related, in part, to the transient nature of epigenetic modifications [16], but clinical data have not been widely reported for this phenomenon. A series of 13 MDS patients who relapsed or progressed after a median of 5.4 months from the end of azacitidine treatment [17] had results similar to those seen in this study. However, on that study, more than 50% of patients discontinued treatment because of adverse events or comorbidities, whereas none of the patients in this study did so. Although grade 3 toxicity was documented in some of our patients, adverse events were not the reason for treatment discontinuation in any case.
We note that these data should be interpreted with caution because this study had a small sample size and thus lacked sufficient power for in-depth statistical analyses. However, because the current standard for HMA treatment is to continue therapy until loss of response, it will likely be difficult to assess larger numbers of patients who discontinue therapy while in response. Furthermore, the fact that early discontinuation of HMA therapy is counter to current recommendations makes patients who both respond to HMA and cease HMA therapy while in response exceedingly rare.
In conclusion, according to these data and currently available information, we strongly recommend the continuation of HMA treatments in the absence of any serious adverse event. In cases of treatment discontinuation, the number of courses received and cytogenetic risk at time of diagnosis seem to be important factors to predict outcome. The impact of number of HMA courses should be taken into account for those patients who undergo an allogeneic stem cell transplant because it could be an important factor that is not currently considered.
Acknowledgments
MC-C is funded by the Fundacion Alfonso Martin-Escudero. GGM is supported by the Edward P. Evans Foundation, the Fundacion Ramon Areces, grant RP100202 from the Cancer Prevention & Research Institute of Texas (CPRIT), and by generous philanthropic contributions to MD Anderson’s MDS/AML Moon Shot Program.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
BIBLIOGRAPHY
- 1.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 2.Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–40. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. The lancet oncology. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2670–7. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:516–23. doi: 10.1200/JCO.2010.31.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. American journal of hematology. 2014;89:97–108. doi: 10.1002/ajh.23642. [DOI] [PubMed] [Google Scholar]
- 7.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. The New England journal of medicine. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 8.Sekeres MA, Cutler C. How we treat higher-risk myelodysplastic syndromes. Blood. 2014;123:829–36. doi: 10.1182/blood-2013-08-496935. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour E, Garcia-Manero G, Batty N, Shan J, O’Brien S, Cortes J, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–4. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–9. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–8. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 14.Coral S, Parisi G, Nicolay HJ, Colizzi F, Danielli R, Fratta E, et al. Immunomodulatory activity of SGI-110, a 5-aza-2′-deoxycytidine-containing demethylating dinucleotide. Cancer immunology, immunotherapy : CII. 2013;62:605–14. doi: 10.1007/s00262-012-1365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruter B, Wijermans PW, Lubbert M. Superiority of prolonged low-dose azanucleoside administration? Results of 5-aza-2′-deoxycytidine retreatment in high-risk myelodysplasia patients. Cancer. 2006;106:1744–50. doi: 10.1002/cncr.21796. [DOI] [PubMed] [Google Scholar]
- 16.Stresemann C, Bokelmann I, Mahlknecht U, Lyko F. Azacytidine causes complex DNA methylation responses in myeloid leukemia. Molecular cancer therapeutics. 2008;7:2998–3005. doi: 10.1158/1535-7163.MCT-08-0411. [DOI] [PubMed] [Google Scholar]
- 17.Voso MT, Breccia M, Lunghi M, Poloni A, Niscola P, Finelli C, et al. Rapid loss of response after withdrawal of treatment with azacitidine: a case series in patients with higher-risk myelodysplastic syndromes or chronic myelomonocytic leukemia. European journal of haematology. 2013;90:345–8. doi: 10.1111/ejh.12079. [DOI] [PubMed] [Google Scholar]