Abstract
Primary central nervous system lymphoma is a rare and distinct subtype of non-Hodgkin's lymphoma that is sensitive to radiation and chemotherapy. Decisions regarding the initial therapeutic approach are influenced by age and risk of therapy-related neurotoxicity. Despite several albeit small phase II studies, and the acknowledged need for larger prospective trials, there is supporting evidence to consider auto-SCT following induction chemotherapy in patients with good performance status. The international extranodal lymphoma study group is conducting a randomized phase II study comparing consolidative radiation therapy to high-dose therapy. Novel therapeutic options including early aggressive approach with upfront auto-SCT and strategies to prevent relapse following transplantation is an area of focus.
Keywords: primary CNS lymphoma, SCT, WBRT, methotrexate
Introduction
Central nervous system (CNS) lymphoma accounts for a small proportion of all CNS tumors and is classified as primary or secondary.1–3 In the absence of systemic lymphoma, involvement of CNS by lymphoma is primary disease. Patients with systemic lymphoma may have concurrent CNS involvement at presentation. CNS disease as a complication of systemic lymphoma is more often seen in the relapse setting, when it may occur in isolation or in association with other sites.1,3–5
According to the US cancer registry data, primary CNS lymphoma (PCNSL) accounts for 3–5% of primary CNS tumors.2,5 Age-adjusted incidence rates continue to rise, even when adjusted for the impact of HIV-related disease.1,2 PCNSL is more common among patients over the age of 65 years, with an incidence rate of 13–16 per million.1 PCNSL is a rare non-Hodgkin's lymphoma that occurs within the cerebrum, spinal cord, leptomeninges or orbits or presents as neurolymphomatosis.5–7 Diffuse large B-cell lymphoma is the most common histological subtype of PCNSL,4,8 though primary T-cell CNSL have been reported.9
Historically, whole brain radiation therapy (WBRT) was offered to patients but was associated with poor long-term outcome.10–12 The availability of more effective chemotherapy regimens has, therefore, relegated single-agent WBRT to salvage or palliative therapy.13 WBRT as consolidation after chemotherapy is complicated by significant neurotoxicity, particularly in patients over the age of 60 years14 and there is ongoing debate about the field and dosage.
The introduction of high dose (HD) MTX at doses sufficient to cross the blood–brain barrier15 has improved outcomes in PCNSL,16 and MTX ≥1g/m2 is regarded as a standard component of chemotherapy regimens in the therapy of PCNSL. When used alone or in combination with other chemotherapy drugs, it is associated with response rates of 52–88% and 70–94%, respectively, with 2-year overall survival (OS) rates of 58–72% and 43–73%.14 Effective chemotherapy combinations with MTX are under clinical investigation, including HD cytarabine and rituximab. The technique of osmotic blood–brain barrier disruption is being investigated in an attempt to improve drug delivery to the CNS for the treatment of malignant brain tumors,17 and promising data have been reported in patients with PCNSL.18,19
With increased risk of relapse following single modality approach, consolidation approaches to improve disease-free interval and survival of patients need to be considered. Consolidation with radiation, further chemotherapy and/or auto-SCT is available. Consolidative therapy with HD MTX has been recently presented by CALGB.20
The risks of disease relapse and therapy-related neurotoxicity are important aspects to consider when initiating therapy for PCNSL. The role of high dose therapy (HDT) followed by auto-SCT in PCNSL, though limited to a few clinical trials, has to a large extent been extrapolated from treatment of diffuse large B-cell lymphoma. The therapeutic benefit of HDT and auto-SCT has not been studied in phase III trials. Therefore, its role in PCNSL in our opinion has been underutilized.
Role of HDT and Auto-SCT in PCNSL at First Response
Several phase II studies among immunocompetent patients with a variety of conditioning regimens have been published on the role of auto-SCT in patients with PCNSL after induction chemotherapy during first remission (Table 1).
Table 1. Auto-SCT outcome in PCNSL.
| Reference | Disease | No. of patients | Induction therapy | Conditioning regimen | CR (%) | Radiation/time | Survival |
|---|---|---|---|---|---|---|---|
| 22 | Primary | 11 | MTX/AraC/ | Bu CY E | 91 | XRT/post | Median survival—NR |
| 23 | Primary | 13 | MTX, Cy, thiotepa | BCNU/thiotepa | 54 | XRT/post | 3-yr OS 77% |
| 24 | Primary | 25 | MTX, etoposide, carmustine | Carmustine, E, Cy and melphalan | 52 | XRT/peri | 4-yr OS 64% |
| 28 | Primary | 23 | MTX | Bu/thiotepa | 70 | XRT/peri | 2-yr OS 48% |
| 21 | Primary | 6 | MBVP, | BEAM | 100 | XRT/peri | 2-yr OS 40% |
| 38 | Recurrent | 43 | Thiotepa, Bu, Cy | Cy, etoposide | 64 | None | 2-yr OS 45% |
| 26 | Primary | 7 | MTX/AraC | Bu/Cy/thiotepa | 86 | None | 3-yr OS 50% |
| 25 | Primary | 28 | MTX/AraC | BEAM | 29 | None | 2-yr OS 55% |
Abbreviations: MBVP = Methotrexate, carmustine, etoposide, methylprednisolone; PCNSL=primary central nervous system lymphoma.
In a study by Brevet et al.21 six patients were treated with BEAM intensive chemotherapy, auto-SCT and radiotherapy. All patients attained a CR at the conclusion of intensive therapy. The median survival among the six patients treated on this regimen was 36 months. Yoon et al.22 investigated the feasibility of upfront auto-SCT in patients with newly diagnosed PCNSL. Treatment consisted of induction chemotherapy with five cycles of HD MTX and two cycles of high-dose cytarabine followed by conditioning with BU, CY, etoposide and auto-SCT. Two patients received WBRT. Of the 11 consecutive PCNSL patients treated with this regimen, 10 achieved CR or CRu. At a median follow-up of 25 months, six patients relapsed with a median EFS of 15 months. Median survival was not yet reached with a 2-year survival rate of 89% at the time of reporting. No long-term data are available, but this aggressive treatment approach was well tolerated by all patients and appeared to be effective in achieving a CR in most patients. Relapse however, remains a major problem and further regimen optimization is necessary.22
Illerhaus et al.23 treated 13 patients with MTX and cytarabine/thiotepa induction chemotherapy followed by HD carmustine/thiotepa and auto-SCT for PCNSL. Radiotherapy was given to patients who did not respond completely to chemotherapy. Sequential chemotherapy was administered in this study with two to four cycles of HD-MTX 8 g/m2 and two cycles of HD-cytarabine and thiotepa. After stem-cell mobilization, carmustine and thiotepa was used as conditioning regimen followed by auto-SCT. Median age was 54 years. Three patients achieved CR and five attained PR after induction therapy. Among the 13 patients, 11 proceeded to HDT and auto-SCT,7 attained a CR and 4 PR. The 3-year disease-free survival (DFS) and OS was 77%.23
Colombat et al.24 reported the safety and efficacy of HD-MTX-based chemotherapy followed by HDT and auto-SCT in patients with newly diagnosed PCNSL. Twenty-five patients received initial two cycles of HD-MTX, etoposide, carmustine and methylprednisolone, and then one cycle of ifosfamide and cytarabine followed by peripheral stem cell collection. Seventeen responsive patients then received HDT using BEAM followed by auto-SCT. After auto-SCT, responding patients received WBRT and non-responders received salvage therapy followed by WBRT (30 Gy). Out of the 21 responding patients, 4 did not have auto-SCT because of toxicity or refusal. With a median follow-up of 34 months, the estimated 4-year EFS was 46% and OS 64%. No evidence of late treatment-related toxicity was observed.24 This is an interesting observation as almost all patients received WBRT at a dose of 30Gy.
In a similar study, Abrey et al.25 reported a phase II study using HD-MTX and cytarabine followed by HDT and auto-SCT in patients with newly diagnosed PCNSL. A total of 28 patients received induction chemotherapy and then 14 patients with chemosensitive disease underwent HDT (BEAM) followed by auto-SCT. The median EFS was 5.6 months for all patients and 9.3 months for 14 patients who underwent transplantation. Out of these 14 patients, 6 (43%) remained disease-free at last follow-up (median follow-up of 28 months). Prospective neuropsychological evaluations have revealed no evidence of treatment-related neurotoxicity.25 Again, this is a small study but the study suggests that this treatment approach is feasible in patients with newly diagnosed PCNSL, without evidence of significant long-term neurotoxicity.
In a pilot study by Cheng et al.26 newly diagnosed CNSL were treated with HD-MTX induction therapy followed by thiotepa, BU and CY and auto-SCT. Six of seven patients achieved a CR, patients tolerated therapy well and achieved remission lasting between 5 to 42 months (Table 1).26 In a multicenter phase II study by Illerhaus et al.27 23 patients received upfront HDT and auto-SCT following three cycles of induction chemotherapy. WBRT (45 Gy) was also administered for consolidation therapy. Five-year relapse-related death was 21% for all patients and 8.7% for patients treated with HDT and auot-SCT. These data support feasibility and excellent outcome using upfront aggressive approach with auto-SCT.27
BU and thiotepa as the conditioning regimen following HD-MTX in PCNSL was reported in 23 patients by Montemurro et al.28 CR and PR rates for all patients were 70 and 13%, respectively. At a median follow-up of 15 months, median EFS after auto-SCT was 27 months and OS was not yet reached. Estimated 2-year EFS and OS were 45 and 48% for all patients vs 56 and 61% for the HDT and auto-SCT group, respectively. There were three deaths due to neurotoxicity among the nine irradiated patients. No persistent neurotoxicity was seen after auto-SCT without subsequent WBRT.28 A relatively higher neurotoxicity noted in patients receiving WBRT, might be related to higher radiation dose used in this study (45 vs 30 Gy).
Tandem auto-SCT in lymphoma has been reported with some success, however, it is associated with significant toxicities among heavily pretreated patients who have received prior radiation therapy.29,30 There is no evidence to support upfront tandem auto-SCT in PCNSL.
Thiotepa has better brain penetration and carries theoretical advantage over other conditioning regimens by effective local blood–brain tissue penetration,31 and needs to be considered in selected patients as it also carries excess toxicities in combination with BU-containing conditioning regimens.32,33
Patients enrolled in phase II trials discussed above are bound to be subject to selection bias. Although firm conclusions cannot be drawn from such studies, early results of upfront HDT and auto-SCT transplantation are encouraging despite confronting disease relapse remains an ongoing challenge. Treatment modalities to sustain response without long-term neuro-cognitive effect of therapy are needed. One approach would be to consider lower radiation dose after auto-SCT, which appears lucrative to decrease relapse rate and minimizing long-term neurocognitive side effects.34 There is also a dire need to consider maintenance therapy in patients who achieve a good response. MTX given every 6 weeks for five cycles has been considered in clinical trials with no clear benefit.35,36 With emerging treatment options, other effective therapies need to be studied further.
As noted earlier, PCNSL is more common among those over the age of 65 years. This provides an increasing challenge to consider other modalities of therapy for patients who may not tolerate the combined modality therapy that includes HDT and auto-SCT.
In the above studies, though there is no increased neurotoxicity reported with induction regimens, systemic toxicity of consolidative auto-SCT needs to be considered.
Role of HDT and Auto-SCT in Relapsed PCMSL
Auto-SCT has also been evaluated among patients who present with relapsed CNS lymphoma.3,37–39 Its recommended use has been limited by the very few reported studies in patients with PCNSL.
Soussain et al.38 presented their results of intensive chemotherapy followed by auto-SCT for refractory, recurrent PCNSL and intraocular lymphoma. Salvage chemotherapy consisted of HD cytarabine and etoposide, and patients received thiotepa, BU and CY conditioning regimen followed by auto-SCT. A total of 43 patients with a median age of 52 years were included in this study. About 15 out of 20 patients with chemosensitive disease and 12 non-responders to CTVE salvage chemotherapy underwent HDT and auto-SCT. All but 1 of the 27 patients obtained a CR. With a median follow-up of 36 months, the median OS of entire group was 18 months, and 59 months for patients who completed the assigned treatment. The respective median PFS times were 11.6 and 41.1 months. The 2-year OS probability was 45% for the whole population vs 69% for 27 patients undergoing auto-SCT.38 These results are encouraging and suggest the group of patients with chemosensitive disease at relapse might benefit from HDT and auto-SCT.
In the absence of curative regimens for patients with relapsed disease, especially in patients who can tolerate intensive chemotherapy we recommend considering HDT and auto-SCT as an alternative approach to prolong disease-free survival.
In patients with secondary CNS lymphoma, there are sparse data to recommend auto-SCT. Eight patients with secondary CNS lymphoma at the time of disease relapse or progression, were treated with HDT and auto-SCT, six patients were in CR before the transplant. Only two of these patients continued to be in CR.40
Current Implications and Future Perspectives of HDT and Auto-SCT in PCNSL
From the above studies, it is evident that HDT followed by auto-SCT in patients with PCNSL is a treatment modality with relatively low long-term toxicity and good early responses. Achieving the best responses before auto-SCT appears to play a role though patients with a PR have appeared to benefit from HDT and auto-SCT. We propose that HDT and auto-SCT need to be considered as an early option after induction therapy. Several conditioning regimens have been used in various studies, however thiotepa-based regimens may have an advantage through better blood–brain penetration. We believe that intensifying multi-agent chemotherapy, to achieve maximal response upfront and subjecting patients to a thiotepa-based conditioning regimen (our preferred regimen is TEAM—thiotepa 300 mg/m2 on day −6, etoposide 200 mg/m2 on days −5 to −2, cytarabine 200 mg/m2 on days−5 to −2 and melphalan 140 mg/m2 on day −1) and auto-SCT is a well-tolerated and effective approach. The efficacy of an additional lower radiation dose (≤30Gy) needs to be explored although hyperfractionated radiation therapy has been reported to be associated with severe neurotoxicity.41 The ongoing randomized phase II study by the International extranodal lymphoma study group is an interesting study with three different regimens with or without the incorporation of rituximab and thiotepa as induction therapy followed by WBRT or HDT and auto-SCT.42 This study is appropriate to reduce selection bias in patients undergoing auto-SCT. This would however not obviate the need to perform a larger randomized study. Extramedullary sites are considered to have limited (often considered as a sanctuary site) graft-vs-tumor effect after allo-SCT.43 However, an allogeneic antitumor effect has been observed in a patient with PCNSL who underwent a non-myeloablative conditioning regimen followed by allo-SCT.44 This report supports the concept of graft vs PCNSL reaction after allo-SCT, and therefore serves as a basis to be considered in future studies and remains an investigational approach.
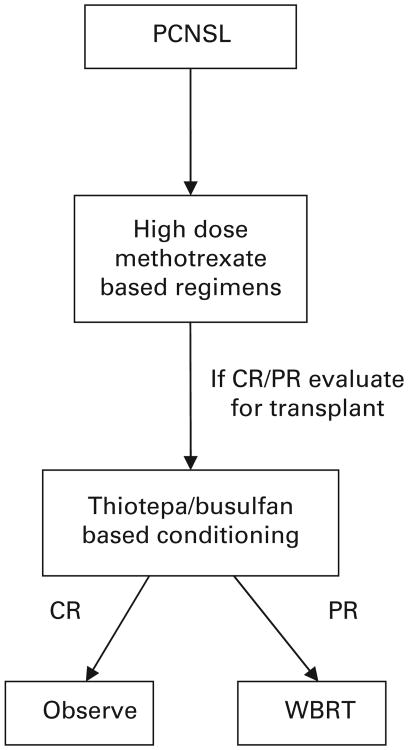
In conclusion, until further prospective studies become available, we propose considering early HDT and auto-SCT after multiagent induction chemotherapy in patients who can tolerate high-dose therapy (the proposed treatment algorithm presented in Figure 1).
Figure 1.
Suggested treatment approach in patients with PCNSL.
Acknowledgments
This study was funded by NR support through Grant No. NIH/5K-12 CA090625-09.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Olson JE, Janney CA, Rao RD, Cerhan JR, Kurtin PJ, Schiff D, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol. 2006;8:27–37. doi: 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Assanelli A, Crocchiolo R, Ciceri F. Central nervous system dissemination in immunocompetent patients with aggressive lymphomas: incidence, risk factors and therapeutic options. Hematol Oncol. 2009;27:61–70. doi: 10.1002/hon.881. [DOI] [PubMed] [Google Scholar]
- 4.Lim T, Kim SJ, Kim K, Lee JI, Lim DH, Lee DJ, et al. Primary CNS lymphoma other than DLBCL: a descriptive analysis of clinical features and treatment outcomes. Ann Hematol. doi: 10.1007/S00277-011-1225-0. e-pub ahead of print 9 April 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstner ER, Batchelor TT. Primary central nervous system lymphoma. Arch Neurol. 2010;67:291–297. doi: 10.1001/archneurol.2010.3. [DOI] [PubMed] [Google Scholar]
- 6.Herrlinger U, Schabet M, Clemens M, Kortmann RD, Petersen D, Will BE, et al. Clinical presentation and therapeutic outcome in 26 patients with primary CNS lymphoma. Acta Neurol Scand. 1998;97:257–264. doi: 10.1111/j.1600-0404.1998.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 7.Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010;115:5005–5011. doi: 10.1182/blood-2009-12-258210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilleri-Broet S, Martin A, Moreau A, Angonin R, Hénin D, Gontier MF, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d'etude des Leucenies et Autres Maladies du Sang (GOELAMS) Am J Clin Pathol. 1998;110:607–612. doi: 10.1093/ajcp/110.5.607. [DOI] [PubMed] [Google Scholar]
- 9.Shenkier TN, Blay JY, O'Neill BP, Poortmans P, Thiel E, Jahnke K, et al. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol. 2005;23:2233–2239. doi: 10.1200/JCO.2005.07.109. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 11.Nelson DF. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL) J Neurooncol. 1999;43:241–247. doi: 10.1023/a:1006206602918. [DOI] [PubMed] [Google Scholar]
- 12.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 13.Bessell EM, Hoang-Xuan K, Ferreri AJ, Reni M. Primary central nervous system lymphoma: biological aspects and controversies in management. Eur J Cancer. 2007;43:1141–1152. doi: 10.1016/j.ejca.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Ferreri AJ, Abrey LE, Blay JY, Borisch B, Hochman J, Neuwelt EA, et al. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol. 2003;21:2407–2414. doi: 10.1200/JCO.2003.01.135. [DOI] [PubMed] [Google Scholar]
- 15.Gabbai AA, Hochberg FH, Linggood RM, Bashir R, Hotleman K. High-dose methotrexate for non-AIDS primary central nervous system lymphoma. Report of 13 cases. J Neurosurg. 1989;70:190–194. doi: 10.3171/jns.1989.70.2.0190. [DOI] [PubMed] [Google Scholar]
- 16.Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: lessons from prospective trials. Ann Oncol. 2000;11:927–937. doi: 10.1023/a:1008376412784. [DOI] [PubMed] [Google Scholar]
- 17.Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Neuwelt EA, Goldman DL, Dahlborg SA, Crossen J, Ramsey F, Roman-Goldstein S, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9:1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 19.Angelov L, Doolittle ND, Kraemer DF, Siegal T, Barnett GH, Peereboom DM, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol. 2009;27:3503–3509. doi: 10.1200/JCO.2008.19.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubenstein JL, Johnson JL, Jung SH, Cheson BD, Kaplan LD. Intensive chemotherapy and immunotherapy, without brain irradiation, in newly diagnosed patients with primary CNS lymphoma: results of CALGB 50202. ASH Annu Meet Abstr. 2010;116:763. [Google Scholar]
- 21.Brevet M, Garidi R, Gruson B, Royer B, Vaida I, Damaj G. First-line autologous stem cell transplantation in primary CNS lymphoma. Eur J Haematol. 2005;75:288–292. doi: 10.1111/j.1600-0609.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoon DH, Lee DH, Choi DR, Sohn BS, Kim S, Kim SW, et al. Feasibility of BU, CY and etoposide (BUCYE), and auto-SCT in patients with newly diagnosed primary CNS lymphoma: a single-center experience. Bone Marrow Transplant. 2011;46:105–109. doi: 10.1038/bmt.2010.71. [DOI] [PubMed] [Google Scholar]
- 23.Illerhaus G, Muller F, Feuerhake F, Schäfer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- 24.Colombat P, Lemevel A, Bertrand P, Delwail V, Rachieru P, Brion A, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38:417–420. doi: 10.1038/sj.bmt.1705452. [DOI] [PubMed] [Google Scholar]
- 25.Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Cheng T, Forsyth P, Chaudhry A, Guttenberger R, Ostertag C, Derigs G, et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant. 2003;31:679–685. doi: 10.1038/sj.bmt.1703917. [DOI] [PubMed] [Google Scholar]
- 27.Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 28.Montemurro M, Kiefer T, Schuler F, Al-Ali HK, Wolf HH, Herbst R, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18:665–671. doi: 10.1093/annonc/mdl458. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos KP, Noguera-Irizarry W, Wiebe L, Hesdorffer CS, Garvin J, Nichols GL, et al. Pilot study of tandem high-dose chemotherapy and autologous stem cell transplantation with a novel combination of regimens in patients with poor risk lymphoma. Bone Marrow Transplant. 2005;36:491–497. doi: 10.1038/sj.bmt.1705103. [DOI] [PubMed] [Google Scholar]
- 30.Glossmann JP, Staak JO, Nogova L, Diehl V, Scheid C, Kisro J, et al. Autologous tandem transplantation in patients with primary progressive or relapsed/refractory lymphoma. Ann Hematol. 2005;84:517–525. doi: 10.1007/s00277-005-1011-y. [DOI] [PubMed] [Google Scholar]
- 31.Abdul-Hai A, Weiss L, Ergas D, Resnick IB, Slavin S, Shapira MY. The effect of high-dose thiotepa, alone or in combination with other chemotherapeutic agents, on a murine B-cell leukemia model simulating autologous stem cell transplantation. Bone Marrow Transplant. 2007;40:891–896. doi: 10.1038/sj.bmt.1705838. [DOI] [PubMed] [Google Scholar]
- 32.Lee SC, Kim SJ, Lee DH, Kim WS, Suh C, Won JH. Excessive toxicity of once daily i.v. BU, melphalan and thiotepa followed by auto SCT on patients with non-Hodgkin' s lymphoma. Bone Marrow Transplant. 2010;45:801–802. doi: 10.1038/bmt.2009.240. [DOI] [PubMed] [Google Scholar]
- 33.Cacchione A, LeMaitre A, Couanet DV, Benhamou E, Amoroso L, Simonnard N, et al. Risk factors for hepatic veno-occlusive disease: a retrospective unicentric study in 116 children autografted after a high-dose BU-thiotepa regimen. Bone Marrow Transplant. 2008;42:449–454. doi: 10.1038/bmt.2008.186. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen PL, Chakravarti A, Finkelstein DM, Hochberg FH, Batchelor TT, Loeffler JS. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23:1507–1513. doi: 10.1200/JCO.2005.01.161. [DOI] [PubMed] [Google Scholar]
- 35.Hoang-Xuan K, Taillandier L, Chinot O, Soubeyran P, Bogdhan U, Hildebrand J, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Taoka K, Okoshi Y, Sakamoto N, Takano S, Matsumura A, Hasegawa Y, et al. A nonradiation-containing, intermediate-dose methotrexate regimen for elderly patients with primary central nervous system lymphoma. Int J Hematol. 2010;92:617–623. doi: 10.1007/s12185-010-0703-9. [DOI] [PubMed] [Google Scholar]
- 37.Hong SJ, Kim JS, Chang JH, Kim KM, Kim SJ, Lee HW, et al. A successful treatment of relapsed primary CNS lymphoma patient with intraventricular rituximab followed by high-dose chemotherapy with autologous stem cell rescue. Yonsei Med J. 2009;50:280–283. doi: 10.3349/ymj.2009.50.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 39.Reddy N, Savani BN. Treatment options for transformed lymphoma: incorporating allogeneic stem cell transplantation in a multimodality approach. Biol Blood Marrow Transplant. 2011;17:1265–1272. doi: 10.1016/j.bbmt.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarnas JC, Negrin RS, Horning SJ, Hu WW, Long GD, Schriber JR, et al. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2000;6:352–358. doi: 10.1016/s1083-8791(00)70060-7. [DOI] [PubMed] [Google Scholar]
- 41.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93–10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Ferreri AJ, Crocchiolo R, Assanelli A, Govi S, Reni M. High-dose chemotherapy supported by autologous stem cell transplantation in patients with primary central nervous system lymphoma: facts and opinions. Leuk Lymphoma. 2008;49:2042–2047. doi: 10.1080/10428190802381238. [DOI] [PubMed] [Google Scholar]
- 43.Clark WB, Strickland SA, Barrett AJ, Savani BN. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Haematologica. 2010;95:860–863. doi: 10.3324/haematol.2010.025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varadi G, Or R, Kapelushnik J, Naparstek E, Nagler A, Brautbar C, et al. Graft-versus-lymphoma effect after allogeneic peripheral blood stem cell transplantation for primary central nervous system lymphoma. Leuk Lymphoma. 1999;34:185–190. doi: 10.3109/10428199909083396. [DOI] [PubMed] [Google Scholar]