SUMMARY
The prognostic value of minimal residual disease (MRD) assessed by multi-parameter flow cytometry (MFC) was investigated among 340 adult patients with B-cell acute lymphoblastic leukaemia (B-ALL) treated between 2004 and 2014 using regimens including the hyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine) backbone. Among them, 323 (95%) achieved complete remission (CR) and were included in this study. Median age was 52 years (range, 15-84). Median white blood cell count (WBC) was 9.35 × 109/l (range, 0.4-658.1 ×109/l). MRD by MFC was initially assessed with a sensitivity of 0.01%, using a 15-marker, 4-colour panel and subsequently a 6-colour panel on bone marrow specimens obtained at CR achievement and at approximately 3 month intervals thereafter. MRD negative status at CR was associated with improved disease-free survival (DFS) and overall survival (OS)(P=0.004 and P=0.04, respectively). Similarly, achieving MRD negative status at approximately 3 and 6 months was associated with improved DFS (P=0.002 and P<0.0001, respectively) and OS (P=0.003 and P<0.0001, respectively). Multivariate analysis including age, WBC at presentation, cytogenetics (standard vs. high risk) and MRD status at CR, 3 months and 6 months, indicated that MRD negative status at CR was an independent predictor of DFS (P<0.05). Achievement of an MRD negative state assessed by MFC is an important predictor of DFS and OS in adult patients with ALL
Keywords: minimal residual disease, acute leukaemia, flow cytometry
INTRODUCTION
Despite achieving high rates of complete remission (CR) with the currently available chemotherapy regimens, the majority of adults with acute lymphoblastic leukaemia (ALL) will relapse.(Pui & Evans, 2006; Bassan & Hoelzer, 2011) Treatment of patients with relapsed disease has been disappointing.(Fielding et al., 2007; O'Brien et al., 2008a; Gokbuget et al., 2012a) Relapse is likely to be related to resistance to the standard cytotoxic agents of residual leukaemia cells, left behind after the initial induction and consolidation strategies.(Fielding et al., 2007; O'Brien et al., 2008a; Gokbuget et al., 2012a) Although allogeneic stem cell transplant (alloSCT) has been successfully used to consolidate a subset of these patients in the first CR, its utility has been limited due to the lack of its universal availability, as well as the morbidity and mortality associated with the procedure itself. Furthermore, a proportion of patients will relapse after an alloSCT, further undermining its ability to cure patients. Therefore, patient and disease-related predictors of outcome, such as age, white blood cell count (WBC) at presentation and karyotypic features, have been used to identify the patients at the highest risk of relapse, in an attempt to select post-remission strategies.(Pui & Evans, 2006; Bassan & Hoelzer, 2011)
The majority of variables used for risk assessment in adult ALL have been pre-therapeutic and do not take into account the complex interaction between the drugs used, the individual patient’s handling of the drugs, and the sensitivity of the leukaemic blasts to the agents. Rapidity of response to the initial therapy, particularly in the paediatric setting, has long been used as a surrogate for the likelihood that the chosen regimen can lead to long-term disease-free and overall survival.(Gajjar et al., 1995; Nachman et al., 1998) More recently, minimal residual disease (MRD) assessment after induction and consolidation therapy has become an established technique and provides an estimate of the reduction of disease burden at various time-points after therapy.(Bruggemann et al., 2006; Campana, 2010; Schrappe, 2014) The MRD level is a reflection of the inherent leukaemia biology (particularly susceptibility to drugs), the adequacy of treatment intensity and other host-drug interactions that influence the response. Clearly, MRD detection is highly dependent on the sensitivity and specificity of the techniques employed, which include multi-parameter flow cytometry (MFC) as well as polymerase chain reaction (PCR) to detect leukaemia-specific fusion transcripts, gene alterations or clone specific immunoglobulin and T-cell receptor genes.(Bruggemann et al., 2012)
MRD monitoring using MFC and PCR has been used extensively in paediatric studies and is well established, being incorporated into large multi-centre trials to assist in selecting therapeutic strategies and making decisions regarding intensification in remission.(Stow et al., 2010; Yeoh et al., 2012; Moorman et al., 2014; Paganin et al., 2014; Vora et al., 2014; Pui et al., 2015) Recently reported adult studies have also begun to determine the utility of identification and quantification of MRD in selecting the most effective and least toxic post-remission strategies.(Bassan et al., 2009; Patel et al., 2010; Gokbuget et al., 2012b; Beldjord et al., 2014; Ribera et al., 2014) Several European trials have utilized patient-specific immunoglobulin and T-cell receptor gene rearrangements but there are few studies using MFC for MRD detection in the adult ALL population.(Ribera et al., 2014)
Beginning in March 2004, we have prospectively collected MRD data in patients with ALL treated at the University of Texas – MD Anderson Cancer Center on various clinical trials using the backbone of the hyperCVAD regimen (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine). Herein, we examine the utility of MRD by MFC in this population in predicting the outcome.
PATIENTS AND METHODS
Patients
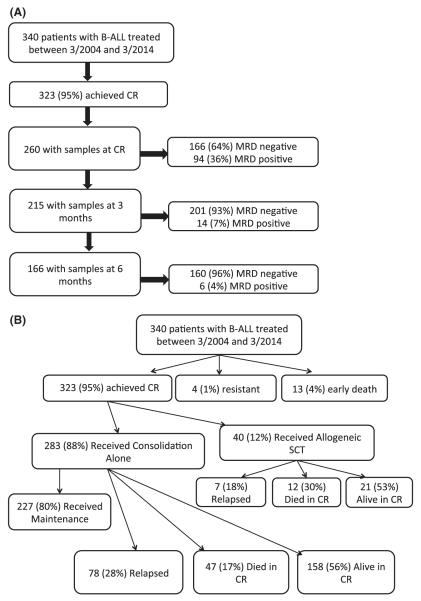
Between March 2004 and March 2014, 340 patients with newly diagnosed B-ALL received therapy with regimens including the hyperCVAD backbone on clinical trials conducted during the period. Among them, 323 (95%) achieved CR or CR without platelet recovery (CRp) and were included in this study (Figure 1a). Median age was 52 years (range, 15-84 years). Median WBC was 9.35 × 109/l (range, 0.4-658.1 × 109/l). Cytogenetics were normal in 62 (18%), Philadelphia+ in 146 (43%), 11q23/rearranged KMT2A (MLL) in 14 (4%), aneuploid in 45 (13%), complex in 29 (9%) and hypodiploid in 13 (4%). Thirty-one (9%) patients had insufficient metaphases or did not have karyotype analysis performed. Patient characteristics are summarized in Table I. All patients signed an informed consent, approved by the University of Texas – M D Anderson Cancer Center Institutional Review Board, to participate in the clinical trials and be evaluated for minimal residual leukaemia at specified intervals. The studies were conducted in accordance with the Declaration of Helsinki.
Figure 1. Patient disposition and sample collection.
a) Patients and sample collection, b) Patient disposition
B-ALL, b-cell acute lymphoblastic leukaemia; CR, complete remission; MRD, minimal residual disease; SCT, stem cell transplant.
Table I.
Patient Characteristics
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median, [range] | 52, [15-84] |
| Male: Female | 180: 160 |
| CNS involvement at diagnosis | 44 |
| WBC at diagnosis (×109/l) | |
| Median, range | 9.35, [0.4 – 658.1] |
| ≥ 30 | 99 (29) |
| Cytogenetics | |
| Normal | 62 (18) |
| Complex | 29 (9) |
| Hypodiploid | 13 (4) |
| Aneuploid | 45 (13) |
| KMT2A rearranged | 14 (4) |
| Philadelphia | 146 (43) |
| Insufficient/Not done | 31 (9) |
CNS, central nervous system; WBC, white blood cell count.
Treatment Regimens and sample collection
Details of the hyperCVAD regimen have been published previously.(Kantarjian et al., 2000) Depending on the presence or absence of specific therapeutic targets, a number of modifications to the regimen were instituted in various clinical trials conducted during the specified period. These mainly involved the addition of tyrosine kinase inhibitors and monoclonal antibodies to the chemotherapy regimen. Details of the regimen as well as the modifications are provided in supplemental Table 1. The numbers of patients treated on the various regimens are shown in Table II. In all studies, bone marrow samples were collected for the evaluation of MRD at the time of achieving CR (approximately day 21 of the first cycle) and subsequently at 3-month intervals thereafter, during the course of consolidation and maintenance therapy.
Table II.
Therapeutic regimens
| Regimen | Number of patients (%) |
|---|---|
| HyperCVAD | 66 (19) |
| HyperCVAD + Rituximab | 75 (22) |
| HyperCVAD + Ofatumumab | 23 (7) |
| HyperCVAD + TKI | |
| Imatinib | 22 (7) |
| Dasatinib | 65 (19) |
| Dasatinib + Rituximab | 15 (4) |
| Ponatinib | 25 (7) |
| Ponatinib + Rituximab | 11 (3) |
| “Mini” HyperCVAD | 2 (0.6) |
| “Mini” HyperCVAD + Rituximab | 22 (7) |
| HyperCMAD | 6 (2) |
| HyperCMAD + Dasatinib | 7 (2) |
| HyperCMAD + Imatinib | 1 (0.3) |
HyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine; TKI, tyrosine kinase inhibitor
Definitions
Achievement of CR necessitated the presence of trilineage haematopoiesis with < 5% blasts in the bone marrow specimen obtained at the time of peripheral blood count recovery with the absence of circulating blasts and extramedullary disease and with an absolute neutrophil count (ANC) >1.0 × 109/l and platelet count > 100 × 109/l. CRp was defined by the achievement of the above mentioned criteria for CR with the exception of lack of platelet recovery to >100 × 109/l. Relapse was characterized as the reappearance of lymphoblasts in the peripheral blood or bone marrow (> 5%) or in any extramedullary site.
Multi-Parameter Flow Cytometry
MFC for assessing MRD was performed on whole bone marrow specimens obtained at the specified time intervals using a standard stain-lyse-wash procedure. 1 × 106 cells were stained per analysis tube, and data were acquired on at least 2 × 105 cells, specimen quality permitting. We excluded specimens containing less than 5 × 104 cells available for analysis. In patients treated earlier on in the course of the studies, data on four-colour staining combinations were acquired on FACSCalibur cytometers using CellQuest software (BD Biosciences, San Diego, CA) and analysed using FlowJo (TreeStar, Ashland, OR). From March 2009 onwards, data on six-colour stains were acquired on FACSCanto cytometers using FACSDiva software (BD Biosciences) and analysed using FCS Express (De Novo Software, Los Angeles, CA). Four-colour combinations contained CD34-fluorescein isothiocyanate (FITC) or CD34-peridinin chlorophyll-cyanin 5.5 (PerCP-Cy5.5) as well as CD19-allophycocyanin (APC) in all tubes, with additional antigens conjugated to FITC and phycoerythrin (PE) including CD10, CD13, CD15, CD20, CD22, CD25, CD33, CD38, CD45, CD58, CD66c and CD81 (all antibodies from BD except CD10 from Beckman Coulter, Fullerton CA and CD66c from Immunotech, Marseilles, France). Six-colour combinations included CD34-PerCP-Cy5.5, CD10-PE-Cy7, and CD19-violet 450 (V450) or CD19-briliant violet 421 (BV421) in each tube (with BV421 yielding a better signal-to-noise ratio than V450), with the additional antigens listed above conjugated to FITC, PE and APC. MRD was identified in comparison with the known patterns of antigen expression by normal maturing CD19+ B cells, as previously described.(Weir et al., 1999) A distinct cluster of at least 20 cells showing altered antigen expression was regarded as an aberrant population, yielding sensitivity for both four-colour and six-colour assays of 1 in 104 cells, or 0.01%. Aberrant expression of at least 2 antigens was needed for assignment of a positive MRD value.
Statistical Analysis
Overall survival (OS) is defined as time from initiation of treatment to death, whereas disease-free survival (DFS) is the time from achievement of CR to disease relapse or death, whichever occurred earlier. Curves for OS and DFS were censored at the time of stem cell transplant or the last follow-up, whichever occurred first. Kaplan-Meier estimators were used to show and compare the survival distributions among different groups and log-rank tests (two-sided at significance level of 0.05) were utilized to evaluate whether the observed differences were statistically significant. Cox proportional hazards models were used for multivariate analysis of OS, DFS and time to disease relapse (death before disease relapse is treated as censoring) using the selected covariates. Statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA.)
To address the issue that MRD status changes over time, the data was analysed by two separate approaches. First, we used landmark analysis (Dafni, 2011) with the MRD status at CR, 3 months and 6 months as landmarks. Separate analyses were conducted for surviving patients at these landmark time points. These results are presented in Tables III, IV and V.
Table III.
Multivariate analysis of covariates for disease-free survival
| Parameter | Hazard ratio (95% confidence interval) |
P value |
|---|---|---|
| At Complete Remission (N=260) | ||
|
| ||
| Age | 1.017 (1.005, 1.029) | 0.006 |
| WBC | 1.003 (1.002, 1.005) | <0.0001 |
| Cytogenetics | 0.89 (0.61, 1.30) | 0.54 |
| MRD at CR | 1.47 (1.003, 2.153) | 0.048 |
|
| ||
| At 3 Months from Therapy Initiation (N=215) | ||
|
| ||
| Age | 1.016 (1.004, 1.028) | 0.01 |
| WBC | 1.003 (1.002, 1.005) | <0.0001 |
| Cytogenetics | 0.92 (0.64, 1.34) | 0.68 |
| MRD at 3 Months | 1.72 (0.79, 3.73) | 0.17 |
|
| ||
| At 6 Months from Therapy Initiation (N=166) | ||
|
| ||
| Age | 1.015 (1.003, 1.027) | 0.014 |
| WBC | 1.003 (1.001, 1.005) | 0.0003 |
| Cytogenetics | 0.98 (0.67, 1.42) | 0.90 |
| MRD at 6 Months | 2.12 (0.70, 6.44) | 0.18 |
WBC, white blood cell count; MRD, minimal residual disease; CR, complete remission
Table IV.
Multivariate analysis of covariates for overall survival
| Parameter | Hazard ratio (95% confidence interval) |
P value |
|---|---|---|
| At Complete Remission (N=260) | ||
|
| ||
| Age | 1.023 (1.011, 1.036) | 0.0002 |
| WBC | 1.002 (1.000, 1.004) | 0.04 |
| Cytogenetics | 0.85 (0.57, 1.25) | 0.40 |
| MRD at CR | 1.4 (0.94, 2.10) | 0.1 |
|
| ||
| At 3 Months from Therapy Initiation (N=216) | ||
|
| ||
| Age | 1.023 (1.010, 1.036) | 0.0004 |
| WBC | 1.002 (1.000, 1.004) | 0.04 |
| Cytogenetics | 0.87 (0.59, 1.28) | 0.47 |
| MRD at 3 Months | 1.96 (0.85, 4.50) | 0.12 |
|
| ||
| At 6 Months from Therapy Initiation (N=166) | ||
|
| ||
| Age | 1.022 (1.010, 1.035) | 0.0005 |
| WBC | 1.001 (1.000, 1.003) | 0.12 |
| Cytogenetics | 0.92 (0.63, 1.35) | 0.67 |
| MRD at 6 Months | 2.68 (0.89, 8.06) | 0.08 |
WBC, white blood cell count; MRD, minimal residual disease; CR, complete remission
Table V.
Multivariate analysis of covariates for time to disease relapse
| Parameter | Hazard ratio (95% confidence interval) |
P value |
|---|---|---|
| At Complete Remission (N=260) | ||
|
| ||
| Age | 1.006 (0.992, 1.021) | 0.39 |
| WBC | 1.005 (1.003, 1.006) | <0.0001 |
| Cytogenetics | 0.815 (0.498, 1.333) | 0.41 |
| MRD at CR | 2.263 (1.432, 3.576) | 0.0005 |
|
| ||
| At 3 Months from Therapy Initiation (N=216) | ||
|
| ||
| Age | 1.003 (0.989, 1.018) | 0.67 |
| WBC | 1.004 (1.003, 1.006) | <0.0001 |
| Cytogenetics | 0.925 (0.570, 1.503) | 0.75 |
| MRD at 3 Months | 1.976 (0.790, 4.944) | 0.145 |
|
| ||
| At 6 Months from Therapy Initiation (N=166) | ||
|
| ||
| Age | 1.001 (0.987, 1.016) | 0.86 |
| WBC | 1.004 (1.002, 1.006) | <0.0001 |
| Cytogenetics | 1.059 (0.653, 1.715) | 0.82 |
| MRD at 6 Months | 3.969 (1.214, 12.691) | 0.02 |
WBC, white blood cell count; MRD, minimal residual disease; CR, complete remission
The second approach was a joint analysis of all time points using the Cox proportional hazard model (Cox, 1972) with MRD status as a time-dependent covariate, and all other factors as time-independent covariates. The data analysis by this approach is presented in Table VI.
Table VI.
Multivariate analysis of time to disease relapse with MRD as a timedependent covariate in Cox proportional hazards model
| Parameter | Hazard ratio (95% confidence interval) |
P value |
|---|---|---|
| Age | 1.003 (0.988, 1.018) | 0.69 |
| WBC | 1.004 (1.003, 1.006) | <0.0001 |
| Cytogenetics | 0.999 (0.618, 1.617) | 0.99 |
| MRD | 6.643 (2.765, 15.956) | <0.0001 |
WBC, white blood cell count; MRD, minimal residual disease; CR, complete remission
RESULTS
Patient disposition
Among the 340 patients treated with B-ALL, 323 (95%) achieved CR; 4 (1%) were resistant to the induction treatment and 13 (4%) died during the induction therapy (Figure 1b). Among the patients who achieved CR, 283 (88%) received consolidation chemotherapy without an alloSCT and 40 (12%) underwent an alloSCT in first CR. Among the patients who did not undergo alloSCT in first CR, 78 (28%) relapsed, 47 (17%) died in CR and 158 (56%) remain alive and in CR. Among these patients, 227 (80%) received maintenance therapy; reasons for not receiving maintenance were relapse in 17 (30%), death in 24 (43%) and other in 5 (9%); 10 patients (18%) were too early in the course of therapy at the time of analysis. Among the 40 patients who underwent alloSCT in first CR, 21 (53%) remain alive and in CR with 7 (18%) having relapsed and 12 (30%) having died in CR.
Outcome prediction by MFC
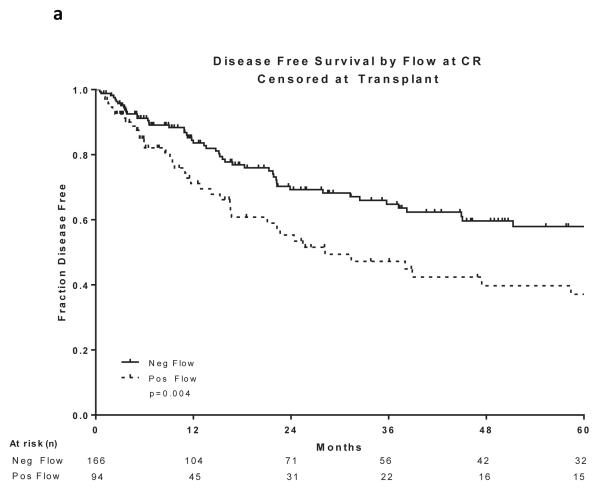
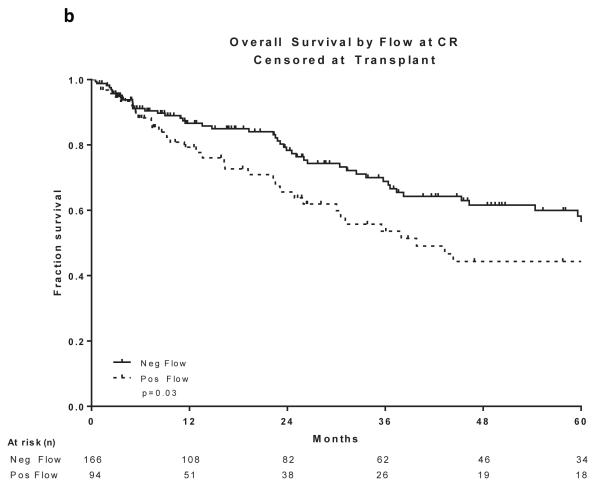
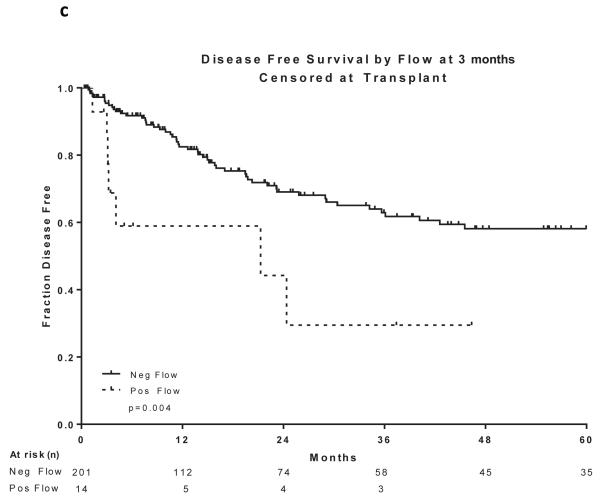
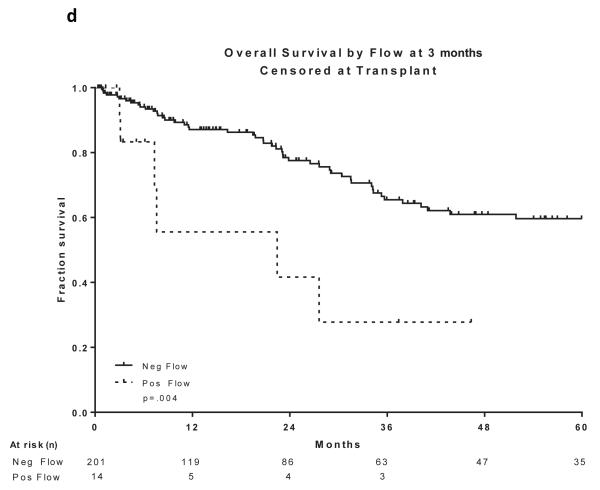
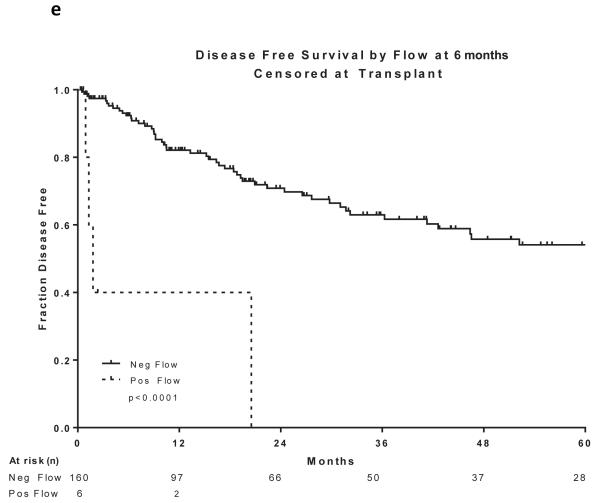
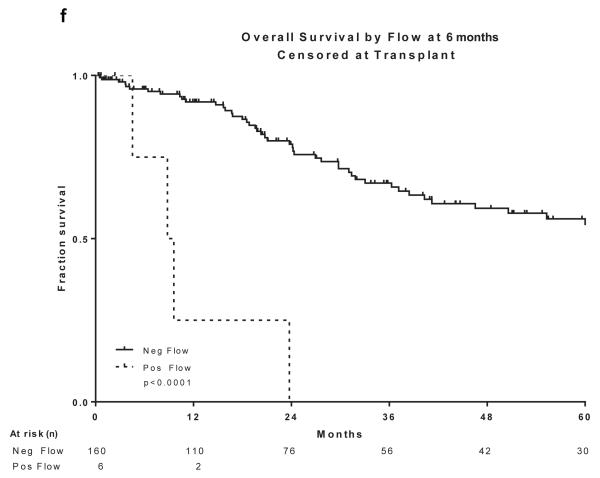
Two hundred and sixty patients had available samples at CR and 166 (64%) became MRD negative. Achieving MRD negative status at CR was associated with a statistically significant improvement in DFS (P =0.004) and OS (P=0.03)(Figure 2a and 2b). 215 patients were evaluated for MRD at approximately 3 months and 201 (93%) became negative. Achieving MRD negative status at approximately 3 months was associated with a statistically significant improvement in DFS (P=0.004) and OS (P=0.004)(Figure 2c and 2d). 166 patients were evaluated for MRD at approximately 6 months and 160 (96%) became negative. Achieving MRD negative status at approximately 6 months was also associated with a statistically significant improvement in DFS (P<0.0001) and OS (P<0.0001)(Figure 2e and 2f). Figure 2 demonstrates the DFS and OS by MRD status at CR (Figure 2a and 2b), at 3 months (Figure 2c and 2d) and at 6 months (Figure 2e and 2f), with patients censored at the time of alloSCT or last follow-up. In order to determine whether the prognostic value of MRD status was independent of other covariates, we performed multivariate analysis including age, WBC at presentation, cytogenetics (standard vs. high risk) and MRD status at CR, 3 months and 6 months as potential factors predicting DFS and OS. Achieving an MRD negative status at CR was an independent predictor of DFS (P=0.048) with age and WBC at presentation remaining independent predictors of both DFS and OS. Achieving an MRD negative status at 6 months was of borderline significance for predicting OS (Tables III and IV). Achieving MRD at CR and at 6 months were independent predictors of relapse with death prior to relapse being censored (Table V). Using a Cox proportional hazard model with joint analysis of all time points and MRD status as a time-dependent covariate, achieving MRD was an independent predictor of relapse (Table VI).
Figure 2. Outcomes by MRD status at CR, 3 and 6 months from treatment.
a) Disease-free survival by flow at CR, b) Overall survival by flow at CR, c) Disease-free survival by flow at 3 months from treatment start date, d) Overall survival by flow at 3 months from treatment start date, e) Disease-free survival by flow at 6 months from treatment start date, f) Overall survival by flow at 6 months from treatment start date
All curves are censored at the time of transplant or last follow-up. All the curves are presented from the time of MRD assessment
CR, complete remission; MRD, minimal residual disease
DISCUSSION
Unlike the paediatric population, progress in the treatment of adult patients with ALL has been limited in the past three decades.(Kantarjian et al., 2012) Whereas most patients with paediatric ALL can be cured with the current multi-agent chemotherapy regimens, most studies conducted in the adult population report survival rates of up to 50%.(Pui & Evans, 2006; Bassan & Hoelzer, 2011)) The reasons underlying these differences are becoming clearer. For example, biological differences between the “adult” versus the “paediatric” disease are better characterized by identification of differences between the incidence of distinct molecular and genetic aberrations in the two populations.(Armstrong & Look, 2005; Pui& Evans, 2006) Some of these biological differences may contribute to the disparity of response to cytotoxic chemotherapy between older and younger patients.(Roberts et al., 2014a) Furthermore, older patients are less able to tolerate intensification of the dose of cytotoxic drugs due to their inherent physiological decline and comorbid conditions.(O'Brien et al., 2008b; Curran & Stock, 2015) Therefore, further progress in treating these patients can occur only after identification of the sub-population of leukaemic cells remaining after induction and consolidation, which are likely to be at least partially resistant to cytotoxic agents. Such minimal residual leukaemia (referred to correctly by some researchers as measurable residual leukaemia, as its detection is entirely dependent on the technology used) ostensibly includes the cells responsible for relapse and hence failure of therapy.(Bruggemann et al., 2006) Design of intensification strategies in selected patients likely to benefit from them, or rational development of agents that can overcome this resistance through alternative mechanisms of action, will then likely reduce the risk of relapse and improve outcomes in general.
Multiple published studies in the paediatric literature have firmly established the significance of detection of MRD using MFC as well as PCR for leukaemia-specific fusion transcripts, gene alterations and clone-specific immunoglobulin and T-cell receptor genes after induction and consolidation. Several paediatric studies have also demonstrated the potential benefit of MRD-directed treatment intensification.(Yeoh et al., 2012; Roberts et al., 2014b; Vora et al., 2014; Pui et al., 2015) In the adult population, there have been few studies that have evaluated this strategy.(Bassan et al., 2009; Gokbuget et al., 2012b; Ribera et al., 2014) Bassan et al (2009) used real time quantitative PCR for known chimeric genes as well as leukaemia-specific probes generated by genomic amplification and sequencing of the VDJ/VJ regions of immunoglobulin heavy chain (IGH) or the kappa light chain (IGK), and T-cell receptor genes for MRD detection. They assigned post-remission maintenance therapy or alloSCT (or autologous transplant followed by maintenance therapy in patients with no available donor), to MRD-negative and MRD-positive patients, respectively. Gokbuget et al (2012b) used a similar strategy to identify patients with a high risk of failure who were candidates for an alloSCT in first remission. Ribera et al (2014) used MFC for detection of MRD after early consolidation therapy in adolescents and younger adults (aged 15-60 years) and demonstrated a favourable outcome without utilization of alloSCT in patients with low flow-MRD levels.
In this study, in a generally older population than was evaluated in the above reports, we have demonstrated the utility of MRD detection by MFC at the time of CR and at 3 and 6 months after initiation of therapy, in identifying patients who are more likely to relapse. On univariate analysis, detection of MRD at all these time-points was significantly associated with a worse DFS and OS. On multivariate analysis, age and WBC at presentation remained highly significant predictors of outcome and detection of MRD at CR was associated with a significantly shorter DFS. Whether MRD assessment by MFC can independently predict OS should be investigated further in larger, prospective trials of adult ALL.
The advantages and disadvantages of MFC for detecting MRD in patients with ALL have been previously extensively discussed.(Bruggemann et al., 2012) The major advantages of this technique are its rapidity and universal availability. This is particularly important in situations in which a decision on therapeutic intervention is needed fairly rapidly as, unlike PCR techniques, the time-consuming characterization of patient-specific markers is not needed.(Bruggemann et al., 2012) However, the limitations of this technique are its limited sensitivity when 4-colour analysis is performed and significant expertise needed when 6 or more colour-MFC is utilized. However, our study demonstrates that despite these limitations, detection of MRD by MFC can provide meaningful information, allowing better stratification of patients for post-remission intensification.
Ultimately, the utility of detection of MRD will depend on the ability to eradicate it with the available strategies, such as intensification of consolidation, or the use of alloSCT or novel agents that have more specific activity against the residual leukemic cells. Furthermore, absence of detectable MRD may allow de-intensification of treatment, thereby reducing the potential toxicity in patients who are destined to do well. Allogeneic stem cell transplantation has been used successfully to improve the outcome of patients with high likelihood of relapse. However, a number of recent reports have suggested a worse outcome for patients with pre-transplant detectable MRD.(Bar et al., 2014; Zhou et al., 2014) Novel therapeutic strategies, such as the use of bi-specific T-cell engaging antibodies or chimeric antigen receptor T-cell therapy, have been successfully employed to eliminate MRD and are likely to be incorporated in the future treatment strategies after cytotoxic chemotherapy and prior to transplantation.(Topp et al., 2011; Grupp et al., 2013) This will probably improve the outcomes and may potentially eliminate the need for transplant in specific populations.
Supplementary Material
Acknowledgments
Supported in part by the MD Anderson Cancer Center Support Grant CA016672
Footnotes
AUTHOR CONTRIBUTIONS
FR, SMB, DAT and HMK designed and conducted the study, treated patients and wrote the manuscript
JLJ, SAW and SK conducted the MRD MFC analyses and wrote the manuscript
RG and XH collected and analysed the data and performed the statistical analysis
EJ, GB, GGM, JAB, AF, WW, TK, NJ, PK, REC, DM, ZE and JEC treated patients on the clinical trials, provided data and reviewed the final manuscript
AUTHOR DISCLOSURES
None of the authors report any relevant disclosures
This study was presented in part at the American Society of Hematology meeting, San Francisco, December 2014
The authors report no competing financial interests
REFERENCES
- Armstrong SA, Look AT. Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol. 2005;23:6306–6315. doi: 10.1200/JCO.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, Appelbaum FR, Gooley TA. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leukemia research and treatment. 2014;2014:421723. doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, Rossi G, Borlenghi E, Pogliani EM, Terruzzi E, Fabris P, Cassibba V, Lambertenghi-Deliliers G, Cortelezzi A, Bosi A, Gianfaldoni G, Ciceri F, Bernardi M, Gallamini A, Mattei D, Di Bona E, Romani C, Scattolin AM, Barbui T, Rambaldi A. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113:4153–4162. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- Beldjord K, Chevret S, Asnafi V, Huguet F, Boulland ML, Leguay T, Thomas X, Cayuela JM, Grardel N, Chalandon Y, Boissel N, Schaefer B, Delabesse E, Cavé H, Chevallier P, Buzyn A, Fest T, Reman O, Vernant JP, Lhéritier V, Béné MC, Lafage M, Macintyre E, Ifrah N, Dombret H, Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123:3739–3749. doi: 10.1182/blood-2014-01-547695. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J, Lüschen S, Pott C, Ritgen M, Scheuring U, Horst HA, Thiel E, Hoelzer D, Bartram CR, Kneba M, German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- Bruggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120:4470–4481. doi: 10.1182/blood-2012-06-379040. [DOI] [PubMed] [Google Scholar]
- Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. 2010;2010:7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34(No. 2) 1972. [Google Scholar]
- Curran E, Stock W. How I Treat: Acute Lymphoblastic Leukemia in Older Adolescents and Young Adults (AYAs) Blood. 2015;125:3702–3710. doi: 10.1182/blood-2014-11-551481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafni U. Primer on Statistical Interpretation and Methods: Landmark Analysis at the 25-Year Landmark Point. Circulation: Cardiovascular Quality and Outcomes. 2011;4:363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, Durrant IJ, Luger SM, Marks DI, Franklin IM, McMillan AK, Tallman MS, Rowe JM, Goldstone AH, Medical Research Council of the United Kingdom Adult ALL Working Party. Eastern Cooperative Oncology Group Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- Gajjar A, Ribeiro R, Hancock ML, Rivera GK, Mahmoud H, Sandlund JT, Crist WM, Pui CH. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995;86:1292–1295. [PubMed] [Google Scholar]
- Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A, Schaich M, Schwartz S, Serve H, Starck M, Stelljes M, Stuhlmann R, Viardot A, Wendelin K, Freund M, Hoelzer D, German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012a;120:2032–2041. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- Gokbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, Fietkau R, Freund M, Ganser A, Ludwig WD, Maschmeyer G, Rieder H, Schwartz S, Serve H, Thiel E, Brüggemann M, Hoelzer D, German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012b;120:1868–1876. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Thomas D, Wayne AS, O'Brien S. Monoclonal antibody-based therapies: a new dawn in the treatment of acute lymphoblastic leukemia. J Clin Oncol. 2012;30:3876–3883. doi: 10.1200/JCO.2012.41.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, Keating MJ, Murphy S, Freireich EJ. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- Moorman AV, Enshaei A, Schwab C, Wade R, Chilton L, Elliott A, Richardson S, Hancock J, Kinsey SE, Mitchell CD, Goulden N, Vora A, Harrison CJ. A novel integrated cytogenetic and genomic classification refines risk stratification in pediatric acute lymphoblastic leukemia. Blood. 2014;124:1434–1444. doi: 10.1182/blood-2014-03-562918. [DOI] [PubMed] [Google Scholar]
- Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- O'Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G, Pierce S, Garcia-Manero G, Kantarjian HM. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008a;113:3186–3191. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008b;113:2097–2101. doi: 10.1002/cncr.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganin M, Fabbri G, Conter V, Barisone E, Polato K, Cazzaniga G, Giraldi E, Fagioli F, Aricò M, Valsecchi MG, Basso G. Postinduction minimal residual disease monitoring by polymerase chain reaction in children with acute lymphoblastic leukemia. J Clin Oncol. 2014;32:3553–3558. doi: 10.1200/JCO.2014.56.0698. [DOI] [PubMed] [Google Scholar]
- Patel B, Rai L, Buck G, Richards SM, Mortuza Y, Mitchell W, Gerrard G, Moorman AV, Duke V, Hoffbrand AV, Fielding AK, Goldstone AH, Foroni L. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. British journal of haematology. 2010;148:80–89. doi: 10.1111/j.1365-2141.2009.07941.x. [DOI] [PubMed] [Google Scholar]
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, Sandlund JT, Ribeiro RC, Rubnitz JE, Inaba H, Bhojwani D, Gruber TA, Leung WH, Downing JR, Evans WE, Relling MV, Campana D. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. The Lancet. Oncology. 2015;16:465–474. doi: 10.1016/S1470-2045(15)70082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera JM, Oriol A, Morgades M, Montesinos P, Sarra J, Gonzalez-Campos J, Brunet S, Tormo M, Fernández-Abellán P, Guàrdia R, Bernal MT, Esteve J, Barba P, Moreno MJ, Bermúdez A, Cladera A, Escoda L, García-Boyero R, Del Potro E, Bergua J, Amigo ML, Grande C, Rabuñal MJ, Hernández-Rivas JM, Feliu E. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32:1595–1604. doi: 10.1200/JCO.2013.52.2425. [DOI] [PubMed] [Google Scholar]
- Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, McCastlain K, Ding L, Lu C, Song G, Ma J, Becksfort J, Rusch M, Chen SC, Easton J, Cheng J, Boggs K, Santiago-Morales N, Iacobucci I, Fulton RS, Wen J, Valentine M, Cheng C, Paugh SW, Devidas M, Chen IM, Reshmi S, Smith A, Hedlund E, Gupta P, Nagahawatte P, Wu G, Chen X, Yergeau D, Vadodaria B, Mulder H, Winick NJ, Larsen EC, Carroll WL, Heerema NA, Carroll AJ, Grayson G, Tasian SK, Moore AS, Keller F, Frei-Jones M, Whitlock JA, Raetz EA, White DL, Hughes TP, Guidry Auvil JM, Smith MA, Marcucci G, Bloomfield CD, Mrózek K, Kohlschmidt J, Stock W, Kornblau SM, Konopleva M, Paietta E, Pui CH, Jeha S, Relling MV, Evans WE, Gerhard DS, Gastier-Foster JM, Mardis E, Wilson RK, Loh ML, Downing JR, Hunger SP, Willman CL, Zhang J, Mullighan CG. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014a;371:1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KG, Pei D, Campana D, Payne-Turner D, Li Y, Cheng C, Sandlund JT, Jeha S, Easton J, Becksfort J, Zhang J, Coustan-Smith E, Raimondi SC, Leung WH, Relling MV, Evans WE, Downing JR, Mullighan CG, Pui CH. Outcomes of children with BCR-ABL1-like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol. 2014b;32:3012–3020. doi: 10.1200/JCO.2014.55.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrappe M. Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. 2014;2014:244–249. doi: 10.1182/asheducation-2014.1.244. [DOI] [PubMed] [Google Scholar]
- Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, Mullighan CG, Zhou Y, Pui CH, Campana D. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115:4657–4663. doi: 10.1182/blood-2009-11-253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Köhne-Volland R, Brüggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsele H, Riethmüller G, Hoelzer D, Zugmaier G, Bargou RC. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, Moorman AV, Wade R. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. The Lancet. Oncology. 2014;15:809–818. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- Weir EG, Cowan K, LeBeau P, Borowitz MJ. A limited antibody panel can distinguish B-precursor acute lymphoblastic leukemia from normal B precursors with four color flow cytometry: implications for residual disease detection. Leukemia. 1999;13:558–567. doi: 10.1038/sj.leu.2401364. [DOI] [PubMed] [Google Scholar]
- Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K, Campana D, Tan PL, Chan MY, Kham SK, Chong LA, Tan AM, Lin HP, Quah TC. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30:2384–2392. doi: 10.1200/JCO.2011.40.5936. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Slack R, Jorgensen JL, Wang SA, Rondon G, de Lima M, Shpall E, Popat U, Ciurea S, Alousi A, Qazilbash M, Hosing C, O'Brien S, Thomas D, Kantarjian H, Medeiros LJ, Champlin RE, Kebriaei P. The effect of peritransplant minimal residual disease in adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Clinical lymphoma, myeloma & leukemia. 2014;14:319–326. doi: 10.1016/j.clml.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.