Abstract
Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT) is the gold standard treatment for pediatric PTSD. Nonetheless, clinical outcomes in TF-CBT are highly variable, indicating a need to identify reliable predictors that allow forecasting treatment response. Here, we test the hypothesis that functional neuroimaging correlates of emotion processing predict PTSD symptom reduction during Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT) among adolescent girls with PTSD. Thirty-four adolescent girls with PTSD related to physical or sexual assault were enrolled in TF-CBT, delivered in an approximately 12 session format, in an open trial. Prior to treatment, they were engaged in an implicit threat processing task during 3T fMRI, during which they viewed faces depicting fearful or neutral expressions. Among adolescent girls completing TF-CBT (n=23), slopes of PTSD symptom trajectories during TF-CBT were significantly related to pre-treatment degree of bilateral amygdala activation while viewing fearful vs neutral images. Adolescents with less symptom reduction were characterized by greater amygdala activation to both threat and neutral images (i.e., less threat-safety discrimination), whereas adolescents with greater symptom reduction were characterized by amygdala activation only to threat images. These clinical outcome relationships with pre-treatment bilateral amygdala activation remained when controlling for possible confounding demographic or clinical variables (e.g., concurrent psychotropic medication, comorbid diagnoses). While limited by a lack of a control group, these preliminary results suggest that pre-treatment amygdala reactivity to fear stimuli, a component of neurocircuitry models of PTSD, positively predicts symptom reduction during TF-CBT among assaulted adolescent girls, providing support for an objective measure for forecasting treatment response in this vulnerable population.
Keywords: adolescence, PTSD, cognitive-behavioral therapy, neuroimaging
Epidemiological studies suggest that ∼50% of adolescents aged 12–17 have been exposed to physical assault, sexual assault, or witnessed violence, with ∼6% of adolescent girls meeting criteria for posttraumatic stress disorder (PTSD) (Kilpatrick et al. 2000;Kilpatrick et al. 2003). Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT) is the gold standard psychological treatment for trauma-exposed youth with symptoms of PTSD (Cohen et al. 2004;Cohen et al. 2010;Cohen et al. 2011). TF-CBT is typically delivered in 12–16 weekly sessions and is composed of modules including: psychoeducation about trauma and PTSD; parenting skills; affect regulation skills; and developing a narrative of the traumatic event and cognitive processing of associated thoughts and feelings. Numerous clinical trials have demonstrated efficacy for TF-CBT in reducing PTSD symptoms, depression, anxiety, and behavior problems (Cary and McMillen 2012).
Despite clear efficacy, clinical response to TF-CBT is highly variable across individuals. These individual differences in clinical response are suggestive of variability in mediating psychopathology mechanisms among youth with PTSD. Identifying objective predictors that characterize variability in core mechanisms and that predict treatment response may help facilitate personalized treatment recommendations and may also help identify both the mechanisms of pediatric PTSD as well as the mechanisms most potently targeted in TF-CBT. Prior research demonstrates the viability of neuroimaging measures of brain function as biomarkers of adult PTSD treatment response Bryant and colleagues found that greater pre-treatment amygdala reactivity to threat predicted less symptom reduction during CBT among a mixed-sex and mixed-trauma adult sample (Bryant et al. 2008a). A separate study among assaulted adult women with PTSD found a positive relationship between pre-treatment ACC activity during anticipation of negative images and treatment response to CBT (Aupperle et al. 2013). Finally, Falconer and colleagues found among a mixed-sex and mixed-trauma adult sample that greater activation of the striatum and ventrolateral PFC during an inhibitory control task predicted better treatment response to CBT (Falconer et al. 2013). Nonetheless, pre-treatment neural processing correlates of symptom reduction has not been investigated among adolescents with PTSD. Investigation of adolescents specifically is important given the substantial neurocognitive development that takes place in adolescence (Blakemore 2012;Crone and Dahl 2012;Paus et al. 2008) and knowledge of development-related difference in intrinsic brain organization (Dosenbach et al. 2010).
Neurocircuitry models of PTSD (Admon et al. 2013;Rauch et al. 2006) emphasize a hyperactive amygdala and dorsal anterior cingulate cortex (ACC) as brain mediators of hypervigilance for threat and heightened anxiety, and a hypoactive medial prefrontal cortex (mPFC) and hippocampus as brain mediators of deficits in emotion regulation and fear extinction. While TF-CBT was not developed to target these brain mechanisms explicitly, the behavioral phenomena targeted in TF-CBT (e.g., heightened anxiety, affect dysregulation, deficits in cognitive coping skills) map closely onto functional attributes of the neurocircuitry of PTSD. Given this overlap between the behavioral targets of TF-CBT and the neuroanatomy of PTSD, it could be expected that clinical response to TF-CBT would be related to pretreatment variability within this neurocircuit.
Here, we test the hypothesis that pre-treatment responsivity within the neurocircuitry mediating threat processing and implicated in PTSD (i.e., amygdala, dACC) predicts symptom reduction during TF-CBT among assaulted adolescent girls with a current diagnosis of PTSD. The prior imaging studies among adults (Aupperle, Allard, Simmons, Flagan, Thorp, Norman, Paulus, & Stein 2013;Bryant, Felmingham, Kemp, Das, Hughes, Peduto, & Williams 2008a;Falconer, Allen, Felmingham, Williams, & Bryant 2013) suggest the hypothesis here among adolescents that lesser amygdala activity and greater ACC and striatum activity should predict greater symptom reduction during TFCBT. We chose fear stimuli as a commonly used probe of threat processing related to the heightened reactivity to threat stimuli associated with PTSD (Bryant et al. 2008b;Felmingham et al. 2010;Rauch et al. 2000). Focus on assault exposure was motivated by 1) the greater risk for psychopathology conferred via assault exposure relative to other types of traumas (Cisler et al. 2012), and 2) restriction to a specific type of trauma increases homogeneity of the sample. Focus on adolescent girls was motivated by 1) increased risk for PTSD following assault among girls relative to boys (Kilpatrick, Ruggiero, Acierno, Saunders, Resnick, & Best 2003), 2) focus on a single sex increases homogeneity of the sample in light of known sex differences in brain function, and 3) adolescence is characterized by heightened stress reactivity and is a developmental period during which many forms of psychopathology, including mood and anxiety disorders, emerge (McCormick et al. 2010;McLaughlin et al. 2011;Ordaz and Luna 2012).
Methods
Participants and assessments
Thirty-four adolescent girls, aged 11–16, meeting DSM-IV criteria for PTSD, having a positive history of assaultive violence exposure, and having a consistent caregiver with whom to participate in treatment, were enrolled in the study and began TF-CBT (see full enrollment flow chart in Supplemental Figure 1). Participants were recruited through networking with local outpatient clinics, child advocacy centers, schools, juvenile justice, churches, and community organizations. Exclusion criteria consisted of MRI contraindications (e.g., internal ferrous metal objects), psychotic symptoms, lack of a consistent caregiver, and presence of a developmental disorder. Concurrent psychotropic medication was not exclusionary. Demographic and clinical characteristics of the sample are provided in Table 1. Adolescents provided assent and a caregiver/legal guardian provided consent. This study was conducted with IRB approval.
Table 1.
Demographic, clinical characteristics, and treatment response of the samples.
| Treatment Completers (n=23) | Completed ≥ 4 TF CBT sessions (n=28) |
|||
|---|---|---|---|---|
| Variable | Mean/frequency (SD) |
Mean/frequency (SD) |
||
| Age | 13.87 (1.77) | 14.04 (1.67) | ||
| Verbal IQ | 95.26 (15.00) | 95.04 (14.22) | ||
| Ethnicity | 39% Caucasian | 39% Caucasian | ||
| 52% African | 50% African | |||
| American | American | |||
| 9% Biracial | 7% Biracial | |||
| 0% Hispanic | 4% Hispanic | |||
| Total number of types of assaults |
5.65 (3.98) | 5.86 (4.34) | ||
| Direct Physical Assault | 96% | 96% | ||
| Sexual Assault | 87% | 89% | ||
| Witnessed Violence | 91% | 93% | ||
| Psychotropic Medication | SSRI - 39% | SSRI - 36% | ||
| Antipsychotic – 17% |
Antipsychotic – 18% |
|||
| SARI – 4% | SARI – 4% | |||
| Alpha blocker – 4% |
Alpha blocker – 4% |
|||
| Pre-Treatment | Post- Treatment |
Pre-Treatment | Post- Treatment |
|
| Current PTSD | 100% | 35% | 100% | - |
| # comorbid diagnoses | 2.74 (2.22) | 1.00 (1.62) | 2.93 (2.40) | - |
| Current Anxiety | 65% | 17% | 64% | - |
| Disorder | ||||
| Current Depressive | 52% | 13% | 50% | - |
| Disorder | ||||
| Current Bipolar Disorder | 4% | 0% | 3% | - |
| Current Alcohol Use (past year) |
8% | 4% | 14% | - |
| Current Substance Use (past year) |
12% | 17% | 17% | - |
| Current Conduct/ODD | 26% | 21% | 32% | - |
| UCLA PTSD Index | 36.04 (17.87) | 18.30 (16.62) |
35.93 (17.74) | 19.64 (16.25) |
| SMFQ | 12.22 (8.25) | 4.61 (6.55) | 12.46 (8.06) | 4.46 (6.00) |
| DERS Nonacceptance | 9.61 (7.06) | 3.48 (5.15) | 10.43 (7.46) | - |
| DERS Goals | 11.65 (5.75) | 4.57 (5.54) | 12.46 (5.79) | - |
| DERS Impulse | 8.30 (6.89) | 3.52 (5.39) | 9.00 (6.67) | - |
| DERS Strategies | 12.57 (8.98) | 5.09 (7.30) | 13.18 (8.99) | - |
| DERS Clarity | 7.83 (5.35) | 3.65 (4.99) | 8.36 (5.46) | - |
Note. SMFQ=Short mood and feelings questionnaire; DERS = Difficulties in Emotion Regulation Scale. Participants who dropped out prior to completing all TF-CBT modules (n=5) did not receive a full post-treatment assessment and their post-treatment data are limited to the UCLA PTSD index and SMFQ.
Participant’s pre- and post-treatment mental health was assessed with the MINI-KID (Sheehan et al. 2010), a structured clinical interview for most Axis I disorders found in childhood and adolescence. Assaultive trauma histories were characterized using the trauma assessment section of the National Survey of Adolescents (NSA) (Kilpatrick, Acierno, Saunders, Resnick, Best, & Schnurr 2000;Kilpatrick, Ruggiero, Acierno, Saunders, Resnick, & Best 2003), a structured interview used in prior epidemiological studies of assault and mental health functioning among adolescents that uses behaviorally specific dichotomous questions to assess sexual assault, physical assault, severe abuse from a caregiver, and witnessed violence. A trained female research coordinator with several years of experience with structured clinical interviews completed the MINI and NSA interviews with participants under the supervision of a licensed clinical psychologist.
The pre- and post-treatment assessment also included measures of verbal IQ (receptive one word picture vocabulary test (Brownell 2000)), PTSD symptom severity (UCLA PTSD Reaction Index (Steinberg et al. 2004)), depression (Short Mood and Feelings Questionnaire (Angold et al. 1995); SMFQ), and emotion regulation ability using the Difficulty in Emotion Regulation Scale (Gratz and Roemer 2004) (DERS). This measure of difficulty with emotion regulation consists of 5 subscales (Bardeen et al. 2012): clarity of emotions, difficulty engaging in goal-directed behavior while experiencing negative emotions, having limited strategies to regulate negative emotions, non-acceptance of negative emotions, and impulse control problems when experiencing negative emotions. Additionally, participants completed these same measures of PTSD and depression symptom severity prior to each therapy visit.
TF-CBT
TF-CBT was delivered by two postdoctoral clinical psychology fellows and a doctoral-level graduate student. The therapists were trained in TF-CBT according to an established protocol approved by Anthony Mannarino, Ph.D., a co-developer of TF-CBT, which included completion of TF-CBTWeb (accessible at www.musc.edu/tfcbt) an online TF-CBT training, three days of in-person TF-CBT training with Dr. Mannarino, and one hour of weekly supervision with a licensed clinical psychologist with expertise in supervising the model. TF-CBT in this study used a 12-week protocol of 60 to 90 minute weekly sessions.
MRI acquisition and Image Preprocessing
MRI acquisition and preprocessing steps are detailed in supplementary material.
fMRI tasks
Implicit Threat Processing Task
During this commonly used task (Williams et al. 2006), participants made button presses indicating gender decisions while viewing faces taken from the NimStim facial stimuli set 20. The faces contained either neutral or fearful expressions, presented either overtly or covertly, in alternating blocks. There were an equal number of female and male faces. Overt faces were presented for 500 ms, with a 1200 ms inter-stimulus-interval displaying a blank screen with a fixation cross, in blocks of 8 presentations for a total block length of ∼17 s. Covert face blocks used a similar design but were presented for 33 ms followed immediately by a neutral facial expression mask for 166 ms from the same actor depicted in the covert image, and the ISI was 1500 ms. Rest blocks that displayed a blank screen with a fixation cross and lasted 10 s were additionally included. The task was presented in two runs, each lasting ∼8 min, during which each block type was presented 5 times. We conducted parallel analyses on the contrasts of covert fear vs covert neutral, overt fear vs overt neutral, and all fear vs all neutral blocks.
Data Analysis
fMRI Data
For the threat processing task, the task design matrix consisted of columns for the four task blocks (overt fear, overt neutral, covert fear, covert neutral). We modeled a 19 s hemodynamic response function explicitly using cubic splines (AFNI’s 3dDeconvolve with ‘CSPLINzero’ option for HRF fitting) with 10 parameters, separately for each block type, and accounting for serial correlation (AFNI’s 3dREMLfit). Functional activation (height and width of HRF) during each block type was characterized with area-under-the-curve analyses using numerical integration (Lenow et al. 2014;Urry et al. 2006).
To assess task-modulated functional connectivity for fear vs neutral trials during the threat processing task, we used generalized psychophysiological interaction (gPPI) analyses (Cisler et al. 2014a;McLaren et al. 2012) using the amygdala clusters identified in the task activation analyses as seed regions (Supplemental Material).
Identifying pre-treatment fMRI predictors of symptom change
Second-level analysis consisted of whole-brain, voxel-wise, robust regression(Wager et al. 2005) analysis, in which the β coefficient representing slope of PTSD symptom change across time for each participant is regressed simultaneously onto 1) the voxel’s contrast value, representing relative % signal change to fear vs neutral trials, and, 2) the intercept from the within-subject regression models representing severity of pre-treatment PTSD symptoms, in order to control for any confounding effects of pre-treatment symptom severity. To correct for multiple comparisons, we maintained a corrected p < .05 using cluster-level thresholding (Forman et al. 1995) defined with Monte Carlo simulation (AFNI’s 3dClustSim), in which a significant cluster (corrected p < .05) is defined as a minimum of 51 contiguous voxels that survive a primary (uncorrected) threshold of p < .01.
We used two additional convergent measures of symptom change: slopes of changes in depression symptoms across treatment as measured by the SMFQ, and magnitude of pre- to post-treatment change in emotion regulation ability, as measured by the subscales of the DERS. Given that the DERS was only collected twice (pre and post-treatment), we could not calculate trajectory slopes comparable to the PTSD or depression symptom slopes.
We conducted parallel analyses for the measures of functional connectivity, in which the β coefficient representing slope of PTSD symptom change was regressed on the gPPI contrast values, again controlling for the intercept from the within-subject regression models representing severity of pre-treatment PTSD symptoms.
We focused analyses on those participants who received all modules of TF-CBT and had usable brain imaging data (n=23). One participant’s fMRI data was unusable due to motion artifact (see supplementary materials), and one participant was excluded due to incomplete brain coverage during fMRI scanning (i.e., excessive OFC and temporal lobe signal dropout). We additionally conducted supplemental analyses among adolescents who completed ≥ four sessions (n=28) (Supplemental Material).
RESULTS
Treatment Outcome
Mean pre- and post-treatment PTSD and depression symptoms, comorbid diagnoses, and DERS scores are reported in Table 1. Mean (SD) slopes of symptom change across treatment were −1.1 (.84) and −1.36 (2.42), among participants completing TF-CBT and participants receiving at least 4 sessions, respectively.
Treatment Outcome and Pre-Treatment Neural Correlates of Threat Processing
Functional Activation
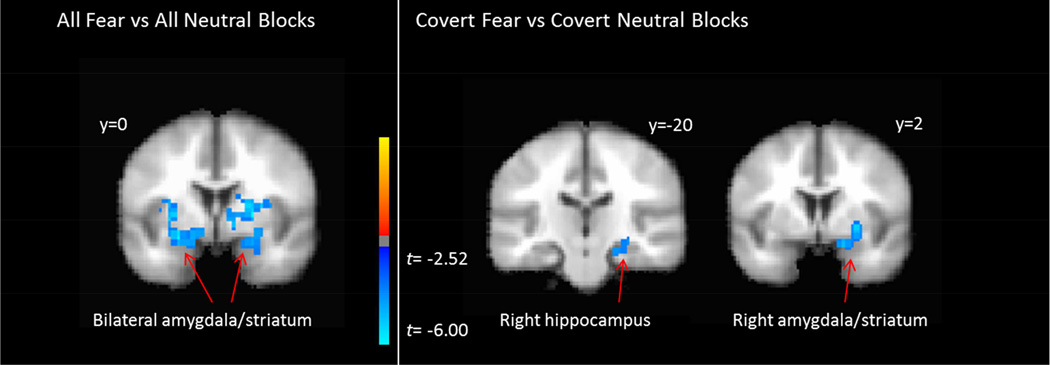
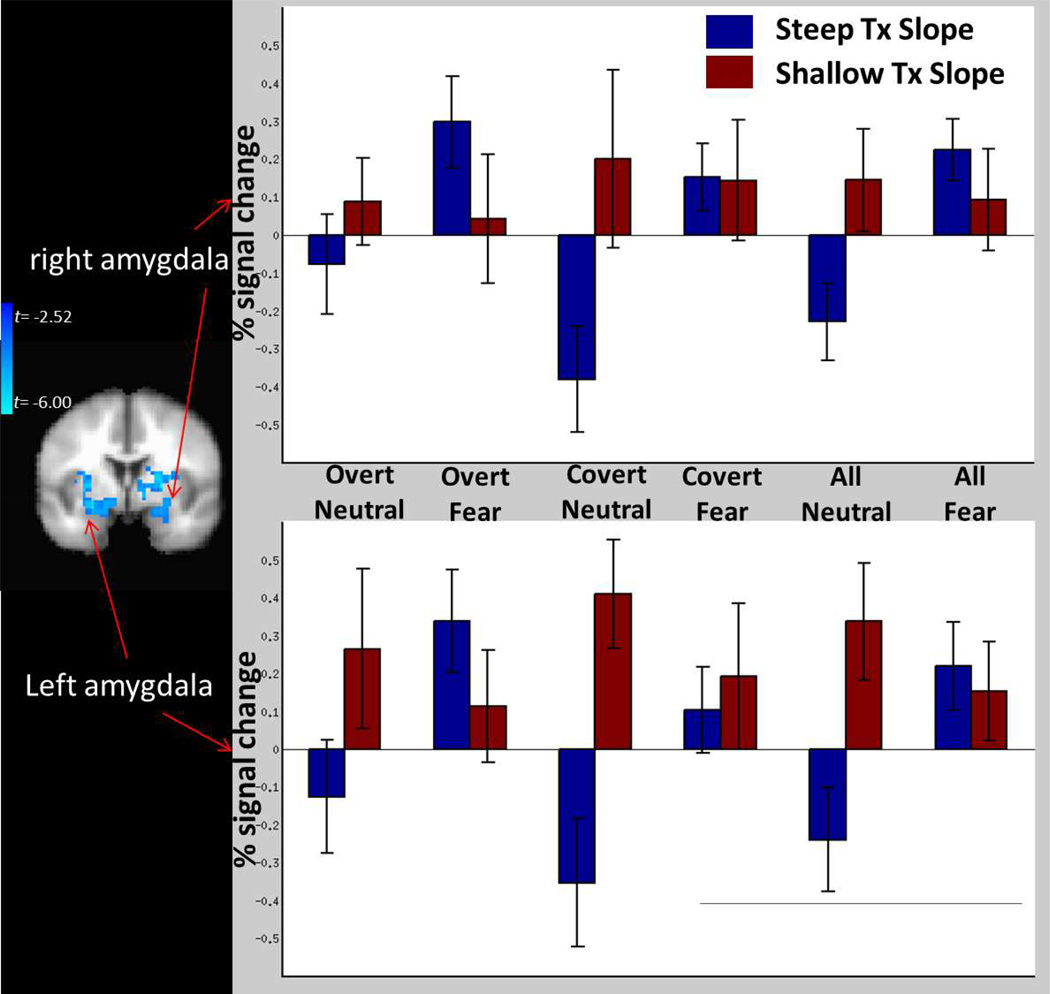
Full results of analyses of functional neuroactivation are depicted in Figure 1 and Table 2. We observed significant clusters of voxels in bilateral dorsal amygdala, extending dorsally into the striatum, where pre-treatment % signal change for all fear vs neutral blocks was significantly negatively related to slope of PTSD symptom change across treatment. For the contrast of covert fear vs neutral blocks, we similarly observed a significant cluster in the right amgydala where greater pre-treatment % signal change was significantly related to larger slope of PTSD symptom reduction during treatment. The contrast of overt fear vs neutral blocks failed to reveal any significant clusters. Figure 2 displays the mean % signal change for fear and neutral blocks across all stimulus presentation formats among those participants with steep and shallow PTSD symptom slope β coefficients based on a median split.
Figure 1.
Graphical depiction of significant clusters of voxels where activity for the contrasts of all fear vs neutral blocks (left) and covert fear vs neutral blocks (right) predicted slope of PTSD symptom trajectories. Negative relationships indicate that greater brain activity at pre-treatment is related to greater declines in PTSD symptoms across treatment.
Table 2.
Significant clusters of voxels where pre-treatment % signal change for contrast of interest is related to PTSD trajectory slopes during TF-CBT.
| X Y Z Center-of-Mass Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Neuroimaging Measure |
Contrast | Anatomical Label | X | Y | Z | Cluster size, voxels |
Peak t value |
| Functional activation | |||||||
| All Fear vs All Neutral |
|||||||
| Right amygdala/striatum | 23 | 2 | −3 | 193 | −9.23 | ||
| Left amygdala/striatum | −22 | −1 | −7 | 137 | −7.63 | ||
| Covert Fear vs | |||||||
| Right hippocampus | 22 | −32 | −5 | 94 | −4.34 | ||
| Right amygdala/striatum | 26 | 3 | −9 | 69 | −5.26 | ||
| Overt Fear vs Overt Neutral |
|||||||
| No significant clusters | |||||||
| Functional connectivity with right amygdala |
|||||||
| All Fear vs All | |||||||
| Dorsal anterior cingulate cortex |
12 | 16 | 32 | 51 | 4.77 | ||
| Covert Fear vs Covert Neutral |
|||||||
| No significant clusters | |||||||
| Overt Fear vs Overt Neutral |
|||||||
| Right mid-posterior insular cortex |
50 | −4 | 4 | 53 | 4.22 | ||
| Functional connectivity with left amygdala |
|||||||
| All Fear vs All Neutral |
|||||||
| Dorsal anterior cingulate cortex |
6 | 14 | 23 | 62 | 5.29 | ||
| Right posterior middle temporal gyrus |
48 | 58 | 9 | 60 | 7.64 | ||
| Covert Fear vs Covert Neutral |
|||||||
| No significant clusters | |||||||
| Overt Fear vs Overt Neutral |
|||||||
| Occipital lobe | −17 | −78 | −13 | 89 | −7.54 | ||
Figure 2.
Graphical depiction of activity for fear and neutral images separately as a function of shallow vs steep PTSD trajectory slopes across treatment and as a function of stimulus presentation formats, and scatter plots of scalar relationships between activity for the contrast of all fear vs neutral blocks and treatment slopes, for the right (top) and left (bottom) amygdala.
We conducted additional analysis to further investigate the specificity of the relationship between % signal change in the amygdala ROIs and symptom reduction across presentation format (covert vs overt) and facial expression (fear vs neutral). These analyses (Supplemental Material) demonstrated similar effects when examining the contrast of covert fear vs covert neutral blocks and overt fear vs overt neutral blocks, and that there was no interaction between presentation format and facial expression.
Finally, given that the amygdala clusters extend dorsally into the striatum, we tested the effect specifically in the bilateral amygdala by using independently selected coordinates for bilateral amygdala taken from our previous study among adult women with PTSD (Cisler et al. 2014b) and again found significant bilateral clusters of activity related to PTSD symptom slopes (Supplemental Material and Supplemental Figure 3).
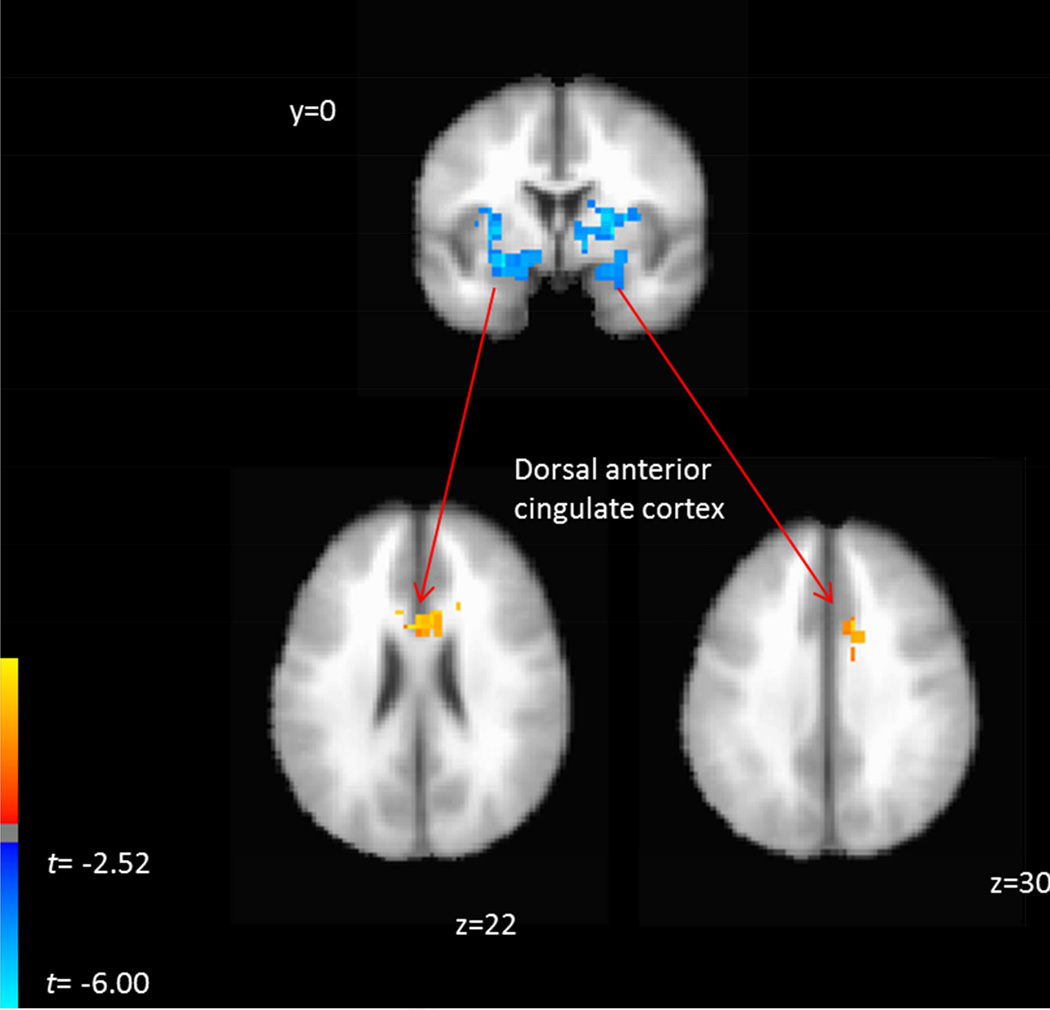
Functional Connectivity with Amygdala during Threat Processing and Treatment Outcome
Given the observed relationships between amygdala activation to fear vs neutral faces and slope of treatment-related PTSD symptom trajectory, we extended the brain-behavior relationship by testing for patterns of whole brain functional connectivity with left and right amygdala during fear vs neutral face blocks that similarly scaled with PTSD symptom trajectory slopes using the gPPI method (Table 2). For both the right and left amygdala, we observed an overlapping significant cluster in the dorsal anterior cingulate cortex (dACC) where greater functional connectivity with the amygdala during all fear vs neutral blocks was associated with shallower slopes of PTSD symptom trajectories (Figure 3). For the contrast of overt fear vs overt neutral blocks, we observe that heightened connectivity between right amygdala and right mid-posterior insular cortex was similarly associated with shallower slopes of PTSD symptom trajectories. For the left amygdala during this same contrast, we observed that greater connectivity with the visual cortex was associated with steeper slopes of symptom trajectory. For the contrast of covert fear vs covert neutral blocks, we did not observe any significant clusters.
Figure 3.
Graphical depiction of significant clusters of voxels where functional connectivity with the left and right amygdala predicted slope of PTSD symptom trajectories. Positive relationships indicate that greater functional connectivity with the seed region was related to shallower slopes of PTSD symptom decline across treatment.
To examine specificity of the dACC-amygdala connectivity with symptom reduction during fear and neutral blocks, we extracted the mean beta coefficients for the fear and neutral blocks separately for each participant in each significant dACC clusters and entered these as simultaneous predictors in the robust regression model (i.e., in place of the contrast variable). This analysis demonstrated, for both dACC clusters, opposing relationships of dACC-amygdala connectivity during fear and neutral blocks. During fear blocks, greater dACC-amygdala connectivity was related to shallower slopes of PTSD symptom trajectories (left amygdala-dACC: β =.13, t = 4.5, p < .001; right amygdala-dACC: β =.25, t = 3.90, p = .001). By contrast, during neutral blocks, greater dACC-amygdala connectivity was related to steeper slopes of PTSD trajectories (left amygdala-dACC: β = −.1, t = −2.6, p = .016; right amygdala-dACC: β = − .22, t −5.22, p < .001).
Convergent Validity for Bilateral Amygdala Response as Predictors of PTSD Trajectories
We extracted mean % signal change values within these two amygdala/striatum clusters for each individual and tested with robust regression whether these values were also significantly related to additional measures of treatment outcome (Supplemental Figures 3 and 4). For the right amygdala, pre-treatment % signal change for the general fear vs neutral contrast was significantly related to the pre- to post-treatment decrease in the DERS subscales of ‘difficulty engaging in goal-directed behavior during negative mood’ (β = 1.2; p = .002), but not significantly related to symptom slopes for depression (β = −.01; p = .99). For the left amygdala, % signal change for the general fear vs neutral contrast was also significantly related to the pre- to post-treatment decrease in the DERS subscales of ‘difficulty engaging in goal directed behavior during negative mood’ (β = 1.01; p = .02), ‘lacking strategies to regulate negative moods’ (β = 1.07; p = .02), but not significantly related to symptom slopes for depression (β = −.44; p = .67).
Addressing Effects of Partial Treatment Completion and Potential Pre-Treatment Confounding Factors
Pre-treatment bilateral amygdala activation to fear vs neutral faces were similarly significantly related to PTSD symptom trajectory slopes when including adolescents who received at least 4 TF-CBT sessions (n=28; Supplementary Figure 5).
When the primary analyses were repeated when including the possible pre-treatment confounding factors of age, verbal IQ, ethnicity, concurrent psychotropic medication (dichotomized into “yes” or “no”), total number of comorbid diagnoses, and assault severity (total number of assaultive event exposures), the observed relationships between pre-treatment left (all ps < .0013) and right amygdala (all ps < .0079) with subsequent PTSD symptom trajectory slopes remained significant. Similarly, the relationship between fear-modulated FC of the dACC with both the right (all ps < .001) and left (all ps < .001) amygdala remained significant when including these potentially confounding variables as covariates.
Aiding in interpretation of the amygdala functional activation findings, we observed opposing relationships between pre-treatment PTSD symptom severity and number of comorbid diagnoses with degree of bilateral amygdala activation: greater PTSD symptom severity was associated with greater fear vs neutral amygdala % signal change (ps < .01 for both left and right amygdala), whereas greater number of comorbid diagnoses (i.e., symptom complexity) was associated with less fear vs neutral amygdala % signal change (ps < .013 for both left and right amygdala).
DISCUSSION
The present data suggest that individual differences within neurocircuitry mediating emotion processing predict symptom reduction during TF-CBT among assaulted adolescent girls with PTSD. Most notably given its role in salience detection, emotion, and centrality to neurocircuitry models of PTSD (Admon, Milad, & Hendler 2013;Davis and Whalen 2001;Rauch, Shin, & Phelps 2006), we found that pre-treatment bilateral amygdala activation in response to fear vs neutral facial expressions was positively related to the course of subsequent PTSD symptom change; depressive symptom change was not predicted by reactivity in the amygdala ROI identified here. While the whole-brain analyses only revealed this relationship for the contrasts including all fear blocks and covert fear blocks, but not for overt fear blocks, the pattern of findings was similar across all stimulus presentation formats (Figure 2) and there was no interaction between stimulus presentation and facial expression. The amygdala ROIs extended dorsally into the bilateral striatum, which is also widely implicated in salience processing (Zink et al. 2003;Zink et al. 2004;Zink et al. 2006), though follow-up ROI analyses did demonstrate the effect in the amygdala specifically. Demonstrating convergent validity, we also observed that pre-treatment bilateral amygdala activation was related to pre- to post-treatment improvements in specific domains of self-rated emotion regulation. Further supporting the robustness of the relationships between amygdala function and PTSD symptom change, this association could not be attributed to pre-treatment PTSD symptom severity, age, IQ, ethnicity, comorbidity, severity of assaultive event exposures, or concurrent medication, and similarly remained significant when including adolescents who received ≥ 4 sessions of TF-CBT. Nonetheless, it should also be explicitly mentioned that the lack of a no-treatment control group precludes inferences about predicting symptom change specifically versus predicting naturalistic/spontaneous symptoms reductions. These caveats should temper the following discussion accordingly.
Interestingly, adolescents who did better in treatment had greater pre-treatment amygdala reactivity to threat stimuli relative to neutral stimuli, whereas adolescents who did not respond as well in treatment demonstrated increased activity to both threat and neutral faces. These results have implications for neurocircuitry models of PTSD and for understanding interindividual variability in response to TF-CBT. Neurocircuitry models of PTSD invariably posit heightened amygdala responding to threat vs neutral (Admon, Milad, & Hendler 2013;Rauch, Shin, & Phelps 2006). Here, we observe that those adolescents who fit this model of neuropathophysiology by demonstrating hyper-reactive amygdala responses also had more severe PTSD symptoms and responded better to a psychological treatment that targets overt behavioral representations consistent with the implicated neurocircuitry. Concurrently, we also observed that assaulted adolescents with PTSD who exhibit increased amygdala activity to both threat and neutral stimuli, suggesting poorer amygdala discrimination between threat and safety, benefited significantly less from the same TF-CBT and tended to have greater symptom complexity (i.e., more comorbid diagnoses). These data highlight individual differences in neurocircuitry mediating PTSD (e.g., amygdala hyperactivity to threat compared to neutral vs non-specific amygdala hyperactivity) and demonstrate their impact on symptom reduction during TF-CBT.
A possible hypothesis regarding poorer symptom change among adolescents with increased amygdala activity to both neutral and threat stimuli is that threat-safety discrimination is a necessary prerequisite for symptom change during TF-CBT. It may be the case that in order for adolescents with PTSD to effectively learn and enact coping skills and to engage and process the traumatic memory, the adolescents first need to be able to discriminate safe signals. Indeed, emerging research among adults with PTSD demonstrates deficient safety signal learning and less contextual modulation of fear extinction memory consolidation (Garfinkel et al. 2014;Jovanovic et al. 2012). Nonetheless, we also observed that amygdala safety-threat discrimination is individually varying in this young PTSD sample, and that these individual differences predicted symptom reduction during TF-CBT. We also observed that at least two variables contributing to the individual differences in neurocircuitry is degree of symptom complexity (number of comorbid diagnoses) and PTSD symptom severity, which were related to lesser and greater differential processing of the amygdala for fear and neutral stimuli, respectively.
The results of the functional connectivity analysis demonstrate that greater fear-related connectivity between the bilateral amygdala and dorsal anterior cingulate cortex (dACC) was associated with worse symptom change, whereas greater connectivity during neutral blocks was associated with better symptom reduction. Functional interpretation of the dACC-amygdala functional connectivity relationships with symptom reduction is not straightforward. On the one hand, the dACC is reported to be hyper-reactive in PTSD (Admon, Milad, & Hendler 2013;Patel et al. 2012) and generally implicated in threat appraisal (Etkin et al. 2011). On the other hand, the dACC also has been theorized to mediate the higher-order process of adaptive control (Shackman et al. 2011), which presumably subsumes more specialized functions to which the dACC is commonly linked (e.g., threat appraisal and conflict monitoring). Under this latter theory, the dACC’s role is to integrate incoming information, such as from the amygdala, in order to bias information processing elsewhere in the brain and respond to task demands. In line with this theory, it might be plausible that the differential predictive relationships between dACC-amgydala connectivity and symptom reduction during fear vs neutral blocks is indicative of ‘flexible’ information processing, such that greater adaptability of the dACC-amygdala circuit to environmental demands (i.e., fear vs neutral stimuli) is a marker of adolescents who are more likely to succeed in cognitive-behavioral therapy that involves changing thinking and behavioral patterns.
To our knowledge, this is the first study examining the neurocircuitry of PTSD as predictors of symptom reduction during treatment for pediatric PTSD, though the study is not without limitation. The current study was limited in power and generalizability by a relatively small sample size, restriction to girls with assault-related PTSD, concurrent psychotropic medication among 43% of the sample, and lack of a no-treatment control group. Interestingly, a previous study among adults with PTSD found that heightened amygdala reactivity to threat relative to neutral stimuli was associated with poorer CBT treatment outcomes (Bryant, Felmingham, Kemp, Das, Hughes, Peduto, & Williams 2008a). While this prior study had a smaller sample size (N=14), focused on a mixed-sex and mixed-trauma adult PTSD sample, and used a somewhat different treatment modality, the discrepant findings observed here among a homogeneous adolescent PTSD population and the previous study among adult PTSD highlights the need for replication and further research in this area. Future research along these lines is needed to elucidate more precisely the brain mechanisms of pediatric PTSD, their relationship with symptom change specifically, and to develop effective personalized treatments.
Supplementary Material
Flow chart of participant enrollment and attrition across the study.
Observed PTSD symptoms across each session for each participant completing treatment (n=23) (top); predicted PTSD symptoms at each session using a simple linear model Y=B0 +B1X, where X is a linear predictor for each session (middle); predicted PTSD symptoms at each session using Yt=B0 +B1X + B2Yt–1, where Yt–1 is the PTSD measure at the previous timepoint and is included to account for autocorrelation is the dependent measure.
Scalar relationships between right amygdala activity for all fear vs neutral faces and pre-post treatment changes in: difficulties engaging in goal directed behavior during negative mood (left), and depression symptoms (bottom).
Scalar relationships between left amygdala activity for fear vs neutral faces and pre-post treatment changes in: difficulties engaging in goal directed behavior during negative mood (top left), having strategies to regulate negative mood (top right), and depression symptoms (bottom).
Relationship between right (top) and left (bottom) amygdala activity and symptom change among those receiving ≥ 4 TF-CBT sessions.
Highlights.
Amygdala reactivity predicted symptom reduction during TF-CBT
Adolescent girls with greater symptom reduction had greater amygdala reactivity to threat versus neutral images
Adolescent girls with poorer symptom reduction had greater amygdala reactivity to both threat and neutral images
Acknowledgements
We thank Scott Steele, Jennifer Lenow, and Ariel Kingsley for help with this project.
Role of Funding
Portions of this work were supported through grants 1R21MH097784-01, 1R21MH106869, and 1R01DA036360-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute for Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no financial conflicts of interest.
Contributions
Ben Sigel and Teresa Kramer were involved in study design, interpretation, and manuscript writing. Karin Vanderzee and Joy Pemberton were data collection, interpretation, and manuscript writing. Sonet Smitherman was involved in study design, data collection, and analysis. Clint Kilts was involved in study design, interpretation, and manuscript writing. Josh Cisler was involved in study design, analysis, interpretation, and manuscript writing.
References
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17(7):337–347. doi: 10.1016/j.tics.2013.05.005. available from: PM:23768722. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Messer SC, Pickles A, Winder F, Silver D. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Method Psych. 1995;5:237–249. available from: <Go to ISI>://A1995TQ48000002. [Google Scholar]
- Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, Paulus MP, Stein MB. Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Res. 2013;214(1):48–55. doi: 10.1016/j.pscychresns.2013.05.001. available from: PM:23916537. [DOI] [PubMed] [Google Scholar]
- Bardeen JR, Fergus TA, Orcutt HK. An examination of the latent structure of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2012;34(3):382–392. [Google Scholar]
- Blakemore SJ. Development of the social brain in adolescence., 2012/03/22. 2012:111–116. doi: 10.1258/jrsm.2011.110221. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22434810. [DOI] [PMC free article] [PubMed]
- Brownell R. Receptive one-word picture vocabulary test. Novato, CA: Academic Therapy Publications; 2000. [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder., 2007/11/17. 2008a:555–561. doi: 10.1017/S0033291707002231. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18005496. [DOI] [PubMed]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study., 2007/05/26. 2008b:517–523. doi: 10.1002/hbm.20415. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17525984. [DOI] [PMC free article] [PubMed]
- Cary CE, McMillen JC. The data behind the dissemination: A systematic review of trauma-focused cognitive behavioral therapy for use with children and youth. Children and Youth Services Review. 2012;34(4):748–757. [Google Scholar]
- Cisler JM, Begle AM, Amstadter AB, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: data from the NSA-R., 2012/02/23. 2012:33–40. doi: 10.1002/jts.21672. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22354506. [DOI] [PMC free article] [PubMed]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014a;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. available from: PM:24055504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Lenow JK, Smitherman S, Everett B, Messias E, Kilts CD. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J Psychiatr Res. 2014b;48(1):47–55. doi: 10.1016/j.jpsychires.2013.09.013. available from: PM:24139810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Bukstein O, Walter H, Benson SR, Chrisman A, Farchione TR, Hamilton J, Keable H, Kinlan J, Schoettle U, Siegel M, Stock S, Medicus J. Practice parameter for the assessment and treatment of children and adolescents with posttraumatic stress disorder., 2010/04/23. 2010:414–430. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20410735. [PubMed]
- Cohen JA, Deblinger E, Mannarino AP, Steer RA. A multisite, randomized controlled trial for children with sexual abuse-related PTSD symptoms., 2004/06/10. 2004:393–402. doi: 10.1097/00004583-200404000-00005. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15187799. [DOI] [PMC free article] [PubMed]
- Cohen JA, Mannarino AP, Iyengar S. Community treatment of posttraumatic stress disorder for children exposed to intimate partner violence: a randomized controlled trial., 2011/01/05. 2011:16–21. doi: 10.1001/archpediatrics.2010.247. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21199975. [DOI] [PubMed]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility., 2012/08/21. 2012:636–650. doi: 10.1038/nrn3313. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22903221. [DOI] [PubMed]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion., 2001/03/13. 2001:13–34. doi: 10.1038/sj.mp.4000812. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11244481. [DOI] [PubMed]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr, Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI., 2010/09/11. 2010:1358–1361. doi: 10.1126/science.1194144. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20829489. [DOI] [PMC free article] [PubMed]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. available from: PM:21167765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Allen A, Felmingham KL, Williams LM, Bryant RA. Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(9):895–901. doi: 10.4088/JCP.12m08020. available from: PM:24107763. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder., 2010/02/10. 2010:241–247. doi: 10.1037/a0017551. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20141261. [DOI] [PubMed]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold., 1995/05/01. 1995:636–647. doi: 10.1002/mrm.1910330508. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7596267. [DOI] [PubMed]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34(40):13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. available from: PM:25274821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav. 2004;26:41–54. available from: <Go to ISI>://000187133200005. [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62(2):695–704. doi: 10.1016/j.neuropharm.2011.02.023. available from: PM:21377482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample., 2000/03/11. 2000:19–30. doi: 10.1037//0022-006x.68.1.19. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10710837. [DOI] [PubMed]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents., 2003/08/20. 2003:692–700. doi: 10.1037/0022-006x.71.4.692. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12924674. [DOI] [PubMed]
- Lenow JK, Scott SJ, Smitherman S, Kilts CD, Cisler JM. Attenuated behavioral and brain responses to trust violations among assaulted adolescent girls. Psychiatry Res. 2014;223(1):1–8. doi: 10.1016/j.pscychresns.2014.04.005. available from: PM:24811608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models., 2009/07/21. 2010:73–85. doi: 10.1016/j.bandc.2009.06.003. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19616355. [DOI] [PubMed]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches., 2012/04/10. 2012:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22484411. [DOI] [PMC free article] [PubMed]
- McLaughlin KA, Hatzenbuehler ML, Mennin DS, Nolen-Hoeksema S. Emotion dysregulation and adolescent psychopathology: A prospective study. Behav Res Ther. 2011;49:544–554. doi: 10.1016/j.brat.2011.06.003. available from: <Go to ISI>://000294531800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37(8):1135–1157. doi: 10.1016/j.psyneuen.2012.01.002. available from: PM:22281210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. available from: PM:22766141. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. available from: PM:19002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future., 2006/08/22. 2006:376–382. doi: 10.1016/j.biopsych.2006.06.004. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16919525. [DOI] [PubMed]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study., 2000/05/17. 2000:769–776. doi: 10.1016/s0006-3223(00)00828-3. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10812035. [DOI] [PubMed]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex., 2011/02/19. 2011:154–167. doi: 10.1038/nrn2994. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21331082. [DOI] [PMC free article] [PubMed]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID)., 2010/03/25. 2010:313–326. doi: 10.4088/JCP.09m05305whi. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20331933. [DOI] [PubMed]
- Steinberg AM, Brymer MJ, Decker KB, Pynoos RS. The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index., 2004/03/25. 2004:96–100. doi: 10.1007/s11920-004-0048-2. vailable from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15038911. [DOI] [PubMed]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. available from: <Go to ISI>://000236912100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression., 2005/05/03. 2005:99–113. doi: 10.1016/j.neuroimage.2005.01.011. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15862210. [DOI] [PubMed]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E. Amygdala-prefrontal dissociation of subliminal and supraliminal fear., 2005/11/11. 2006:652–661. doi: 10.1002/hbm.20208. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16281289. [DOI] [PMC free article] [PubMed]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29(3):977–983. doi: 10.1016/j.neuroimage.2005.08.006. available from: PM:16153860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. available from: PM:12954871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. available from: PM:15134646. [DOI] [PubMed] [Google Scholar]
- Bush K, Cisler JM. Decoding neural events from fMRI BOLD signal: A comparison of existing approaches and development of a new algorithm. Magnetic Resonance Imaging. 2013;31(6):976–989. doi: 10.1016/j.mri.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. available from: PM:24055504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution., 2003/06/05. 2003:200–207. doi: 10.1016/s1053-8119(03)00058-2. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12781739. [DOI] [PubMed]
- Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70(11):1181–1189. doi: 10.1001/jamapsychiatry.2013.2430. available from: PM:24173657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches., 2012/04/10. 2012:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22484411. [DOI] [PMC free article] [PubMed]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. available from: PM:23994314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of participant enrollment and attrition across the study.
Observed PTSD symptoms across each session for each participant completing treatment (n=23) (top); predicted PTSD symptoms at each session using a simple linear model Y=B0 +B1X, where X is a linear predictor for each session (middle); predicted PTSD symptoms at each session using Yt=B0 +B1X + B2Yt–1, where Yt–1 is the PTSD measure at the previous timepoint and is included to account for autocorrelation is the dependent measure.
Scalar relationships between right amygdala activity for all fear vs neutral faces and pre-post treatment changes in: difficulties engaging in goal directed behavior during negative mood (left), and depression symptoms (bottom).
Scalar relationships between left amygdala activity for fear vs neutral faces and pre-post treatment changes in: difficulties engaging in goal directed behavior during negative mood (top left), having strategies to regulate negative mood (top right), and depression symptoms (bottom).
Relationship between right (top) and left (bottom) amygdala activity and symptom change among those receiving ≥ 4 TF-CBT sessions.