Abstract
The complex carbohydrates accessible to the distal gut microbiota (DGM) are key drivers in determining the structure of this ecosystem. Typically, plant cell wall polysaccharides and recalcitrant starch (i.e. dietary fiber), in addition to host glycans are considered the primary nutrients for the DGM; however, we recently demonstrated that α-mannans, highly branched polysaccharides that decorate the surface of yeast, are also nutrients for several members of Bacteroides spp. This relationship suggests that the advent of yeast in contemporary food technologies and the colonization of the intestine by endogenous fungi have roles in microbiome structure and function. Here we discuss the process of yeast mannan metabolism, and the intersection between various sources of intestinal fungi and their roles in recognition by the host innate immune system.
Keywords: catabolism, carbohydrate active enzyme, distal gut microbiota, evolution, fungal cell wall, mannooligosaccharide, polysaccharide, symbiosis, yeast mannan
Background
Domesticated yeast has transformed the quality and festivity of mealtimes for thousands of years. Antiquated texts put the dawn of yeast applications in human food and drink production around 3,000–5,000 BC; some evidence however, suggests that they likely originated earlier in the Neolithic era.1 Recently, we discovered that yeast mannan, a cell wall carbohydrate of Saccharomyces cerevisae (i.e., baker's yeast; Fig. 1) and other fungi, is specifically metabolised by several members of the distal gut microbiota (DGM).2 The term mannan was derived from ‘manna’ (Hebrew: ℸη) – the flaky sweet substance that the Israelites consumed during their exodus in the desert (Numbers 11:1–9); similarly, yeast mannan today still clearly contributes to the nutrition of the DGM as the genes required for its degradation are still broadly represented in the gut metagenomes of healthy human subjects. Yeast mannan defines the outermost boundary between the fungus and its environment, and is involved in a number of physiological events related to fungal cell wall stability and remodeling.3 Fungal carbohydrates also define key elements for host immune recognition and defense (Fig. 1;4,5).
Figure 1.
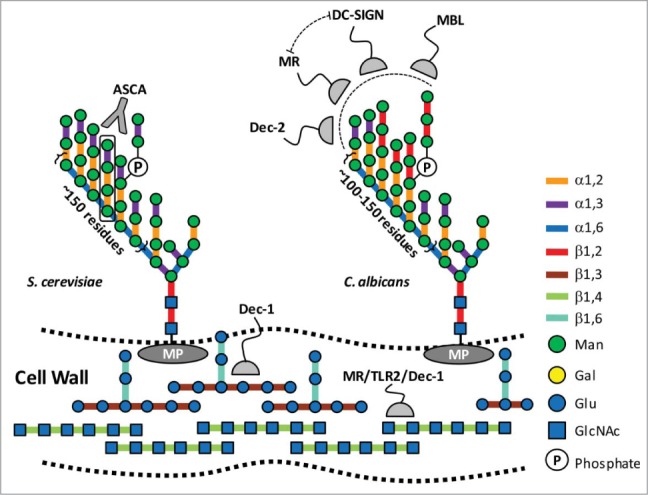
Structures of immunogenic carbohydrates within fungal cell walls. The composition of the cell wall of yeast and filamentous fungi varies between species; however, there are structural similarities. These include chitin (β-1,4-N-actetylglucosamine) and β-1,3(β-1,6)-glucans, which are core elements that provide tensile strength to the cell wall; and surface mannoproteins (MP). Yeast mannan is a highly branched complex carbohydrate an α-1,6 backbone, with α-1,2; and α-1,3-linked mannose sidechains. Some sidechains are further branched though phosphate ester bonds. C. albicans mannan has a conserved core structure with S. cerevisiae, but displays signature β-1,2 mannosyl residues at the termini of its sidechains. Numerous host immune responses to these polysaccharides have been documented,4,5 and several examples are displayed here. β-Glucans and chitin are important targets because they represent conserved elements across a spectrum of fungal species. Dectin-1 (Dec-1) is a C-type lectin receptor with high affinity for pure β-1,6 branched β-1,3 glucans.34 Immune responses to chitin are complex and yet to be fully elucidated, but they have been shown to involve macrophage mannose receptor, TLR2, and Dec-1.27 α-Mannans represent the outermost feature of the fungus, and have been linked to a variety of innate and adaptive responses. The mannotetraose (Man-α1,3→Man-α1,2→α-1,2→Man) sidechains of S. cerevisiae are antigens for ASCA, an antibody which is found in higher titres in individuals with CD (black box;32). Several proteins are known to interact with C. albicans mannans, including the C-type lectin receptor dectin-2 (Dec-2); mannose binding lectin (MBL);28 and MR29 and dendritic cell receptor (DC-SIGN),30 which have combinatorial effects.
The ‘sugar-coated’ surface of yeast
Yeast mannan is synthesized onto a GlcNAc2-Man8–9 acceptor, a highly conserved core oligosaccharide in eukaryotic glycoproteins, in the Golgi apparatus by a series of specific glycosyl transferases.6 The first steps involve the M-Pol I complex, which contains 2 family 62 glycosyl transferases Mnn9p and Van1p.7,8 Mnn9p catalyzes the first reaction and primes Man8GlcNAc2 with a single α-1,6 decoration, which is then extended into a highly conserved α-1,6-mannan backbone, comprising ∼200 Man units, by Van1p. This backbone is decorated by species-specific sidechains. For example, S. cerevisiae sidechains contain Man-α-1,3→Man-α-1,2→Man-α-1,2→Man with occasional phosphomannoligosaccharide branches; whereas the invasive pathogen Candida albicans displays Man-β-1,2 oligosaccharides of varying length that cap the α-linked Man side chains (Fig. 1). These terminal structures represent 2 of the growing list of signature carbohydrate structures that are being linked to fungal perception and host immunity.5
Orchestrated metabolism of yeast mannan by the DGM
The human genome contains only a handful of CAZymes known to be active on dietary carbohydrates, and the majority of those are involved in the digestion of α-glucans (e.g. isomaltooligosaccharides, starch) and sucrose within the upper alimentary canal.9 The vast bulk of dietary polysaccharides are impervious to human digestion and they transit to the colon where they are depolymerized and fermented into short chain fatty acids by the DGM, which are subsequently utilized by the host. One of the hallmark features of the DGM is the prevalence of bacteria such as Bacteroides spp whose genomes encode a large number of carbohydrate active enzymes (CAZymes) dedicated to the metabolism of dietary fiber.10 The colon is a highly competitive microbial ecosystem with diverse species competing for limited nutrients. In order to persist, intestinal residents occupy select niches or adapt successful metabolic strategies. One such strategy has been exploited by the Bacteroidetes, which has many members that operate as nutritional ‘generalists’.11,12 Generalists harness catabolic machinery targeting a wide variety of complex carbohydrates, the major nutrient available to the DGM. Thus the extensive repertoire of CAZymes enable the organisms of the DGM to respond to the variation in available dietary nutrients. To optimize efficiency and limit metabolic cost, Bacteroides spp. organize their carbohydrate metabolic pathways into Polysaccharide Utilization Loci (PULs).13 PULs are independently regulated functional units. Each targets a specific glycan. SusC/D-like proteins (named after the starch utilization system, the first characterized glycan degrading system in Bacteroides) function as outer-membrane bound protein complexes that recruit carbohydrates at the cell surface and facilitate transport in a predicted TonB activated process. Other features of PULs include regulatory proteins that sense the presence of a targeted substrate and activate expression of a pathway consisting of depolymerising enzymes, which release oligosaccharides and monosaccharides from polysaccharide substrates. Investigating the molecular basis of how CAZymes from Bacteroides spp dismantle structurally complex dietary carbohydrates has become a highly-successful strategy for enzyme discovery.
Similar to the metabolism of dietary glycans such as fructans,14 xylan15,16 and xyloglucan,17 B. theta contains PULs that are dedicated for yeast mannan metabolism; however, it is a more complex process and represents an exception to the ‘one PUL for one substrate’ paradigm.13 Three PULs (MAN-PUL1/2/3) are induced by yeast mannan, and a fourth PUL (HMNG-PUL) that targets structurally related high mannose N-glycans is induced by Man8GlcNAc2. Using a series of biochemical approaches and targeted gene disruption, we were able to define the function of the majority of genes products encoded within MAN-PUL1/2/3 (Table 1). These enzymes work interdependently to completely saccharify yeast mannan in a process that initiates on the cell surface and culminates with intracellular mannose. Intriguingly, MAN-PUL1 and MAN-PUL2 display a high level of synteny; however, only MAN-PUL2 is indispensable, suggesting that there is functional specialization that has co-evolved within these pathways. This is exemplified by the capacity of the mannan PULs to accommodate diverse sugars and/or linkages found in other fungal species, such as Candida albicans18 and Schizosaccharomyces pombe.19 These findings suggest that high-mannose containing complex carbohydrates are valuable nutrients for B. theta proliferation or it exposes an important role for the symbiotic turnover of fungal carbohydrates in the intestine; 2 roles that may not be mutually exclusive. Indeed, there is a strong selective pressure for yeast mannan degradation and its significance for host health may extend beyond nutrient acquisition. Thus, while MAN-PULs are activated in the mammalian host, deletion of these genetic loci confers a significant advantage over the wild type bacterium in diets lacking the yeast glycan, and this metabolic trait is very highly conserved in different strains of B. theta.
Table 1.
MAN-PUL1/2/3 gene products involved in yeast mannan metabolism
| Gene | Family | Location1 | Actvity2 | Gene | Family | Location1 | Actvity2 |
|---|---|---|---|---|---|---|---|
| PUL-Man1 | PUL-Man2 | ||||||
| BT2620 | GH97 | P | exo-α-galactosidase | BT3773 | GH92 | P | exo-α-1,3 mannosidase3 |
| BT2621 | ORF | C | unknown | BT3774 | GH38 | P | exo-α-mannosidase |
| BT2622 | GH67 | C | unknown | BT3775 | GT32 | C | α-1,3 glycosyl transferase |
| BT2623 | GH76 | E | endo-α-1,6 mannanase | BT3776 | GT32 | C | α-1,6 glycosyl transferase |
| BT2624 | ORF | E | unknown | BT3777 | ORF | P | unknown |
| BT2625 | SusD-like | E | transport | BT3778 | ORF | C | unknown |
| BT2626 | SusC-like | OM | transport | BT3779 | ORF | C | unknown |
| BT2627 | ORF | E | unknown | BT3780 | GH130 | P | unknown |
| BT2628 | HTCS | IM | regulator | BT3781 | GH125 | P | exo-α-1,6 mannosidase |
| BT2629 | GH92 | P | exo-α-1,3 mannosidase | BT3782 | GH76 | P | endo-α-1,6 mannanase |
| BT2630 | PTase | P | mannose-6-P phosphatase | BT3783 | PTase | P | mannose-6-P phosphatase |
| BT2631 | GH76 | P | endo-α-1,6 mannanase | BT3784 | GH92 | P | exo-α-1,2 mannosidase3 |
| BT2632 | GH125 | P | exo-α-1,6 mannosidase | BT3785 | ORF | C | unknown |
| PUL-Man3 | BT3786 | HTCS | IM | regulator | |||
| BT3853 | SARP/OmpR | IM | regulator | BT3787 | ORF | E | unknown |
| BT3854 | SusC-like | OM | transport | BT3788 | SusC-like | OM | transport |
| BT3855 | SusD-like | E | transport | BT3789 | SusD-like | E | transport |
| BT3856 | ORF | E | unknown | BT3790 | ORF | E | unknown |
| BT3857 | ORF | C | unknown | BT3791 | SGBP | E | unknown |
| BT3858 | GH92 | P | exo-α-1,3 mannosidase3 | BT3792 | GH76 | E | endo-α-1,6 mannanase |
| BT3859 | ORF | E | unknown | ||||
| BT3860 | ORF | E | SGBP | ||||
| BT3861 | ORF | E | SGBP | ||||
| BT3862 | GH99 | E | endo-α-1,2 mannosidase |
Mannan utilization is a selfish process
Depolymerisation of yeast mannan is metabolically expensive. The complex, highly branched substrate greatly restricts enzyme access and its degradation requires a large number of enzymes (Table 1). In the metabolism of other complex carbohydrates, some organisms simplify transport by centralizing depolymerisation outside of the cell. For example, the ‘cellulosome’ is a large multienzyme complex found within some members of Clostridia that often tethers an extensive repertoire of plant cell wall degrading glycanases on the cell surface (Fig. 2). Extracellular processing comes at cost, however, as simple sugars released from complex substrates by ‘keystone species’ become accessible to other local residents.20 In these scenarios, carbohydrate metabolism is a ‘shared’ process and individuals can occupy ecological niches along catabolic cascades by specializing in stratified levels of carbohydrate or metabolite utilization.16,21 The model of yeast mannan metabolism by B. theta represents a ‘selfish’ alternative to this theme as limited processing occurs outside of the cell (Fig. 2). Complex mannooligosaccharides are generated by first exposing and cleaving the α-1,6 mannan backbone at sparse sites and then shuttling these branched fragments across the outer membrane. Importantly, the extracellular enzymes that cleave the backbone operate at noticeably slower rates than their counterparts within the periplasm. This ensures that fragments are produced at a rate that will not saturate the transport equilibrium, which would result in loss of excess products to the environment.
Figure 2.
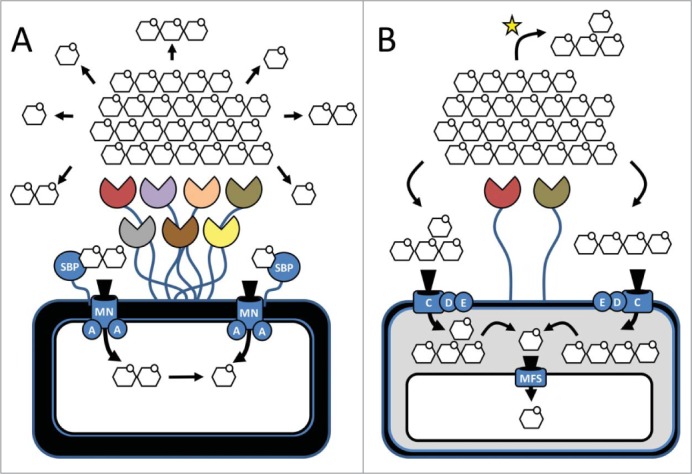
Strategies for extracellular digestion of complex carbohydrates. (A) The ‘sharing’-strategy: extracellular enzymes or enzyme complexes (e.g., Cellulosomes) process complex carbohydrate substrates into simple sugars and small oligosaccharides. These substrates can be readily utilized by any proximal bacterium. In Gram (+) bacteria products are bound by solute binding proteins (SBPs) and transported across the cell wall by ATP-dependent transporters MNA2. (B) The ‘selfish’-strategy: minimal extracellular processing of the substrate. Complex oligosaccharides are transported into the periplasm through the Sus-like transport system where the bulk of depolymerisation occurs. Monosaccharides enter the cytoplasm through a major facilitator superfamily (MFS) transporter. Variations of this pathway exist as come complex oligosaccharides released during extracellular processing may be selectively used by other bacteria in the ecosystem (indicated with a yellow star); however, they remain inaccessible to the majority of bacteria within proximity.
Co-culturing experiments with B. theta and Bacteroides xylanisolvens and Bacteroides cellulosilyticus, 2 species that cannot metabolize yeast mannan but can grow on mannose, support this selfish model.2 When provided with only yeast mannan as a carbon source, B. theta growth rates are unperturbed in co-culture whereas both of its relatives do not exhibit significant growth. This finding underpins that B. theta deploys a selfish mode of yeast mannan metabolism and prevents the superfluous release of mannose or mannooligosaccharides into the media to be shared with other species. Significantly, this relationship may be substrate specific, and one needs to be cautious before extrapolating whether B. theta is solely selfish in its metabolism, as a selective sharing relationship was recently demonstrated between Bacteroides ovatus and Bifidobacterium adolescentis16, and several members of Bacteroidales with differing growth aptitudes were able to liberate oligosaccharides (i.e. “public goods”) from multiple carbohydrate sources into solution for utilization by other species (21; Fig. 2). The tendency of PULs to concentrate complex oligosaccharide depolymerising enzymes within the periplasm,14,17,22,23 however, suggests that B. theta is primarily concerned with self-nourishment and symbioses with other species may be dependent on the chemistry or complexity of the substrate.
A coupled catabolic-biosynthetic cascade to reprofile exogenous mannose?
MAN-PUL2 contains the first characterized example of coupled mannan catabolism and mannooligosaccharide biosynthesis in nature. A putative biosynthetic cassette contains 2 intracellular family 32 glycosyl transferases (GT32s; BT3775 and BT3776;) that sequentially catalyze the synthesis of a branched α-mannose trisaccharide that is likely decorated further by other biosynthetic components encoded within MAN-PUL2 or inherent capsular polysaccharide synthesis pathways. To ensure that nascent α-Man3 products are not indiscriminately digested the catabolic and anabolic stages of mannose processing are compartmentalized into the periplasm and cytoplasm, respectively.
Although the downstream role of α-Man3 remains to be resolved, it is likely either a component of a storage molecule or a capsular polysaccharide. The typansomatid protozoan Leishmania spp. synthesizes an intracellular β-1,2 mannan that is harvested when glucose becomes limiting,24 and bacterial glycogen (i.e., α-1,4(α-1,6)-glucan) is a storage reserve in over 40 different bacterial species.25 Alternatively, α-Man3 may be incorporated into the capsule of B. theta. A previous study determined that disrupting Bt3775 or genes encoding the capsular polysaccharide 4 (CPS4) biosynthetic pathway removed a surface epitope recognized by 225.4, an IgA monoclonal antibody raised in a germ-free mouse colonized with B. theta.26 The potential interplay between yeast mannan metabolism, α-mannooligosaccharide synthesis, and capsular remodeling represents a unique response of B. theta to dietary yeast and warrants further investigation.
Fungal immune perception and B. theta
Chitin (β-1,4-N-acetylglucosamine) and β-1,3(β-1,6)-glucans are the primary structural polysaccharides of the fungal cell wall. These repetitive structures are not found within human glycans, and therefore, make ideal targets for the recognition of foreign symbionts or opportunistic pathogens (Fig. 1). In this regard, the perception of fungal polysaccharides has been linked to numerous innate and adaptive immune responses involving surveillance proteins, such as dectin-1 (Dec-1), mannose receptor (MR), and toll-like receptor 2 (TLR2).4,5,27 Yeast mannan on the other hand shares compositional and stereochemical similarity with human high mannose N-glycans; although, it is substantially larger and its sidechains are capped with Man-α-1,3-Man instead of Man-α-1,2-Man (Fig. 1). In addition, carbohydrate heterogeneity within fungal mannans, such as the β1,2 mannosyl caps that decorate the surface of the opportunistic pathogen Candida albicans, are structural signatures that can be detected by other immune sentinel proteins, such as the C-type lectin receptor dectin-2 (Dec-2); mannose binding lectin (MBL);28 and MR29 and dendritic cell receptor (DC-SIGN),30 a process which can involve combinatorial interactions (Fig. 1).
The sidechains of yeast mannan have been linked to perturbed immune responses in patients with Crohn's disease (CD), a debilitating inflammatory autoimmune disorder that presents in the small bowel and colon. Anti-S. cerevisiae antibodies (ASCA) preferentially recognize the sidechain structure of yeast mannan (Man-α-1,3→Man-α-1,2→Man-α-1,2→Man),31 and have been found in higher titres in the sera of CD patients. Intriguingly, the DGM of CD patients has a marked reduction in the abundance of B. theta in comparison with healthy individuals (∼35%).32 Additionally, higher levels of Bacteroides spp have been associated with CD patients in remission when compared to those that have relapsed.33 It is tempting to speculate that this relationship may result, at least in part, from a reduced ability of a compromised DGM (i.e. lacking B. theta) to tolerate and digest mannans from dietary or endogenous yeasts.
In culture B. theta PUL-MAN expression is high within its carbohydrate metabolism hierarchy, and these pathways are actively expressed in vivo,2 underpinning the importance of yeast mannan responsive enzymes. For example, BT3862 is an extracellular endo-α-1,2-mannosidase from MAN-PUL3 that prunes yeast mannan sidechains by specifically cleaving within the ASCA epitope and releasing Man-α-1,2→Man2 Exploiting this relationship may have therapeutic potential for alleviating ASCA-associated exacerbations and B. theta has received orphan drug status by the FDA for the treatment of pediatric CD. In this light, harnessing the mechanistic roles of naturally occurring members of the DGM for improving intestinal health and developing applications for offsetting dysbioses that result from improper regulation of DGM community structure is a promising research avenue for next generation ‘live-culture’ biologics.
Conclusion
The analysis of yeast mannan degradation by B. theta provides a model for the depolymerisation and utilization of complex sterically constrained carbohydrates by Bacteroides. The bacterium has evolved a surface enzyme system that is optimized to produce a large number of oligosaccharides for import into the periplasm where depolymerisation is completed. This mechanism minimises the energy used in the import process and the loss of nutrients to the environment, and thus represents a selective advantage for the bacterium. While this cellular deployment pattern of enzyme systems is conserved for other glycans, the discriminating factor between selfish and sharing metabolism appears to be substrate dependent. Extracellular digestion of ‘accessible’ glycans (i.e., sterically unconstrained) results in the release of oligosaccharides into the environment where they become available to other organisms in the DGM.16,21
Both the sources of yeast mannan molecules and the significance of their turnover by the DGM for host health may extend beyond nutrient acquisition. For example, recent efforts have been aimed at defining and enumerating the fungal members of the microbiota (“mycobiome”) during health and disease and it is possible that B. theta has evolved to utilize mannans from a variety of endogenous yeasts that colonize the gut. In addition, while cultural consumption of foods that are fermented with yeast is a uniquely human endeavor, yeasts are naturally present in a variety of foods such as ripe and spoiled fruits and may therefore have impacted microbiota evolution in other animals as well. Regardless of the source, it should be emphasized that there is apparently a strong selective pressure for yeast mannan degradation in B. theta. This evidence emanates from our observation that these 3 PULs are consistently activated in the mammalian host even in the absence of usable mannan, perhaps in response to an inducing signal derived from endogenous N-glycans or another source that mimics the presence of the yeast polysaccharide. Surprisingly, deletion of these loci in B. theta conveys a substantial competitive advantage over the wild type bacterium in diets lacking the yeast glycan, while wild-type bacteria compete best when bona fide mannan is present as a nutrient. Thus, while deployment of this ‘expensive’ metabolic trait decreases the fitness of B. theta, it has remained highly present in nearly all strains of this species that were analyzed.
Funding Statement
DWA is supported by the Beef and Cattle Research Council, AAFC Growing Forward 2 Grant (No. FDE.15.13). ECM is supported by awards from the NIH (No. DK084214, No. GM099513), the Global Probiotics Council Young Investigator Grant for Probiotics Research, and funds provided by the University of Michigan Biological Sciences Scholars Program. HJG is supported by grants from the European Research Council (No. 322820) and The Wellcome Trust (WT097907AIA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Patrick E, McGovern DLG, Exner LJ, Voigt MM. Neolithic resinated wine. Nature 1996; 381:480-1; http://dx.doi.org/ 10.1038/381480a0 [DOI] [Google Scholar]
- 2.Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al.. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015; 517:165-9; PMID:25567280; http://dx.doi.org/ 10.1038/nature13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays 2006; 28:799-808; PMID:16927300; http://dx.doi.org/ 10.1002/bies.20441 [DOI] [PubMed] [Google Scholar]
- 4.Barreto-Bergter E, Figueiredo RT. Fungal glycans and the innate immune recognition. Front Cell Infect Microbiol 2014; 4:145; PMID:25353009; http://dx.doi.org/ 10.3389/fcimb.2014.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog 2010; 6:e1000758; PMID:20421940; http://dx.doi.org/ 10.1371/journal.ppat.1000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science 2001; 291:2364-9; PMID:11269317; http://dx.doi.org/ 10.1126/science.291.5512.2364 [DOI] [PubMed] [Google Scholar]
- 7.Stolz J, Munro S. The components of the Saccharomyces cerevisiae mannosyltransferase complex M-Pol I have distinct functions in mannan synthesis. J Biol Chem 2002; 277:44801-8; PMID:12235155; http://dx.doi.org/ 10.1074/jbc.M208023200 [DOI] [PubMed] [Google Scholar]
- 8.Striebeck A, Robinson DA, Schuttelkopf AW, van Aalten DM. Yeast Mnn9 is both a priming glycosyltransferase and an allosteric activator of mannan biosynthesis. Open Biol 2013; 3:130022; PMID:24026536; http://dx.doi.org/ 10.1098/rsob.130022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 2013; 11:497-504; PMID:23748339; http://dx.doi.org/ 10.1038/nrmicro3050 [DOI] [PubMed] [Google Scholar]
- 10.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 2014; 42:D490-5; PMID:24270786; http://dx.doi.org/ 10.1093/nar/gkt1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, et al.. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 2011; 9:e1001221; PMID:22205877; http://dx.doi.org/ 10.1371/journal.pbio.1001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martens EC, Kelly AG, Tauzin AS, Brumer H. The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J Mol Biol 2014; 426:3851-65; PMID:25026064; http://dx.doi.org/ 10.1016/j.jmb.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem 2009; 284:24673-7; PMID:19553672; http://dx.doi.org/ 10.1074/jbc.R109.022848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 2010; 141:1241-52; PMID:20603004; http://dx.doi.org/ 10.1016/j.cell.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Chekan JR, Dodd D, Hong PY, Radlinski L, Revindran V, Nair SK, Mackie RI, Cann I. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc Natl Acad Sci U S A 2014; 111:E3708-17; PMID:25136124; http://dx.doi.org/ 10.1073/pnas.1406156111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Basle A, Morland C, Day AM, Zheng H, et al.. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun 2015; 6:7481; PMID:26112186; http://dx.doi.org/ 10.1038/ncomms8481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, Klinter S, Pudlo NA, Urs K, Koropatkin NM, et al.. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 2014; 506:498-502; PMID:24463512; http://dx.doi.org/ 10.1038/nature12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall RA, Gow NA. Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol 2013; 90:1147-61; PMID:24125554; http://dx.doi.org/ 10.1111/mmi.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osumi M, Sato M, Ishijima SA, Konomi M, Takagi T, Yaguchi H. Dynamics of cell wall formation in fission yeast, Schizosaccharomyces pombe. Fungal Genet Biol 1998; 24:178-206; PMID:9742201; http://dx.doi.org/ 10.1006/fgbi.1998.1067 [DOI] [PubMed] [Google Scholar]
- 20.Ze X, Le Mougen F, Duncan SH, Louis P, Flint HJ. Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 2013; 4:236-240; PMID:23549436; http://dx.doi.org/ 10.4161/gmic.23998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakoff-Nahoum S, Coyne MJ, Comstock LE. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol 2014; 24:40-49; PMID:24332541; http://dx.doi.org/ 10.1016/j.cub.2013.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renzi F, Manfredi P, Mally M, Moes S, Jeno P, Cornelis GR. The N-glycan glycoprotein deglycosylation complex (Gpd) from Capnocytophaga canimorsus deglycosylates human IgG. PLoS Pathog 2011; 7:e1002118; PMID:21738475; http://dx.doi.org/ 10.1371/journal.ppat.1002118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nihira T, Suzuki E, Kitaoka M, Nishimoto M, Ohtsubo K, Nakai H. Discovery of beta-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase involved in the metabolism of N-glycans. J Biol Chem 2013; 288:27366-74; PMID:23943617; http://dx.doi.org/ 10.1074/jbc.M113.469080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ralton JE, Naderer T, Piraino HL, Bashtannyk TA, Callaghan JM, McConville MJ. Evidence that intracellular beta1-2 mannan is a virulence factor in Leishmania parasites. J Biol Chem 2003; 278:40757-63; PMID:12902334; http://dx.doi.org/ 10.1074/jbc.M307660200 [DOI] [PubMed] [Google Scholar]
- 25.Preiss AAIAJ. Bacterial glycogen and plant starch biosynthesis. Biochem Edu 2010; 20:196-203 [Google Scholar]
- 26.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2007; 2:328-39; PMID:18005754; http://dx.doi.org/ 10.1016/j.chom.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 27.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol 2009; 182:3573-82; PMID:19265136; http://dx.doi.org/ 10.4049/jimmunol.0802113 [DOI] [PubMed] [Google Scholar]
- 28.Brummer E, Stevens DA. Collectins and fungal pathogens: roles of surfactant proteins and mannose binding lectin in host resistance. Med Mycol 2010; 48:16-28; PMID:19639514; http://dx.doi.org/ 10.3109/13693780903117473 [DOI] [PubMed] [Google Scholar]
- 29.Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, Kullberg BJ, Torensma R, Williams DL, Figdor CG. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem 2008; 283:20590-9; PMID:18482990; http://dx.doi.org/ 10.1074/jbc.M709334200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cambi A, Gijzen K, de Vries l J, Torensma R, Joosten B, Adema GJ, Netea MG, Kullberg BJ, Romani L, Figdor CG. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 2003; 33:532-8; PMID:12645952; http://dx.doi.org/ 10.1002/immu.200310029 [DOI] [PubMed] [Google Scholar]
- 31.Sendid B, Colombel JF, Jacquinot PM, Faille C, Fruit J, Cortot A, Lucidarme D, Camus D, Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol 1996; 3:219-26; PMID:8991640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007; 104:13780-5; PMID:17699621; http://dx.doi.org/ 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter De Cruz SK, Wagner J, Buckley M, Sim WH, Prideaux L, Lockett T, McSweeney C, Morrison M, Kirkwood CD, Kamm MA. Association between specific mucosa-associated microbiota in Crohn's disease at the time of resection and subsequent disease recurrence: A pilot study. J Gastroenterol Hepatol 2014; 30:268-78; PMID:25087692; http://dx.doi.org/ 10.1111/jgh.12694 [DOI] [PubMed] [Google Scholar]
- 34.Adams EL, Rice PJ, Graves B, Ensley HE, Yu H, Brown GD, Gordon S, Monteiro MA, Papp-Szabo E, Lowman DW, et al.. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J Pharmacol Exp Ther 2008; 325:115-23; PMID:18171906; http://dx.doi.org/ 10.1124/jpet.107.133124 [DOI] [PubMed] [Google Scholar]
- 35.O Rahman SPC, Harrington DJ, Sutcliffe IC. Methods for the bioinformatic identification of bacterial lipoproteins encoded in the genomes of Gram-positive bacteria. World J Microbiol Biotechnol 2008; 24:2377-82; PMID:15539077; http://dx.doi.org/ 10.1007/s11274-008-9795-215539077 [DOI] [Google Scholar]
- 36.Zhu Y, Suits MD, Thompson AJ, Chavan S, Dinev Z, Dumon C, Smith N, Moremen KW, Xiang Y, Siriwardena A, et al.. Mechanistic insights into a Ca2+-dependent family of alpha-mannosidases in a human gut symbiont. Nat Chem Biol 2010; 6:125-32; PMID:20081828; http://dx.doi.org/ 10.1038/nchembio.278 [DOI] [PMC free article] [PubMed] [Google Scholar]


