Abstract
This investigation assessed the potential of isolating novel probiotics from mothers and their infants. A subset of 21 isolates among 126 unique bacteria from breast milk and infant stools from 15 mother-infant pairs were examined for simulated GI transit survival, adherence to Caco-2 cells, bacteriocin production, and lack of antibiotic resistance. Of the 21 selected isolates a Lactobacillus crispatus isolate and 3 Lactobacillus gasseri isolates demonstrated good profiles of in vitro GI transit tolerance and Caco-2 cell adherence. Bacteriocin production was observed only by L. gasseri and Enterococcus faecalis isolates. Antibiotic resistance was widespread, although not universal, among isolates from infants. Highly similar isolates (≥ 97% similarity by barcode match) of Bifidobacterium longum subsp. infantis (1 match), Lactobacillus fermentum (2 matches), Lactobacillus gasseri (6 matches), and Enterococcus faecalis (1 match) were isolated from 5 infant–mother pairs. Antibiotic resistance profiles between these isolate matches were similar, except in one case where the L. gasseri isolate from the infant exhibited resistance to erythromycin and tetracycline, not observed in matching mother isolate. In a second case, L. gasseri isolates differed in resistance to ampicillin, chloramphenicol and vancomycin between the mother and infant. In this study, gram positive bacteria isolated from mothers' breast milk as well as their infants exhibited diversity in GI transit survival and acid inhibition of pathogens, but demonstrated limited ability to produce bacteriocins. Mothers and their infants offer the potential for identification of probiotics; however, even in the early stages of development, healthy infants contain isolates with antibiotic resistance.
Keywords: antibiotic resistance, bacterial, breast milk, fecal, infant, microbiota, probiotics
Abbreviations
- GI
gastrointestinal
- MRS
deMan, Rogosa, and Sharpe.
Introduction
The human microbiome is a vast system of microbial communities that inhabit various niches within the human body and play an essential role in human physiology and health. Constituents of the GI microbiota help establish mucosal barrier function and absorb nutrients , produce metabolites, contribute to xenobiotic metabolism, support the immune system and prevent pathogen colonization (reviewed in1,2).
Colonization of the infant gut is thought to begin during birth as the infant is exposed to the mother's vaginal and fecal microbiota.3 It is further modulated by exposure to colostrum and breast milk,4,5 by the introduction of formula and other foods, and by exposure to skin6 and environmental microbes.7 This early development plays a critical role in gut barrier formation and immune system maturation.8 Alterations in this process may affect the risk of disease in later life.9,10
Commensal constituents of the GI microbiota have been investigated as probiotics, live microbes that can be beneficial to the health when administered in adequate amounts. Early research focused on gastrointestinal health, such as reducing the duration of rotavirus-associated acute infantile diarrhea,11 mitigating the risk of antibiotic-associated diarrhea in children, 12 and alleviating symptoms of irritable bowel syndrome.13-16 More recently, evidence has emerged for therapeutic potential in a wider range of conditions, such as mitigating the risks of eczema,17,18 atopic dermatitis,19 obesity and metabolic syndrome,20,21 and female urogenital infections.22-24
It has further been suggested that probiotics should not contain antibiotic resistance35 as the establishment of the GI microbiota during infancy plays a critical role in long-term health.25,26 The microbiota from mothers and their infants, especially those organisms transmitted from mother to child, are likely a repository of potential probiotics. This study reports on the characteristics of unique bacterial isolates from breast milk and infant fecal microbiota sampled from 15 mother-infant pairs and screened for potential properties that may impact their use as probiotics, such as antibiotic resistance, resistance to gastric acids, bacteriocin production, and adherence to intestinal epithelial cells (Caco-2). These are common tools utilized in the assessment of probiotic technologies.
Results
Identification of unique bacterial isolates from mother-infant pairs
A total of 60 breast milk samples and 30 infant fecal samples were obtained from 15 healthy mothers and their breast-fed infants over the course of 2 consecutive days. Isolation of bacterial colonies on selective agar was followed by 16S sequencing to identify genus and species, and Barcode genome typing to identify distinctive isolates. An isolate was declared distinct if its genome barcode banding pattern matched no other isolate or any ATCC strains from the Diversilab strain typing database. A “match” was called when there was approximately 97% or greater similarity between 2 isolates in genome barcode typing (Fig. 1). The breast milk and fecal samples yielded 126 distinct bacterial isolates representing the genera Bifidobacterium, Lactobacillus, Corynebacterium, Propionibactrium, Rothia, Enterococcus, Staphylococcus, and Streptococcus (Table 1).
Figure 1.
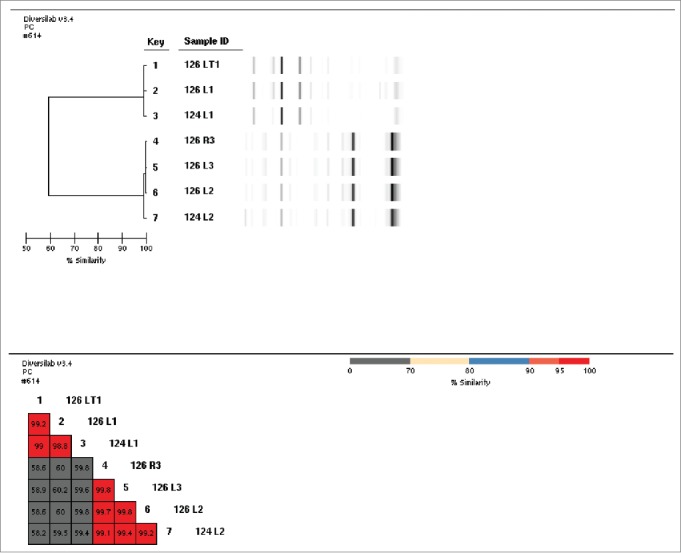
Example of bacterial barcodes and isolate matching for Lactobacillus isolates from infant-mother pairs. Isolate IDs labeled with 126 are from infant stool and those labeled 124 are from the mother's breast milk.
Table 1.
Unique bacterial isolates by genus and species isolated from breast milk and infant stool samples obtained from 15 mothers and their infants aged 1, 2 or 3 months (n= 5 per infant age group)
| Bacterial genus and species | Source | No of unique isolates | Comments |
|---|---|---|---|
| Bifidobacterium boum | Fecal | 2 | |
| Milk | 2 | From same individual | |
| Bifidobacterium breve | Fecal | 5 | From same infant (age 1 month) |
| Bifidobacterium longum | Fecal | 5 | From 3 infants (ages 1 and 2 months) |
| Bifidobacterium longum subsp. infantis* | Fecal | 6 | From 4 infants (ages 1, 2, 3 months). One isolate match with mother. |
| Milk | 1 | Matched that of her 3-month old infant | |
| Bifidobacterium longum subsp longum | Fecal | 2 | |
| Bifidobacterium scardovii | Fecal | 2 | |
| Lactobacillus crispatus | Fecal | 2 | From same infant |
| Lactobacillus fermentum | Fecal | 1 | Matched isolate from mother |
| Lactobacillus gasseri | Fecal | 10 | From 5 infants |
| Milk | 8 | From 4 mothers. One isolate from each of 3 mothers matched a isolate from her infant | |
| Lactobacillus paracasei | Fecal | 2 | From same infant |
| Lactobacillus rhamnosus | Fecal | 10 | From 4 infants |
| Enterococcus casseliflavus | Fecal | 3 | From same infant |
| Milk | 1 | ||
| Enteroccocus faecalis | Fecal | 9 | One isolate matched in mother and infant |
| Milk | 2 | From same individual. One match in mother and infant | |
| Corynebacterium variabile | Milk | 1 | |
| Proprionibacterium acnes | Fecal | 1 | |
| Milk | 3 | From 2 individuals | |
| Propionibacterium avidum | Fecal | 1 | |
| Milk | 1 | ||
| Rothia mucilaginosa | Milk | 1 | |
| Staphylococcus epidermidis | Milk | 10 | 4 from a single mother |
| Streptococcus gordoniI | Milk | 1 | |
| Streptococcus mitis | Milk | 14 | |
| Streptococcus oralis | Milk | 1 | |
| Streptococcus parasanguinis | Milk | 4 | From 3 individuals |
| Streptococcus salivarius | Milk | 13 | From 9 individuals |
| Streptococcus vestibularis | Milk | 2 | |
| Total number of unique isolates | 126 |
aBold shaded rows denote mother-infant isolate matches among isolates.
bIsolated from separate individuals unless otherwise noted. C:\broker\TFJ-US\KGMI\KGMI_A_1103425\KGMI_A_1103425\KGMI_A_1103425.
In five mother-infant pairs (A-E), one or more distinct isolates were conserved between both breast milk and fecal samples, indicating transmission between mother and baby (Table 2). Four of these 5 mother-infant pairs (A, C, D, and E) exhibited matching isolates of Lactobacillus gasseri. In two of the 5 mother-infant pairs, isolate matches were isolated on consecutive days of sampling, although those from each day were distinct. Pair A yielded distinct isolates of both L. gasseri and L. fermentum each day; pair C yielded a distinct isolate -match of L. gasseri isolates at each sampling. Matching isolates of Lactobacillus fermentum, Bifidobacterium breve, and Enterococcus faecalis were also represented among the mother-infant pairs. These mother-infant pair isolates were distinct and none were found in common between nonrelated mother-infant pairs.
Isolate matches between mother-infant pairs identified among 126 unique isolates derived from breast milk and infant stool samples collected from 15 mother-infant pairs on 2 consecutive days
| Mother- Infant Pair | Colony ID | Subject ID | Source | Isolate | Barcode Match (%) | Ampicillin | Chloramphenicol | Clindamycin | Erythromycin | Gentamicin | Streptomycin | Tetracycline | Vancomycin | Quinupri/Dalfopri |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIRST SAMPLING DAY | ||||||||||||||
| E | 396 R1 | 306 | Fecal | Lactobacillus gasseri | 97.7 | 0.19 | 8* | 16* | 2* | 64* | 16 | 6* | 4* | 4 |
| 361 L1 | 3066 | Milk | Lactobacillus gasseri | 0.032 | 4 | 48* | 0.75 | 24* | 16 | 1 | 2* | 1 | ||
| SECOND SAMPLING DAY | ||||||||||||||
| A | 291 L1 | 102 | Fecal | Lactobacillus fermentum | 98.5 | 0.125 | 6* | 0.125 | 0.5 | 6 | 96* | 4 | n.r. (>256) | 0.38 |
| 289 LT2 | 1022 | Milk | Lactobacillus fermentum | 0.19 | 8* | 0.194 | 0.5 | 8 | 128* | 4 | n.r. (>256) | 0.5 | ||
| 291 R3 | 102 | Fecal | Lactobacillus gasseri | 98.2 | 0.19 | 6* | 4* | 0.19 | 6 | 12 | 0.75 | 1 | 0.25 | |
| 289 L1 | 1022 | Milk | Lactobacillus gasseri | 0.19 | 6* | 6* | 0.19 | 8 | 12 | 0.75 | 2 | 0.5 | ||
| C | 312 LT4 | 204 | Fecal | Lactobacillus gasseri | 97.6 | 0.25 | 4 | 3* | 0.125 | 12 | 8 | 1 | 2 | 0.5 |
| 310 L1 | 2044 | Milk | Lactobacillus gasseri | 0.38 | 6* | 3* | 0.25 | 8 | 8 | 2 | 4* | 1 |
Asterisk indicates isolate has a MIC higher than the breakpoint for the antibiotic listed and is therefore considered resistant according to EFSA guidelines. n.r. indicates that the antibiotic test is not required due to intrinsic resistance and no breakpoints are listed in EFSA guidelines.
Table 2.
Isolate matches and antibiotic resistance patterns between mother-infant pairs identified among 126 unique isolates derived from breast milk and infant stool samples collected from 15 mother-infant pairs on 2 consecutive days
| Mother-Infant Pair |
Colony ID |
Subject ID |
Source |
Isolate |
Barcode Match (%) |
Ampicillin |
Chloramphenicol |
Clindamycin |
Erythromycin |
Gentamicin |
Streptomycin |
Tetracycline |
Vancomycin |
Quinupri/Dalfopri |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIRST SAMPLING DAY | ||||||||||||||
| A | 126 L2 | 102 | Fecal | Lactobacillus fermentum | 99.2 | 0.94 | 4 | 0.064 | 0.5 | 4 | 64 | 6 | n.r. (>256) | 0.38 |
| 124 L2 | 1022 | Milk | Lactobacillus fermentum | 0.94 | 3 | 0.064 | 0.5 | 6 | 96* | 4 | n.r. (>256) | 0.038 | ||
| 126 LT1 | 102 | Fecal | Lactobacillus gasseri | 98.8 | 0.19 | 8 | 12* | 0.25 | 8 | 12 | 1 | 1.5 | 0.25 | |
| 124 L1 | 1022 | Milk | Lactobacillus gasseri | 0.19 | 12* | 48* | 0.38 | 16 | 12 | 4 | 2 | 0.5 | ||
| B | 141 R4 | 202 | Fecal | Enterococcus faecalis | 99.1 | 0.25 | >256* | >256* | >256* | >256* | >256* | 16* | 2 | 4 |
| 139 R2 | 2022 | Milk | Enterococcus faecalis | 0.19 | 8 | >256* | 96* | 128* | 256* | 16* | 2 | 8* | ||
| C | 147 L2 | 204 | Fecal | Lactobacillus gasseri | 98.1 | >256* | 8* | 2* | 0.38 | 6 | 12 | 1.5 | 2 | 0.38 |
| 145 L1 | 2044 | Milk | Lactobacillus gasseri | >256* | 16* | 4* | 0.19 | 12 | 8 | 3 | 3* | 0.5 | ||
| D | 159 B1 | 303 | Fecal | Bifidobacterium longum infantis (breve) | 97.4 | 0.5 | 0.5 | 0.125 | 0.094 | 8 | 24 | 0.38 | 0.38 | 0.125 |
| 157 B1 | 3033 | Milk | Bifidobacterium longum infantis (breve) | 0.64 | 0.019 | 0.032 | 0.019 | 6 | 8 | 0.19 | 0.38 | 0.125 | ||
| 159 L1 | 303 | Fecal | Lactobacillus gasseri | 98.3 | 0.19 | 4 | 12* | 0.19 | 8 | 12 | 1 | 1 | 2 | |
| 157 L1 | 3033 | Milk | Lactobacillus gasseri | >256* | 8* | 4* | 0.19 | 8 | 12 | 1 | 3* | 2 | ||
Antimicrobial susceptibility of isolates
One hundred and 14 (114) distinct isolates displayed acceptable growth in liquid medium and were further characterized for susceptibility to 9 antimicrobials (Table 2). Of 55 isolates from infant stools, 71% (n=39) exhibited resistance to one or more antibiotics based on the minimum inhibitory concentration breakpoints (MIC) established by the European Food Safety Association (Table 2). The Enterococcus faecalis isolates all displayed resistance to one or more antibiotics. For example, 2 E. faecalis isolates from a 1-month old infant were resistant to chloramphenicol, clindamycin, erythromycin, gentamicin, and tetracycline and a third isolate from the same infant was resistant to these antibiotics as well as to vancomycin and quinupri/dalfopri. Most Lactobacillus isolates from infant stools were resistant to one to 3 antibiotics tested. By contrast, bifidobacteria isolates from infants had comparatively lower frequencies of antibiotic resistance: unique isolates of Bifidobacterium breve collected from the same 1-month old infant were resistant to tetracycline; 2 unique isolates of Bifidobacterium longum subsp infantis (breve) from a 2-month old infant were resistant to erythromycin; 3 other unique isolates of bifidobacteria collected from individual infants were susceptible to all antibiotics tested.
Breast milk also yielded isolates of E. faecalis, Lactobacillus species, and Bifidobacterium. Fifty-seven (57) of the 59 unique bacterial strains from breast milk characterized were resistant to one or more of the antibiotics assessed. Distinctive isolates of bifidobacteria (B. boum) from breast milk were resistant to tetracycline; other distinct bacterial isolates found in breast milk were resistant to between 3 and 7 antibiotics. Novel isolates of commensal skin bacteria, such as Propionibacterium species, staphylococci and streptococci, were more numerous in the breast milk samples
GI transit tolerance in simulated bile, gastric juice, and intestinal juice
The potential for a subset of 13 Lactobacillus and Enterococcus isolates with minimal antibiotic resistance were evaluated for the capacity to survive GI tract conditions by in vitro susceptibility to 0.3% bile, simulated gastric juice (saline containing pepsin at pH 2) and simulated intestinal juice (saline containing bile and pancreatin at pH 8) (Table 3). None of the isolates tested were resistant to both simulated gastric juice and simulated intestinal juice. The most tolerant of simulated in vitro GI conditions were isolates 330 L1 (L. crispatus), 297 R1 (L. fermentum), and 289 LT2 (a mother-infant isolate match of L. fermentum). These isolates survived low pH conditions for about 30 minutes and were resistant to more alkaline simulated intestinal conditions for approximately 2 hours (Table 3). In general, the L. rhamnosus isolates survived intestinal conditions comparatively well, but were rapidly inactivated by low pH. Among them, isolate 321 L3 (L. rhamnosus) had the best survival profile. By contrast, L. gasseri isolates tolerated gastric acid comparatively well, but grew poorly in 0.3 % bile and did not survive in vitro simulated intestinal conditions. Enterococcus isolates were comparatively tolerant of bile and intestinal conditions, but did not survive low pH.
Table 3.
Survival of selected unique bacterial isolate from infant stool and breast milk in simulated bile, gastric juice and intestinal juicea
| Simulated bile | Simulated gastric juice | Simulated intestinal juice | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD 600 |
% survival |
% survival |
|||||||||||||
| Isolate/ID | Source | 5 hr | 10 hr | 24 hr | Overall | 0 | 0.5 hr | 1 hr | 2 hr | Overall | 0 | 1 hr | 2 hr | 4 hr | Overall |
| L. crispatus 330 L1 | Fecal | 0.2 | 0.6 | 1.2 | ++ | 100 | 92 | 0.8 | 0 | + | 100 | 70 | 40 | 10 | + |
| L. fermentum 289 LT2 | Isolate match | 0.3 | 0.65 | 0.9 | ++ | 100 | 103 | 0 | 0 | + | 100 | 95 | 95 | 34 | ++ |
| L. fermentum 297 R1 | Fecal | 0.2 | 0.4 | 0.6 | + | 100 | 187 | 25 | 0 | + | 100 | 78 | 106 | 86 | ++ |
| L. gasseri 163 R1 | Milk | 0.2 | 0.3 | 0.3 | + | 100 | 109 | 93 | 27 | ++ | 100 | 55 | 27 | 2.3 | + |
| L. gasseri 147 LT2 | Fecal | 0 | 0 | 0.1 | — | 100 | 101 | 40 | 30 | ++ | 100 | 32 | 1 | 0.3 | — |
| L. gasseri 291 R3 | Isolate match | 0 | 0 | 0.3 | — | 100 | 99 | 81 | 17 | ++ | 100 | 7 | 2 | 0.2 | — |
| L. gasseri 312 LT4 | Isolate match | 0.06 | 0.15 | 0.05 | — | 100 | 78 | 67 | 11 | ++ | 100 | 2 | 1 | 0.4 | — |
| L. gasseri 163 L1 | Milk | 0.15 | 0.3 | 0.35 | + | 100 | 100 | 0 | 0 | + | 100 | 60 | 35 | 3 | + |
| L. rhamnosus 141 L1 | Fecal | 0.1 | 0.6 | 1.1 | + | 100 | 0.2 | 0 | 0 | — | 100 | 80 | 74 | 63 | + |
| L. rhamnosus 156 L1 | Fecal | 0.1 | 0.2 | 0.7 | + | 100 | 6 | 0 | 0 | — | 100 | 180 | 120 | 140 | ++ |
| L. rhamnosus 153 LT1 | Fecal | 0.05 | 0.1 | 0.8 | + | 100 | 0.2 | 0 | 0 | — | 100 | 120 | 95 | 40 | ++ |
| L. rhamnosus 321 L3 | Fecal | 0.06 | 0.1 | 0.65 | + | 100 | 75 | 0 | 0 | + | 100 | 105 | 70 | 100 | ++ |
| E. casseliflavus 153 R3 | Fecal | 0.5 | 0.3 | 0.2 | + | 100 | 0.1 | 0.1 | 0 | — | 100 | 54 | 74 | 40 | ++ |
| E. casseliflavus 153 R4 | Fecal | 0.09 | 0.35 | 0.3 | + | 100 | 0 | 0 | 0 | — | 100 | 135 | 90 | 85 | ++ |
| E. faecalis 141 R4 | Isolate match | 0.3 | 0.5 | 0.8 | ++ | 100 | 1 | 0 | 0 | — | 100 | 117 | 100 | 97 | ++ |
Simulated bile: MRS + 0.3% oxgall; simulated gastric juice: NaCl + and pepsin (pH 2); simulated intestinal juice: NaCl + oxgall + pancreatin (pH 8)
Adherence to Caco-2 intestinal cells
Twenty one isolates of bifidobacteria, lactobacilli and enterococci were tested for adherence to Caco-2 intestinal epithelial cells relative to a reference strain from the species (Table 4). Bifidobacteria showed low adherence relative to standard (20-60%). Adherence of E. casseliflavus isolates exceeded the standard and E. faecalis 141 R4 reached 80% of standard (data not shown). Lactobacillus adherence results are shown in Figure 2. Adherence of isolates 330 L1 (L. crispatus) and 153 LT1 (L. rhamnosus) were comparable to the standard. Adherence of Lactobacillus gasseri isolates 312 LT4 and 147 LT2 exceeded the standard.
Comparative analysis of isolates screened for potential probiotic properties
| Isolate | ID | Source | GI transit profile | Bacteriocin activity | Caco-2 adherence |
|---|---|---|---|---|---|
| L. rhamnosus | 141 L1 | Fecal | Does not survive acid, tolerates bile, intestinal conditions | Against Lactobacillus | Lower than standard |
| L. rhamnosus | 156 L1 | Fecal | Does not survive acid, tolerates bile, intestinal conditions | None | Lower than standard |
| L. rhamnosus | 153 LT1 | Fecal | Does not survive acid, tolerates bile, intestinal conditions | None | Comparable to standard |
| L. rhamnosus | 321 L3 | Fecal | Does not survive acid, tolerates bile, intestinal conditions | None | Lower than standard |
| E. casseliflavus | 153 R3 | Fecal | Does not tolerate acid, survives bile and intestinal conditions | None | Greater than standard |
| E. casseliflavus | 153 R4 | Fecal | Does not tolerate acid, survives bile and intestinal conditions | None | Greater than standard |
| E. faecalis | 141 R4 | Isolate match | Does not tolerate acid, survives bile and intestinal conditions | Against Vancomycin resistant enterococci (VRE) | Lower than standard |
Pathogens tested: Listeria monocytogenes, E. coli 0157:H7, MRSA (Methicillin Resistant Staphylococcus aureus, Salmonella Typhimurium, Shigella boydii, Streptococcus mutans and Streptococcus pyogenes.
Table 4.
Comparative analysis of isolates screened for potential probiotic properties
| Isolate | ID | Source | GI transit profile | Antimicrobial activity | Caco-2 adherence |
|---|---|---|---|---|---|
| Bifidobacterium boum (breve) | 396 B1 | Fecal | Not tested | None | Lower than standard |
| Bifidobacterium longum subsp infantis (breve) | 159 B1 | Isolate match | Not tested | None | Poor |
| L. crispatus | 330 L1 | Fecal | Limited acid tolerance, good tolerance of bile and intestinal conditions | Acid - Against | |
| Listeria, E. coli | Greater than standard | ||||
| L. fermentum | 297 R1 | Fecal | Limited acid tolerance, good tolerance of bile and intestinal conditions | Acid | Not tested |
| L. fermentum [NOT IN LIST OF UNIQUE ISOLATES] | 289 LT2 | Isolate match | Limited acid tolerance, good tolerance of bile and intestinal conditions | Acid | Not tested |
| L. gasseri | 147 LT2 | Isolate match | Tolerates acid but not bile and intestinal pH | Acid Against MRSA, Listeria, E. coli, Salmonella | Greater than standard |
| L. gasseri | 291 R3 | Isolate match | Tolerates acid but not bile and intestinal pH | Acid Against L. delbrueckii, | |
| E. Coli | Lower than standard | ||||
| L. gasseri | 312 LT4 | Fecal | Tolerates acid but not bile and intestinal pH | Acid Against MRSA, Listeria, E. coli, Salmonella | Greater than standard |
| L. gasseri | 163 L1 | Milk | Limited acid tolerance, fair survival in bile and intestinal pH | Acid Against E. coli, Salmonella | Lower than standard |
| L. gasseri | 163 R1 | Milk | Acid tolerance, fair survival in bile and intestinal pH | None | Lower than standard |
Figure 2.
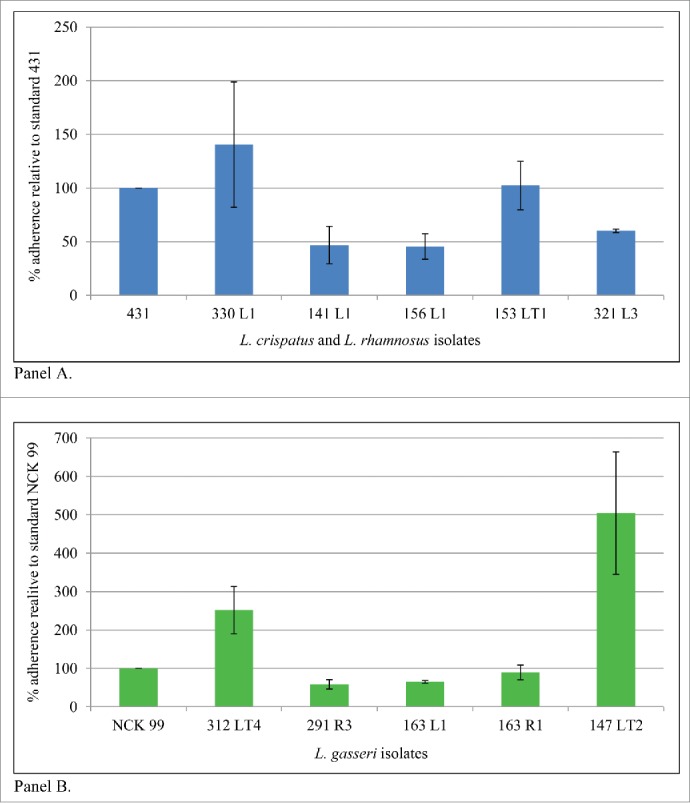
Caco-2 adherence of selected isolates of L. crispatus and L. rhamnosus (Panel A) and L. gasseri (Panel B) from infant stool and breast milk from infant-mother pairs. Reference strains: L. rhamnosus NCK 431 and L. gasseri NCK 99.
Bacteriocin production
Antimicrobial and bacteriocin activity was assessed on a subset of 21 isolates against E.coli 0157:H7 (human isolate), Shigella boydii, Salmonella Typhimurium, Campylobacter jejuni, Listeria monocytogenes 184 and 187, Streptococcus mutans, Streptococcus pyogenes, Vancomycin-Resistant Enterococcus faecalis (VRE) and Methicillin Resistant S. aureus (MRSA) by the direct spot overlay assay, using proteases, sodium hydroxide, and catalase to distinguish possible inhibitory mechanisms (production of a protein/peptide bacteriocin, acid, or hydrogen peroxide) (Table 4). The majority of the isolates tested showed significant inhibition due to acid production (Fig. 3). Protease- sensitive bacteriocin production was found in only 2 strains: L. gasseri 291 R3 (against L. delbruecki) and E. faecalis 141 R4 (against VRE enterococcus) (Fig. 3, Panels A & D). None of the Lactobacillus isolates demonstrated confirmed bacteriocin activity against Listeria monocytogenes, E. coli, Salmonella Typhimurium and MRSA the common pathogens used to assess bacteriocin production in probiotics. Bifidobacteria and L. fermentum isolates did not exhibit antimicrobial activity against indicator pathogens (data not shown); however, in the future it would be important to expand the list of pathogens to include a more comprehensive list those associated with pediatric infections. L. gasseri isolates 291 R3 and 312 LT4 as well as L. crispatus 330 L1 exhibited acid-based inhibition against multiple pathogens (shown in Figure 3 , Panel C, for E. coli 0157:H7).
Figure 3.
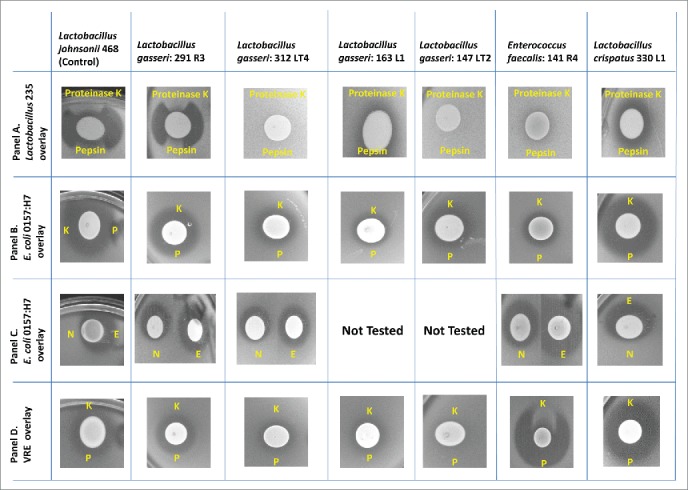
Bacteriocin screening. Only those isolates, pathogens and indicator combinations for which a zone of inhibition was observed are shown. All other combinations of isolate and pathogens did not result in a zone of inhibition in this spot-overlay detection method. Panels A & B: L. gasseri 291R3 produces a protease (Proteinase K (K) and Pepsin (P)) sensitive bacteriocin against the indicator L. delbeueckii 235, as indicated in Panel A. However, 291 R3's zone of inhibition against E. coli 0157:H7 is NOT sensitive to proteases. (Panel B). Further investigation was required to rule out acid production or an unusual bacteriocin structure. Panel C: Treatments: N- 10 N NaOH (3 μL) E- catalase (3 μL 10 mg/mL) Placing catalase in the zones had NO EFFECT; this ruled out hydrogen peroxide antimicrobial activity. But, placing 3 μL of 10 N NaOH inside the Zones of Inhibition (ZOI), diminished the antimicrobial activity by neutralizing the acid. Therefore, we conclude that the antimicrobial ZOIs against E. coli 0157:H7 are due to acid production. Panel D: The only isolate that produces a bacteriocin against a pathogen is E. faecalis 141 R4. This isolate produces a bacteriocin against Vancomycin Resistant Enterococcus (VRE) and is sensitive to Proteinase K, but not Pepsin (P).
Comparative assessment
Of the unique isolates evaluated, isolates 330 L1 from infant stool (L. crispatus) and breast milk isolate 163 L1 (L. gasseri) showed the greatest range of desirable probiotic properties (Table 4). Isolate 330 L1 exhibited some acid tolerance, relatively good survival under simulated intestinal conditions, greater adherence to Caco-2 cells than the standard, and demonstrated acid inhibition against potential pathogens such as Listeria, and E. coli 0157:H7. Isolate 163 L1 displayed limited acid tolerance and fair survival under simulated intestinal conditions; its adherence level was lower than that of the standard. Isolates 147 LT2 (a mother-infant isolate match) and fecal isolate 312 LT4, both L. gasseri isolates, exhibited strong adherence to Caco-2 cells. Generally, L. gasseri isolates exhibited relatively poor survival under simulated in vitro intestinal conditions.
Discussion
Establishment of the GI tract microbiota in the infant is thought to be critical to developing homeostasis and maintaining health and breast milk is recognized as the single most important postnatal factor in microbiome, metabolome, and immunological programming.27 In this investigation, we isolated and characterized unique bacterial isolates present in the fecal stools from breast-fed infants aged 1, 2 and 3 months, and in breast milk from their mothers. As the intestinal microbiota is likely to be a rich source of probiotics, we hypothesized that microbes found in the stool of young infants, especially if transferred from mother to child, might ultimately prove beneficial for screening as potential probiotic cultures. Isolates from breast milk and infant stools were screened for antibiotic resistance and subsets were evaluated in vitro for simulated GI transit survival, adherence to Caco-2 cells, and bacteriocin production, all of which are characteristics that are important aspects of any potential probiotic.
Lactobacilli, bifidobacteria, and enterococci were the most common genera found among the unique isolates in the stools of breast-fed infants. This is consistent with the fact that lactobacilli, enterobacteria, and coliforms are the first microbes to colonize the infant gut after birth,28 and that breast milk, which contains significant amounts of lactic acid bacteria and bifidobacteria, is a source of commensal bacteria to the infant gut.29,30 In this investigation, B. longum, B. infantis and B. breve, were the most common bifidobacteria in infant feces.31,32 L. gasseri and L. rhamnosus, common intestinal biota, were significantly represented among unique isolates from infants. In addition to bifidobacteria, lactobacilli, and enterococci, commensal skin bacteria such as propionibacteria, staphylococci, and streptococci were significantly represented among isolates from breast milk.
Five mother-infant pairs shared matching isolates (≥ 97% homology between bacterial barcodes). These matches included one isolate of Bifidobacterium longum subsp infantis (breve), 2 isolates of L. fermentum, 6 isolates of L. gasseri, and one isolate of E. faecalis. Twenty one (21) isolates, including some that matched between mother-infant pairs, were assessed in vitro for tolerance to GI conditions and evaluated for Caco-2 adherence, and bacteriocin production. Isolate 330 L1, a L. crispatus from infant stool, had the most promising profile with respect to all 3 properties; notably, it demonstrated acid inhibition against the food-borne pathogens Listeria and E. coli 0157:H7. Three L. gasseri isolates also were of potential interest. Isolate 163 L1 from breast milk significantly inhibited Salmonella and E. coli, but its adherence was not as strong as the reference strain. Isolate 147 LT2, an infant-mother isolate match, and isolate 312 LT4, from infant stool, both were strongly adherent to Caco-2 cells but did not survive simulated intestinal conditions. Interestingly, L. crispatus and L. gasseri isolates were well represented in a study of bacteriocin producing isolates from intestinal microbiota of 266 Irish people over age 65, but those reportedly were inactive against Listeria monocytogenes, Listeria innocua, or S. aureus.33
Isolates of interest may also exhibit other desirable properties such as immunostimulating effects. Strains of B. infantis, for example, have been demonstrated to promote intestinal health. 34 Isolate 157 B1 (a mother-infant isolate match of B. longum subsp infantis) and fecal isolates 297 R1 and 289 LT2 (L. fermentum isolates that displayed good intestinal transit tolerance) could be examined for beneficial metabolic activity (e.g. β-galactosidase cleavage to assist lactose digestion, bile acid cleavage to reduce cholesterol synthesis) or for immunological effects (e.g., dendritic cell activation).
Surprisingly, a significant proportion of isolates from infant stools were resistant to one or more than 9 clinically relevant antibiotics, as characterized by MIC values that exceeded the breakpoints recommended by EFSA.35 This is not likely to have resulted from postnatal exposure to antibiotics. The greatest degree of multidrug resistance was displayed by isolate 396 R1, a L. gasseri from a 3-month old infant, which was resistant to chloramphenicol, clindamycin, erythromycin, gentamycin, tetracycline, and vancomycin. Among isolates that displayed potential probiotic properties, L. crispatus isolate 330 L1, from a 3-month old infant, was resistant to clindamycin and tetracycline; L. gasseri isolate 147 LT2, an infant-mother isolate match, was resistant to chloramphenicol and clindamycin; L. gasseri 312 LT4, from a 2-month old infant, was resistant to clindamycin and displayed MICs at the breakpoints for chloramphenicol and vancomycin; and isolate 163 L1, an L. gasseri isolate from breast milk, was resistant to vancomycin. Isolate 159 B1, the B. infantis longum isolate-match from a mother and her 3-month old infant, was sensitive to all 9 antibiotics evaluated.
Other investigators have found high levels of antibiotic resistance in infant fecal microbiota.36 In a study of Greek infants, enterococci exhibited high frequencies of resistance to rifampicin, tetracycline, erythromycin and vancomycin, and multi-resistant strains were prevalent.37 Among fecal lactobacilli isolated from breast- and formula-fed infants, high frequencies of resistance to vancomycin were observed;38 investigators have found vancomycin resistance to be intrinsic to some species of lactobacilli, notably L. rhamnosus.39,40
Parents' intestinal and skin microbiota are a likely reservoir of antibiotic resistance. Microbiota from the mother's gastrointestinal tract are transmitted to the newborn, which raises the possibility that infants may be colonized by antibiotic-resistant bacteria in early infancy. For example, transfer of bifidobacteria from mother to infant has been confirmed at the strain level in pre-delivery stool samples from the mother and in infant feces from 0 (meconium) through 90 days after birth, a time frame that overlaps the age range of infants in our study.3 In the evaluation of bifidobacteria transfer alluded to above, 11 strains from 8 mother-infant pairs were monophyletic for mothers' and infants' feces and 2 monophyletic strains were transferred from mother to infant in breast milk. Besides transfer from the mother's milk and GI tract, transfer of skin microbiota from parents to the infant gut also occurs early in life. A study of 50 Swedish families found that the presence of S. aureus in infant stool was highly correlated with S. aureus carriage on parental skin, and 90% of S. aureus in the stool of 3-day-old infants were identical to parental skin strains.6
An interesting finding was that matching isolates in 2 mother infant pairs exhibited different antibiotic resistance profiles. In one instance, an isolate of L. gasseri from a 3-month old infant exhibited tetracycline and erythromycin resistance not present in the matching isolate from its mother. Other investigators have identified tetracycline resistance in the gut microbiota of a single, healthy mother-infant pair in which the infant was exclusively breast-fed for 5 months.41 This resistance was encoded by different genes and microorganisms in mother and infant; moreover, the microbiota from the child yielded a novel composite transposon bearing both tetracycline and erythromycin resistance genes, suggesting horizontal transfer via a mobile genetic element and raising the possibility of gene transfer among distantly related bacteria co-inhabiting the GI tract of the same individual.41 An initial safety screen of potential probiotic candidates may exclude isolates that harbor transferable resistance genes as well as excluding isolates with putative virulence factors.
In summary, this study identified 126 unique bacterial isolates among mother-infant pairs; 10 isolate matches were collected from 5 of the pairs. Twenty-one isolates were further characterized for putative probiotic properties. A Lactobacillus crispatus isolate and 3 Lactobacillus gasseri isolates demonstrated desirable profiles of comparatively good in vitro GI tolerance conditions and intestinal cell adherence. Some isolates produced large inhibitory zones against pathogens but those zones were largely due to acid production which has been shown to inhibit pathogen growth.42 A broader assessment of health modulating properties may uncover additional isolates which may be of interest. Antibiotic resistance was common, though not universal, among isolates from infants and was unlikely to have resulted from environmental exposures of the bacteria prior to colonization of the infant. Further work is necessary to fully characterize the probiotic potential of these unique gram positive bacteria from mother-infant pairs.
Methods
Sample collection and ethics statement
This was single-center study with 15 young, healthy breast-fed infants and their mothers performed over the course of 3 successive days. The objectives were to identify and characterize novel bacterial isolates from breast milk and infant stool in terms of potential probiotic properties and to screen for antibiotic resistance.
The study population comprised 3 groups of mother-infant pairs (n=5 pairs per group) that included infants of 1 month, 2 month, and 3 months of age, respectively. Mothers and infants were in good health (self-reported). Subjects were excluded if the mother or infant had a gastrointestinal disorder or had taken antibiotics in the previous 14 days; if the infant had been ill in the previous 4 days; or if the mother consumed yogurt with active cultures or took oral probiotics. After enrollment, self-obtained breast milk samples (4 oz. from each breast) and fecal samples from the first diaper change of the day were collected on the next 2 successive days. Infant subjects were identified by a 3-digit code, with the first digit corresponding to the infant's age in months. Mothers were identified by 4-digit code in which the first 3 digits were identical to the identification code of her infant. Samples were placed in labeled collection bags and stored at -80ºC prior to analysis. The study was approved by an Investigational Review Board and performed in compliance with the US Code of Federal regulations on Good Clinical Practices (21 CFR 10.90, 50, 56 and 812) and the World Medical Association Declaration of Helsinki (1996 amendment). All participants signed informed consent prior to study enrollment.
Bacterial isolate collection
Samples collected were shipped on dry ice to North Carolina State University (NCSU, TRK where isolation and initial identification of bacteria was completed. Isolation agar media used included MRS (deMan, Rogosa, and Sharpe) - code R; LBS (Lactobacillus selective medium) - code L; LBS + Tomato Juice (Lactobacillus selective medium plus tomato juice) – code LT; and BSM (Bifidobacterium selective medium: MRS + 0.05% cysteine + 0.005% murpirocin) - code B. For identification, morphologically unique colonies were chosen from the agar media, re-streaked for isolation, grown in broth and viewed under a phase contrast microscope. Colonies were identified by a sample number, the agar used in isolation (R, L, LT, or B), and the number of colonies isolated on a specific agar plate (1-7 maximum). As an example, isolate 141 L1, derived from an infant fecal sample, grew on LBS agar and was the first colony chosen on that plate. Glycerin stocks were made from isolates and stored frozen at -80oC for subsequent analyses.
Colonies from the isolation step were further identified by sequencing of the 16S rRNA gene from a 500 bp amplicon using 16S primers.43 The sequence obtained was then taken through the BLAST database for identification. Most identification was to the genus level. For corroboration, further identification of glycerin stocks was performed in Cincinnati (KK and DC) by16S 500 bp MicroSeq PCR/Sequencing kits (Life Technologies, #4370489 and #4346480) on a 3130xl Genetic Analyzer (Life Technologies, Carlsbad, CA).
Genotyping of isolates
All isolates were genotyped for the purpose of comparison, and to move forward with testing distinct isolates only. For isolates recovered from both the mothers' breast milk and the infants' fecal samples, the DiversiLab system (bioMérieux, Durham NC) was utilized to identify similarity indices for matches between mother-infant pairs. Isolates were grown on the appropriate agar media (MRS or RC Reinforced Clostridial) and DNA extracted using the Mo-Bio UltraClean Microbial DNA Isolation Kit Protocol (MoBio #12224-250, Carlsbad CA). DNA was quantified using the Nanodrop 1000 spectrophotometer (Thermo-Scientific, Waltham, MA) and normalized to 25 ng/μL using1x Tris-EDTA. Master Mix from the Diversilab Kit (bioMérieux, #410963: Bifidobacterium, #410982 Lactobacillus, # 410969 Enterococcus) was added to a 96-well plate (23 µl per well) followed by 2 µl of the normalized DNA (25 ng/μl). The plate was sealed and placed onto a 9700 fast thermal cycler (Life Technologies, Carlsbad, CA) with the appropriate thermal cycling program according to the kit used. The PCR product was then added to the DiversiLab system chip along with the Diversilab DNA reagents and supplies (bioMérieux, # 270670) according to kit protocol. The chip was analyzed using the Diversilab software version 3.4 and highly similar matches were called if ≥ 97% homology was seen between the barcodes of 2 or more isolates. If no barcode match to any other isolate was found, the isolate was declared distinct and potentially unique. A subset of distinct isolates progressed to antimicrobial susceptibility testing, in vitro assessment of GI transit tolerance, bacteriocin production and adherence to Caco-2 cells.
Antimicrobial susceptibility testing
The minimum inhibitory concentration (MIC) in mg/mL for distinct isolates was determined quantitatively using the E-test strips with a predefined antibiotic gradient (bioMérieux, Marcy-l’Étoile, France). Antibiotic strips tested include Ampicillin (bioMérieux # 501558), Chloramphenicol (bioMérieux #507558), Clindamycin (bioMérieux #509558), Erythromycin (bioMérieux #510558) Gentamicin (bioMérieux #512558), Streptomycin (bioMérieux #526848), Tetracycline (bioMérieux #522558), Vancomycin (bioMérieux #525558), and Quinupri/Dalfopri (bioMérieux #528758). Antimicrobial strip concentrations ranged from 0.016 to 256 μg/ml for all antibiotics except Streptomycin which had a range of 0.064 to1024 μg/ml and Quinupri/Dalfopri which had a range of 0.002 to 32 μg/ml.
Distinct isolates from mothers and infants were grown from glycerin stocks anaerobically or aerobically on MRSA, RCA, or TSA (Tryptic Soy Agar) plates for 48-72 hours, depending on the isolate. Cultures were adjusted to 1 × 108 CFU/mL in diluent. For the agar test plates, a 90% Iso-Sensitest broth (Thermo Scientific, #CM0473B), 10% (MRSB, RCB or TSB), 0.3g/L-cysteine (Sigma-Aldrich, #30089 + 15g Bacto Agar (BD, #214030) medium was used. Sterile, Polyester tipped swabs (Puritan #25-806 1PD, Guilford, ME) were dipped into the bacterial isolate solution and streaked on an entire agar plate with a fresh tip 3 times, rotating the plate 60° each time and allowing excess moisture to absorb into the agar plate before applying the E-test strips. The E-test strips were placed onto the agar plate with sterile forceps and bubbles removed by gently pushing on the strip with the forceps. Inverted plates were incubated anaerobically or aerobically, depending on the isolate, at 35+2°C until a distinct lawn of organisms appeared, typically 24-48 hours. The MIC was read where the zone of inhibition merged with the strip and compared to EFSA guidelines for resistance cut-off values according to genus.35
In vitro assays for tolerance to bile and simulated gastric and intestinal juice
The in vitro methodology developed by Charteris et al. was used, which mimics conditions encountered during in vivo human upper gastrointestinal transit. 44 In brief, cells (1% inoculum) were grown overnight in MRS, spun down and washed 3 times in sterile distilled water. The tolerance of isolates was determined by exposing washed cell suspensions at 37°C to either (1) MRS + 0.3% oxgall (Difco), monitoring aliquots at OD600 over time; or (2) a simulated gastric juice (pH 2·0), a sterile solution of sodium chloride (0·5% w/v) containing pepsin (0·3% w/v); and (3) simulated small intestinal juice (pH 8·0), a sterile solution of sodium chloride (0·5% w/v) and Difco Bovine Oxgall ( 0.3%) containing pancreatin USP (1 g L−1), monitoring changes in total viable count periodically.
Bacteriocin production assay
The spot-overlay detection method was used to test isolates for activity against E.coli 0157:H7 (human isolate), Shigella boydii, Salmonella Typhimurium, Campylobacter jejuni, Listeria monocytogenes 184 and 187, Streptococcus mutans, Streptococcus pyogenes, Vancomycin-Resistant Enterococcus faecalis (VRE) and Methicillin Resistant S. aureus (MRSA). Lactobacillus delbrueckii 235 was used as target/indicator bacteria. Test (isolate) organisms (5 µL) were spotted onto MRS or BHI plates; the applied culture was allowed to absorb onto agar and the plates incubated for 24-48 hr. until a disc of growth appeared. The indicator (pathogen) isolate (10-100 µL depending on turbidity of the overnight culture) was added to a tube of optimum growth medium-overlay agar (0.75%, 10 mL), gently inverted to mix, and then immediately poured over the producer plate. Agar was allowed to solidify and plates were re-incubated under appropriate conditions to allow for optimal indicator/pathogen growth. Zones of inhibition were noted. Producer strains that demonstrated an inhibition zone were retested, applying various proteases within the zone of inhibition on the plate. Proteases inactivate bacteriocins of a peptide or protein nature, thereby eliminating inhibition zone formation. Isolates were also tested for antimicrobial activity by production of acid and hydrogen peroxide using NaOH and catalase, respectively, in place of proteases.
Adherence assays
Adherence Assays were conducted as previously described. 45 Caco-2 cells were grown in MEM with 10% FBS and antibiotics (Penicillin, Streptomycin and Amphotericin B). Each well of a 12-well plate was seeded with 6.5 × 104 cells and grown for 21 days in order to differentiate for the adherence assay. Cells were washed with PBS followed by addition of antibiotic-free medium, then incubated (37⁰C, 5% CO2). Isolates of interest (1 × 108 cells) were added to each well (in triplicate) and allowed to adhere for 1 hour at 37oC (5% CO2). Following a rinse with PBS, adherent cells were quantified by plating on MRS. Percent adherence was reported relative to the appropriate reference strains, which were L gasseri ADH (NCK 99); L. rhamnosus GG (NCK 431); Enterococcus faecalis (NCK337); and Bifidobacterium lactis (NCK 1573
Statistical Analyses
For the adherence to Caco-2 assays were run in triplicate in the same experiment and averaged and Standard Deviations determined (Excel Microsoft). For MIC determinations, each isolate was examined in duplicate on different days and averaged. For survival in simulated GI environments, each isolate was tested in 3 independent observations and the means and standard deviations were calculated. Error bars represent the Standard Deviation of the Mean (Excel Microsoft).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Deborah Hutchins, PhD ELS (Hutchins & Associates LLC) for technical writing assistance.
Funding
The study was funded by the Procter & Gamble Company. Writing support provided by Deborah Hutchins, PhD ELS was funded by The Procter & Gamble Company.
References
- 1.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 2001; 292:1115-8; PMID:11352068; http://dx.doi.org/ 10.1126/science.1058709 [DOI] [PubMed] [Google Scholar]
- 2.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430-5; PMID:8950812; http://dx.doi.org/ 10.1016/0966-842X(96)10057-3 [DOI] [PubMed] [Google Scholar]
- 3.Makino H, Kushiro A, Ishikawa E, Muylaert D, Kubota H, Sakai T, Oishi K, Martin R, Ben Amor K, Oozeer R, et al. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl Environ Microbiol 2011; 77:6788-93; PMID:21821739; http://dx.doi.org/ 10.1128/AEM.05346-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albesharat R, Ehrmann MA, Korakli M, Yazaji S, Vogel RF. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol 2011; 34:148-55; PMID:21300508; http://dx.doi.org/ 10.1016/j.syapm.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Martin V, Maldonado-Barragan A, Moles L, Rodriguez-Banos M, Campo RD, Fernandez L, Rodríguez JM, Jiménez E. Sharing of bacterial strains between breast milk and infant feces. J Hum Lact 2012; 28:36-44; PMID:22267318; http://dx.doi.org/ 10.1177/0890334411424729 [DOI] [PubMed] [Google Scholar]
- 6.Lindberg E, Adlerberth I, Hesselmar B, Saalman R, Strannegard IL, Aberg N, Wold AE. High rate of transfer of Staphylococcus aureus from parental skin to infant gut flora. J Clin Microbiol 2004; 42:530-4; PMID:14766812; http://dx.doi.org/ 10.1128/JCM.42.2.530-534.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007; 5:e177; PMID:17594176; http://dx.doi.org/ 10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker WA. Role of nutrients and bacterial colonization in the development of intestinal host defense. J Pediatr Gastroenterol Nutr 2000; 30 Suppl 2:S2-7; PMID:10749395; http://dx.doi.org/ 10.1097/00005176-200000002-00002 [DOI] [PubMed] [Google Scholar]
- 9.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003; 361:512-9; PMID:12583961; http://dx.doi.org/ 10.1016/S0140-6736(03)12489-0 [DOI] [PubMed] [Google Scholar]
- 10.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90:859-904; PMID:20664075; http://dx.doi.org/ 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 11.Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991; 88:90-7; PMID:1905394 [PubMed] [Google Scholar]
- 12.Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics 1999; 104:e64; PMID:10545590; http://dx.doi.org/ 10.1542/peds.104.5.e64 [DOI] [PubMed] [Google Scholar]
- 13.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128:541-51; PMID:15765388; http://dx.doi.org/ 10.1053/j.gastro.2004.11.050 [DOI] [PubMed] [Google Scholar]
- 14.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101:1581-90; PMID:16863564; http://dx.doi.org/ 10.1111/j.1572-0241.2006.00734.x [DOI] [PubMed] [Google Scholar]
- 15.Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther 2005; 22:387-94; PMID:16128676; http://dx.doi.org/ 10.1111/j.1365-2036.2005.02579.x [DOI] [PubMed] [Google Scholar]
- 16.Kajander K, Myllyluoma E, Rajilic-Stojanovic M, Kyronpalo S, Rasmussen M, Jarvenpaa S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther 2008; 27:48-57; PMID:17919270; http://dx.doi.org/ 10.1111/j.1365-2036.2007.03542.x [DOI] [PubMed] [Google Scholar]
- 17.Wickens K, Stanley TV, Mitchell EA, Barthow C, Fitzharris P, Purdie G, Siebers R, Black PN, Crane J. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin Exp Allergy 2013; 43:1048-57; PMID:23957340; http://dx.doi.org/ 10.1111/cea.12154 [DOI] [PubMed] [Google Scholar]
- 18.Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, Purdie G, Crane J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy 2012; 42:1071-9; PMID:22702506; http://dx.doi.org/ 10.1111/j.1365-2222.2012.03975.x [DOI] [PubMed] [Google Scholar]
- 19.Folster-Holst R. Probiotics in the treatment and prevention of atopic dermatitis. Ann Nutr Metab 2010; 57 Suppl:16-9; PMID:20829588; http://dx.doi.org/ 10.1159/000309054 [DOI] [PubMed] [Google Scholar]
- 20.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog 2012; 53:100-8; PMID:22634320; http://dx.doi.org/ 10.1016/j.micpath.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 21.Million M, Raoult D. Species and strain specificity of Lactobacillus probiotics effect on weight regulation. Microb Pathog 2013; 55:52-4; PMID:23332210; http://dx.doi.org/ 10.1016/j.micpath.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 22.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 2011; 52:1212-7; PMID:21498386; http://dx.doi.org/ 10.1093/cid/cir183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudha MR, Maurya AK. Effect of oral supplementation of the probiotic capsule UB-01BV in the treatment of patients with bacterial vaginosis. Benef Microbes 2012; 3:151-5; PMID:22433661; http://dx.doi.org/ 10.3920/BM2011.0054 [DOI] [PubMed] [Google Scholar]
- 24.Vicariotto F, Del Piano M, Mogna L, Mogna G. Effectiveness of the association of 2 probiotic strains formulated in a slow release vaginal product, in women affected by vulvovaginal candidiasis: a pilot study. J Clin Gastroenterol 2012; 46 Suppl:S73-80; PMID:22955364; http://dx.doi.org/ 10.1097/MCG.0b013e3182684d71 [DOI] [PubMed] [Google Scholar]
- 25.Collado MC, Cernada M, Bauerl C, Vento M, Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012; 3:352-65; PMID:22743759; http://dx.doi.org/ 10.4161/gmic.21215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol 2011; 31 Suppl 1:S29-34; PMID:21448201; http://dx.doi.org/ 10.1038/jp.2010.172 [DOI] [PubMed] [Google Scholar]
- 27.Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. Eur J Clin Nutr 2011; 65:10-9; PMID:20948557; http://dx.doi.org/ 10.1038/ejcn.2010.225 [DOI] [PubMed] [Google Scholar]
- 28.Mshvildadze M, Neu J. The infant intestinal microbiome: friend or foe? Early Hum Dev 2010; 86 Suppl 1:67-71; PMID:20116944; http://dx.doi.org/ 10.1016/j.earlhumdev.2010.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin R, Heilig GH, Zoetendal EG, Smidt H, Rodriguez JM. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol 2007; 13:2638-44; PMID:18045446; http://dx.doi.org/ 10.1111/j.1365-2672.2007.03497.x [DOI] [PubMed] [Google Scholar]
- 30.Martin R, Heilig HG, Zoetendal EG, Jimenez E, Fernandez L, Smidt H, Rodríguez JM. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res Microbiol 2007; 158:31-7; PMID:17224259; http://dx.doi.org/ 10.1016/j.resmic.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 31.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 2010; 156:3329-41; PMID:20864478; http://dx.doi.org/ 10.1099/mic.0.043224-0 [DOI] [PubMed] [Google Scholar]
- 32.Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology 2010; 156:3317-28; PMID:20829292; http://dx.doi.org/ 10.1099/mic.0.041913-0 [DOI] [PubMed] [Google Scholar]
- 33.Lakshminarayanan B, Guinane CM, O'Connor PM, Coakley M, Hill C, Stanton C, O'Toole PW, Ross RP. Isolation and characterization of bacteriocin-producing bacteria from the intestinal microbiota of elderly Irish subjects. J Appl Microbiol 2013; 114:886-98; PMID:23181509; http://dx.doi.org/ 10.1111/jam.12085 [DOI] [PubMed] [Google Scholar]
- 34.Brenner DM, Chey WD. Bifidobacterium infantis 35624: a novel probiotic for the treatment of irritable bowel syndrome. Rev Gastroenterol Disord 2009; 9:7-15; PMID:19367213 [PubMed] [Google Scholar]
- 35.Technical guidance prepared by the panel on additives and products or substances used in animal feed (FEEDAP) on the update of the criteria used in the assessment of bacterial resistance to antibiotics of human and veterinary importance. EFSA J 2008; 732:1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gueimonde M, Salminen S, Isolauri E. Presence of specific antibiotic (tet) resistance genes in infant faecal microbiota. FEMS Immunol Med Microbiol 2006; 48:21-5; PMID:16965348; http://dx.doi.org/ 10.1111/j.1574-695X.2006.00112.x [DOI] [PubMed] [Google Scholar]
- 37.Kirtzalidou EI, Mitsou EK, Pramateftaki P, Kyriacou A. Screening fecal enterococci from Greek healthy infants for susceptibility to antimicrobial agents. Microb Drug Resist 2012; 18:578-85; PMID:22827719; http://dx.doi.org/ 10.1089/mdr.2012.0028 [DOI] [PubMed] [Google Scholar]
- 38.Kirtzalidou E, Pramateftaki P, Kotsou M, Kyriacou A. Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe 2011; 17:440-3; PMID:21621627; http://dx.doi.org/ 10.1016/j.anaerobe.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 39.Charteris WP, Kelly PM, Morelli L, Collins JK. Gradient diffusion antibiotic susceptibility testing of potentially probiotic lactobacilli. J Food Prot 2001; 64:2007-14; PMID:11770631 [DOI] [PubMed] [Google Scholar]
- 40.Danielsen M, Wind A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 2003; 82:1-11; PMID:12505455; http://dx.doi.org/ 10.1016/S0168-1605(02)00254-4 [DOI] [PubMed] [Google Scholar]
- 41.de Vries LE, Valles Y, Agerso Y, Vaishampayan PA, Garcia-Montaner A, Kuehl JV, Christensen H, Barlow M, Francino MP. The gut as reservoir of antibiotic resistance: microbial diversity of tetracycline resistance in mother and infant. PLoS One 2011; 6:e21644; PMID:21738748; http://dx.doi.org/ 10.1371/journal.pone.0021644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolling GL, Wu M, Warren CA, Durmaz E, Klaenhammer TR, Guerrant RL. Lactic acid production by Streptococcus thermophilus alters Clostridium difficile infection and in vitro Toxin A production. Gut Microbes. 2012 November 1; 3(6): 523-529; http://dx.doi.org/ 10.4161/gmic.21757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullen MJ, Sanozky-Dawes RB, Crowell DC, Klaenhammer TR. Use of the DNA sequence of variable regions of the 16S rRNA gene for rapid and accurate identification of bacteria in the Lactobacillus acidophilus complex. J Appl Microbiol 2000; 89:511-6; PMID:11021584; http://dx.doi.org/ 10.1046/j.1365-2672.2000.01146.x [DOI] [PubMed] [Google Scholar]
- 44.Charteris W, Kelly P, Morelli L, Collins J. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 1998; 84:759-68; PMID:9674129; http://dx.doi.org/ 10.1046/j.1365-2672.1998.00407.x [DOI] [PubMed] [Google Scholar]
- 45.Yong GJ, Azcárate-Peril AM,, O'Flaherty S, Durmaz E, Valence F, Jardin J, Lortal S, Klaenhammer TR. Development and Application of a upp-Based Counterselective Gene Replacement System for the Study of the S-Layer Protein SlpX of Lactobacillus acidophilus NCFM. Appl Environ Microbiol 2009; 75:3093-105; PMID:19304841; http://dx.doi.org/ 10.1128/AEM.02502-08 [DOI] [PMC free article] [PubMed] [Google Scholar]


