Of the dizzying maze of pathways regulating the epithelial Na channel (ENaC), one of the most unusual is activation by proteases. Vallet et al.1 first discovered this phenomenon by identifying a channel activating protease later identified as a homolog of prostasin in a screen of gene products able to alter channel function in the Xenopus oocyte expression system. Subsequent studies identified a variety of serine proteases that could activate the channels, as well as consensus sites for cleavage on the channel subunits themselves.2 Independent lines of investigation revealed cleavage of the α and gamma subunits of ENaC in kidney tissue in the extracellular domain near the N-terminal transmembrane segment (Fig. 1), and showed that the extent of cleavage correlated with the upregulation of the channels in vivo by the mineralocorticoid aldosterone.3 This finding underscores the likely physiological relevance of proteolytic activation. However the reason for having such a step remains obscure. It may add a point of hormonal regulation, keep pores closed during their biosynthesis and trafficking, or just be an awkward way that evolution has stumbled upon to produce a functional channel.
Figure 1.
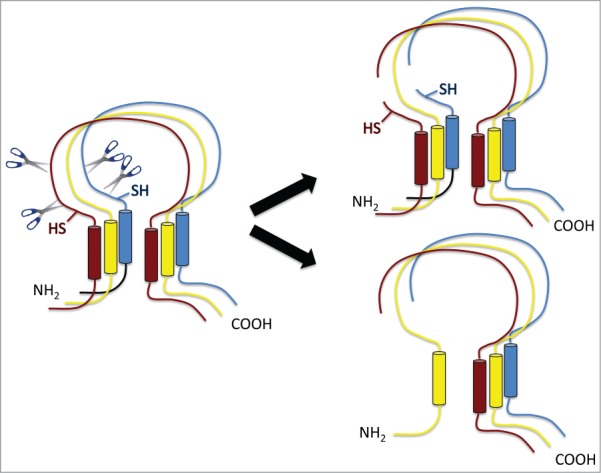
Schematic view of ENaC composed of 3 homologous subunits α (red), β (yellow) and gamma (blue) subunits with similar transmembrane topologies. The α and gamma subunits are cleaved at least twice in the extracellular domain near the N-terminal membrane-spanning segment (left). This divides the subunits into 2 segments. The shorter N-terminal part could stay within the channel structure (upper right) or separate (lower right). SH marks sites of engineered cysteines that affect channel function.
The precise structural basis of channel activation is unknown; the closest solved structures, those of the related acid-sensing channel ASIC1, differ in the relevant portion of the peptides. However the work of Kleyman, Hughey, and colleagues4 provides intriguing clues. They reported that activation requires at least 2 cleavage steps for each subunit, with at least one of these occurring intracellularly. Furthermore peptides predicted to be released in the process inhibited the activated channels when added to the extracellular side of the membrane. This suggests that in the inactive state an internal particle blocks the pore, analogous to inactivation of voltage-gated Na channels5 albeit from the extracellular rather than the intracellular side. Here relief from inactivation requires proteolytic removal of the blocking particle and is presumably irreversible in vivo. A more complicated effect involving relaxation of the whole channel structure to a more open state is also possible.
In either case, the fates of the individual parts of the cleaved subunits are uncertain. Do they stay together or drift apart? The larger C-terminal fragments of α and γENaC are essential for channel function as they contain key amino acids that confer selectivity and sensitivity to amiloride,6 the canonical blocker of these channels. The N-termini, however, might be expendable.
Berman et al.7 take up this question in an article in the current issue of Channels. They added cysteines on either side of the putative cleavage domains of α and γENaC. When expressed in oocytes, the α subunits at the surface of the cell were in both cleaved and intact forms. After activation with the protease subtilisin, modification of the N-terminal engineered cysteines on the α subunit by the thiol reagent MTSEA inhibits channel activity. This implies that the N-terminal fragment remains part of the structure of the conducting channels. Studies of the γENaC reached a similar conclusion. This subunit appears to be nearly all in the cleaved state at the surface of the cell. Here the N-terminal cysteine mutations themselves reduce the basal channel current. Their modification with MTSEA further inhibited activity. These findings indicate that the γENaC N-terminus is also an essential component of the active channel. However, biochemical analysis by Western blot indicates a low abundance of the αENaC N-terminal fragment relative to the C-terminal portion. This implies that many of the channels may indeed shed this moiety, presumably also losing activity.
The results make good sense from the perspective of protein structure. Although the C-terminal portions of the subunits may form most of the conducting pore, the N-terminus and its membrane-spanning domain are likely to be important for stabilizing the structure in the lipid bilayer.8 A full understanding of the interactions between the pieces of the channel in the closed and active states awaits more direct structural information.
References
- 1.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. Nature 1997; 389:607-10; PMID:9335501; http://dx.doi.org/ 10.1038/39329 [DOI] [PubMed] [Google Scholar]
- 2.Rossier BC, Stutts MJ. Annu Rev Physiol 2008; 71:361-79; PMID: 18928407; http://dx.doi.org/22038262 10.1146/annurev.physiol.010908.163108 [DOI] [PubMed] [Google Scholar]
- 3.Palmer LG, Patel A, Frindt G. Clin Exp Nephrol 2012; 16:35-43; PMID:22038262; http://dx.doi.org/ 10.1007/s10157-011-0496-z [DOI] [PubMed] [Google Scholar]
- 4.Kleyman TR, Carattino MD, Hughey RP. J Biol Chem 2009; 284:20447-51; PMID:19401469; http://dx.doi.org/ 10.1074/jbc.R800083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catterall WA. Exp Physiol 2014; 99:35-51; PMID:24097157; http://dx.doi.org/ 10.1113/expphysiol.2013.071969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellenberger S, Schild L. 2015; 67:1-35; PMID:25287517; http://dx.doi.org/ 10.1124/pr.114.009225 [DOI] [PubMed] [Google Scholar]
- 7.Berman JM, Awayda RG, Awayda MS. Channels 2015; 9(5): 281–90; PMID: 26218672; http://dx.doi.org/19641589 10.1080/19336950.2015.1073869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzales EB, Kawate T, Gouaux E. Nature 2009; 460:599-604; PMID:19641589; http://dx.doi.org/ 10.1038/nature08218 [DOI] [PMC free article] [PubMed] [Google Scholar]


