ABSTRACT
Mating plugs are hardened structures—typically a coagulation of seminal fluid components—that are transferred to, or formed within, the female reproductive tract of numerous animal species (both mammals and insects). Analysis of the role(s) of the mating plug in reproduction has been conducted in a wide array of diverse species. These structures have been proposed to have a multitude of functions, which include altering female re-mating rate, acting as a barrier to re-mating and being required for sperm storage or sperm movement to occur in mated females. A recent analysis of the Drosophila melanogaster mating plug has shown that proper formation of the structure is required for optimal fertility in flies: the Drosophila mating plug is required to retain the ejaculate within the female reproductive tract once mating has terminated. Here, we discuss the possible implications of the Drosophila mating plug in the fertility of this species in light of these new results.
Keywords: Drosophila reproduction, mating plug, PEBme, sperm storage
Introduction
Mating plug formation—a coagulation of seminal fluid components transferred to, or formed within, the female reproductive tract—is a phenomenon that occurs during or shortly after mating in several taxonomically diverse species.1,2 These structures are proposed to have numerous functions, which include preventing sperm loss,3 facilitating the storage of sperm in the mated female,4 decreasing or eliminating female sexual receptivity 3,5-7 and/or acting as a physical barrier to prevent female re-mating, thereby reducing the risk of sperm competition.8 Evidence for the latter includes a correlation of mating plug formation in primate species with multiple mating9 and the accelerated rate of evolution of a major mating plug gene in polyandrous primates relative to monandrous primates.10
However, while many studies have focused on the mating plug's role in female re-mating and in sperm storage/sperm competition parameters, recent studies have suggested that in some species, mating plugs may have additional and somewhat unexpected roles in reproductive processes. For example, in matings by mutant male mice that lack the prostate-expressedtransglutaminase, “copulatory plugs” do not form, and this prevents sperm migration to the sites of fertilization.11 In addition, the mouse seminal protein SVS2, which is also required for proper copulatory plug formation, is required for sperm survival in the uterus.12 These data suggest that the copulatory plug may have a sperm protective function, as well as facilitating sperm motility, in the female mouse reproductive tract. In another example, presence of a mating plug in anopheline mosquitoes has been suggested to associate with malaria-vectorial capacity.13 In species where malaria transmission is high, coagulated mating plugs form, correlating with transfer of the steroid hormone 20E to females during mating. 20E interacts with a female protein that regulates expression of lipid transporters resulting in increased oogenesis and favoring development of Plasmodium, the malaria parasite.13-15 Examples such as these suggest that the mating plug likely functions in processes aside from the oft-cited roles of preventing re-mating and effecting sperm storage.
The Drosophila mating plug
Little is known about mating plug formation across Drosophila species. Alonso-Pimental et al.16 distinguished among several types of structures formed in mated female reproductive tracts of different Drosophila species. For example, they reported that D. hexastigma has a hardened mating plug whose description seems similar to what Bairati and Perotti17 observed in D. melanogaster. A mating plug has also been reported in D. hibisci, but it differs from D. melanogaster's in being a gelatinous structure that fills the lumen of the female reproductive tract and remains for several days post-mating.3,7 Alonso-Pimental et al. also describe structures that they call “sperm sacs” in D. mettleri and in D. nigrospiracula. They describe these structures as softer and less distending than true mating plugs. (They suggest that the D. melanogaster plug may fall into this category, but Bairati and Perotti's,17 and our (18; see below), data favor its fitting into their “matng plug” category.) Finally, they describe the insemination reaction mass that forms after mating in the female reproductive tract of some cactophilic Drosophila.19 The insemination reaction mass also fills the female reproductive tract after mating and prevents egg-laying and re-mating until it is cleared by the female. Its size and persistence are heightened in interspecific and intra-specific interpopulation matings.20
Drosophila melanogaster affords the possibility of taking a genetic approach to dissect mating plug function. The mating plug of D. melanogaster consists of 2 distinct regions (Fig. 1) that form sequentially and are composed of proteins from different tissues of the male reproductive tract. The rapid-forming posterior mating plug (PMP) is derived from proteins secreted from the male's ejaculatory bulb6,21-23 while the later-forming anterior mating plug (AMP) is derived primarily from proteins secreted from the male's accessory glands.23,24 Removal of the accessory gland-derived seminal protein Acp36DE from the ejaculate prevents AMP formation24 and hinders sperm storage.24-26 However, because Acp36DE localizes to the sperm mass as well as to female reproductive tract tissues,24 it is not yet known whether the sperm storage defects observed in mates of Acp36DE null males are due to the lack of AMP formation or to additional functions of Acp36DE in mated females.
Figure 1.
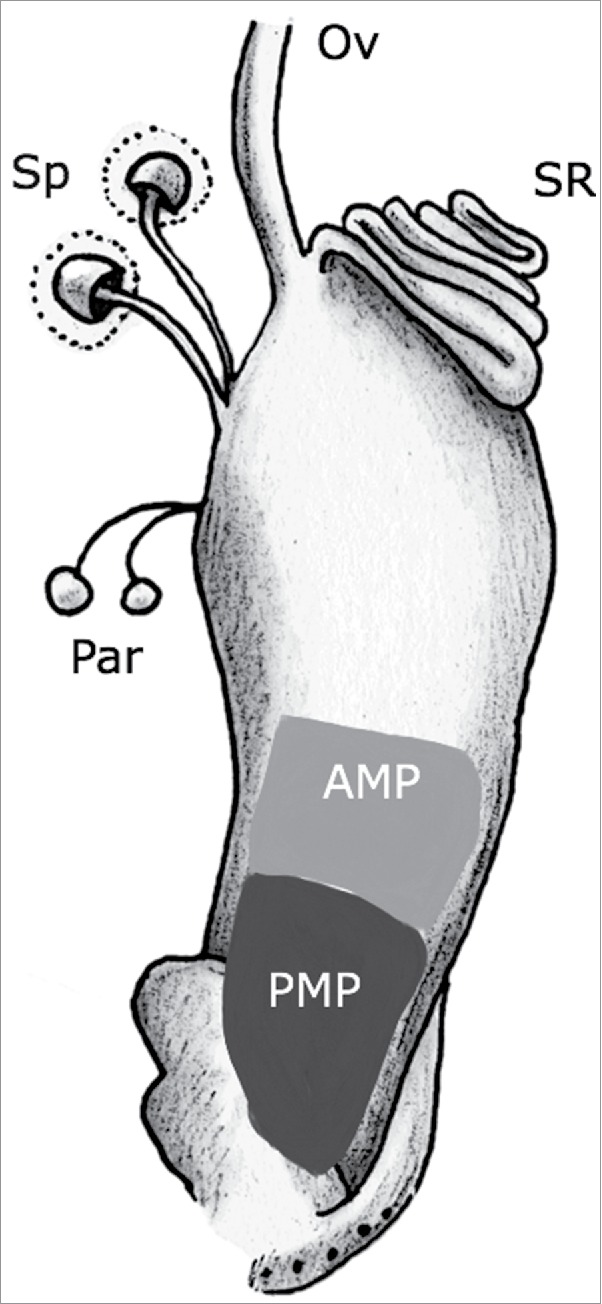
Diagram of the mating plug in the Drosophila melanogaster female lower reproductive tract. The uterus is shown containing the mating plug (AMP + PMP; for anterior + posterior mating plug, respectively). The figure also shows the sperm storage organs (seminal receptacle (SR), spermathecae (ST)), the parovaria (Pa), and the lower common oviduct (Ov). The upper reproductive tract (ovaries, lateral oviducts, upper common oviduct) is not shown, for simplicity.
The role of the PMP in reproductive events was even less well understood. The rapid formation of the PMP (∼5 min),21 its density, and its formation immediately inside the entrance to the female reproductive tract23 made dissecting its role in reproduction of particular interest. To test the role of the PMP we initially attempted to suppress the production of all secreted proteins of the ejaculatory bulb.18,22 From the Drosophila Fly Lights GAL4 collection,27,28 we identified a GAL4 driver that expressed strongly in the ejaculatory bulb. With this CREB-GAL4 driver we generated male flies that express a misfolded rhodopsin in the ejaculatory bulb; this genotype induced ER stress in the ejaculatory bulb and thereby prevented production of its secreted proteins.18,22 Unfortunately, CREB-GAL4; UAS-Rh1G69D transgenic males had behavioral abnormalities, likely due to expression of the driver (and hence induction of ER stress) outside the ejaculatory bulb, that made their reproductive phenotype difficult to interpret. Functional tests of the role of the ejaculatory bulb thus had to focus on specific ejaculatory bulb proteins. Proteomic analysis of the D. melanogaster mating plug identified potential candidates that could be targeted for RNAi knockdown.18 The first ejaculatory bulb/PMP protein targeted for RNAi-mediated knockdown was Protein of the Ejaculatory Bulb II (PEBII).6 Removal of PEBII from the ejaculate (via ubiquitous RNAi knockdown) reduced mating plug size and affected the short-term sexual receptivity of recently mated females but had no observable effect on female fertility.6
Dissecting D. melanogaster mating plug function
To attempt to get a broad view of ejaculatory bulb function, we decided to study the Protein of the Ejaculatory Bulb in melanogaster (PEBme).29 PEBme, a UV autofluorescent protein,21 has sequence features consistent with a structural role, including repetitive PGG motifs similar to those found in homopolymer-forming proteins.21 Putative orthologs of PEBme, defined here as reciprocal best BLAST hits, are detectable throughout the subgenus Sophophora. Orthologs are not detectable in the subgenus Drosophila, including the desert Drosophila, or in other dipterans. PEBme has well-conserved N- and C-terminal regions, with a highly variable central region spanning amino acids 127–273 of the 377 aa of the D. melanogaster protein (Fig. 2). This variable region is highly repetitive, and is particularly rich in G, L, P and S residues. Alignments among putative PEBme orthologs from D. melanogaster, D. simulans, D. erecta, D. yakuba, D. ananassae, and D. elegans suggest a high frequency of insertions and deletions in this central region. This high sequence variability could in principle result from diversifying selection, perhaps due to effects of PEBme in sperm competition or sexual conflict.30 Alternatively, if the central region of PEBme is needed to function in a largely structural role, there may be low levels of constraint on its sequence; perhaps any (or various) repeated homopolymer-generating sequence will do, allowing for high levels of neutral divergence. Unfortunately, the repetitive and simple-sequence nature of the central region of PEBme makes sequence alignments highly gapped and uncertain, preventing standard tests for positive selection based on between-species divergence, such as those implemented in PAML.31 Alternative, population-based approaches may instead allow for formal neutrality tests if alignments are more trustworthy.
Figure 2.
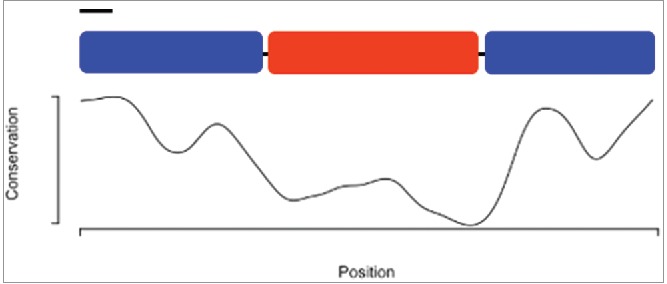
A schematic of the PEBme open reading frame, above a graph of amino acid sequence conservation. The protein is drawn to-scale, with the black bar indicating the predicted signal sequence. PEBme's ends (blue) are more highly conserved in sequence than its central region (red), which contains the repeated motifs. Conservation scores were annotated using the physico-chemical scores of Livingstone et al.,53 as implemented in JalView.54 The graph was generated in R.55 Scores range from 0 (no conservation) to 11 (full conservation among all species).
Due to its potential structural role, we reasoned that knockdown of PEBme might disrupt mating plug formation. PEBme's role in mating plug formation and fertility were unknown and null mutations of the gene are unavailable. We attempted to knock down PEBme ubiquitously, but this resulted in late-pupal lethality. This lethality may reflect a requirement for PEBme expression in other tissues (i.e. the digestive and/or respiratory system, where PEBme is also expressed),32 or could be due to a non-specific, dominant effect for this particular line, similar to effects reported for a subset of KK RNAi lines when crossed to a GAL4 driver.33 To circumvent the lethality of ubiquitous PEBme knockdown, we used the CREB-GAL4 driver to target PEBme for RNAi knockdown in (or primarily in) the ejaculatory bulb.18
Knocking down PEBme to ∼25% of wild-type levels was sufficient to nearly eliminate mating plug formation and had drastic consequences on female fertility.18 When mating plug formation was perturbed or prevented in this way, mated females were significantly compromised in their ability to increase egg production and to store sperm after mating.18 Moreover these females did not properly undergo the changes in uterine conformation 18 that normally occur after mating and are essential for sperm storage.25,34 The breadth of phenotypes suggested that the reduction in fertility in these females was likely due to a phenomenon that affected the entire ejaculate, such as loss of ejaculate in females mated to PEBme knockdown males. Indeed, in the absence of PMP formation, the ejaculate was often not maintained in the female reproductive tract. Rather, it failed to coagulate properly and thus was incidentally pulled from the female when males uncoupled. These results suggested that coagulation of the PMP may allow the males to disengage cleanly once mating has ended. Thus, our experiments showed that in D. melanogaster, mating plug formation is crucial for fertility and for aspects of mating itself.
At first glance, these results support previous reports about the requirement of a mating plug to prevent sperm “leakage” or “backflow” from the female reproductive tract.3 However, careful consideration of the observed phenotypes suggests that mating plug formation may support fertility in numerous additional ways, some of which are analogous to mating plug functions in other organisms.
Potential roles of the D. melanogaster mating plug in mating and fertility
The most notable phenotype that we observed in mates of PEBme knockdown males was complete loss of the ejaculate from the reproductive tracts of mated females.18 This phenotype was significantly exacerbated when the mating animals were shaken lightly. This level of agitation, sufficient to prematurely terminate matings by knockdown and control males, did not impact the fertility of control males, but greatly exacerbated the fertility defects of knockdown males. These results suggest that the PMP may support copulating males by “holding” them in place for the duration of copulation. If this hypothesis is correct, it suggests that mutations that perturb mating plug formation may have drastic consequences in wild D. melanogaster populations. In the wild, mating often occurs in proximity to rotting fruit, which attracts large numbers of flies 35 including competitor males that are likely to jostle copulating pairs. Moreover, under such crowded conditions, the threat of sperm competition would be high—a situation in which sperm and/or seminal fluid transfer may be increased.36,37 In such a situation the male benefits of increased ejaculate allocation would still require maintenance of the ejaculate in the female reproductive tract and therefore necessitate proper mating plug formation.
Mating plugs have been hypothesized to act as a form of mate guarding. In D. melanogaster, the mating plug does not prevent sperm transfer from subsequent competitor males—sperm from both males can be observed in the few females that re-mate shortly after an initial mating.38 Further, females that mate to males lacking sex peptide (a seminal protein that suppresses female re-mating1) will often quickly re-mate with a second male (FWA, unpublished observations) even though these females have fully formed PMPs.23 Thus, mating-induced refractoriness to re-mating resulting from seminal protein receipt and the formation of the mating plug may simply ensure the storage and subsequent usage of an initial male's sperm.
Upon mating, sperm storage in the female reproductive tract occurs rapidly, beginning ∼25 min after copulation begins.38 Females ultimately expel the remainder of the ejaculate (mating plug plus non-stored sperm) several hours after mating ends.38,39 The timing of ejaculate expulsion affects sperm competitive success in multiply-mated females.40 Given the phenotypes of PEBme knockdown males, we suggest that PMP coagulation may provide a mechanism to delay ejaculate ejection, ensuring that the males' sperm are stored in maximal numbers and providing sufficient time for seminal fluid proteins to elicit their effects (more sperm in storage results in more sequestered sex-peptide in the female reproductive tract41 which will prolong female remating-refractoriness). In view of potential conflicts between the reproductive strategies and interests of males and females, it will be interesting to investigate whether some proteases secreted by the female reproductive tract42-44 might work to degrade the mating plug allowing it to be expelled more quickly.
In addition to keeping the ejaculate contained within the female reproductive tract, it is likely that the PMP, or the MP as a whole, acts as a scaffold to support sperm storage and, possibly, seminal protein function. The PMP forms quickly, ∼5min after mating begins21 and is followed by the ‘opening’ of the uterus,25,34 formation of the AMP,24 the organization and movement of sperm toward the female sperm storage organs34 and, finally, active sperm storage.25,34 PMP formation may be the first step in supporting male-derived action in the female reproductive tract by providing a structure to support subsequent AMP formation. In addition it is intriguing to speculate that the MP might facilitate sperm storage, for example by providing a scaffold, structure, or meshwork for sperm to move against, or to ‘disentangle’ individual sperm from the sperm mass. However, the effects of the mating plug on the characteristics and rate of sperm entry into storage are as yet unknown and require future exploration, for example in PEBme knockdown situations.
The mating plug may play an additional structural role. Once the ejaculate has been transferred to the female, the uterus undergoes a series of conformational changes that depend on the receipt of seminal proteins from the male's accessory gland.25,34 However, in the absence of transfer of accessory gland proteins, the posterior mating plug still forms and causes an expansion in volume of the posterior uterus.22,23 It has been proposed that sensory neurons of the Drosophila uterus are mechanosensory and therefore act as stretch receptors, sensing that mating has occurred and coordinating post-mating processes such as fertilization and egg-laying.45-47 The volume and solidification of the mating plug may contribute to distending the uterus, thereby activating the stretch receptors to signal an initiation of female post-mating responses.
In addition to secreting PMP proteins, the ejaculatory bulb is also the site of synthesis of male pheromones, including triacylglycerides48 and cis-vaccenyl acetate (cVA).49 These molecules are transferred to the female reproductive tract during mating.48,50 While triacylglycerides are not expressed sex-specifically in D. melanogaster, cVA is part of the hydrocarbon profile of Drosophila males and influences courtship and aggression behaviors.50,51 It is possible that mating plug formation supports the positioning or concentration of pheromones at the appropriate place in the female reproductive tract.
Conclusion
Mating plugs actively contribute to fertility. In addition to the well-defined roles of mating plugs in supporting sperm storage and affecting sexual receptivity, the phenotypes of PEBme knockdown males showed that the Drosophila melanogaster mating plug is required for the maintenance of the ejaculate within the post-mated female reproductive tract, and for allowing males to uncouple cleanly after the termination of copulation. However, many fascinating questions about mating plugs remain to be addressed. For instance, now that constituents of the D. melanogaster mating plug, and some of their functions, are known it will be interesting to compare the biochemistry of such post-mating structures across Drosophila species, including whether and how PEBme-like proteins are involved in the formation of other mating plug-like structures, such as the insemination reaction masses that form in heterospecific matings among desert Drosophila species.52 These questions are particularly interesting given the diversity of such post-mating structures even within species (among populations)20 as well as between species. Relatedly, the roles of the many other proteins identified by proteomic analysis of the mating plug will be interesting to discover, as these are still unknown even in D. melanogaster.18 It will also be interesting to determine the extent of variation in mating plug coagulation in wild Drosophila melanogaster (or across laboratory strains), and whether and how this affects fertility. Another interesting set of questions relates to the likely role of mating plugs in sexual conflict as well as in fertility.20 In this context, a detailed evolutionary analysis of PEBme may identify whether its putative structure-generating region is under positive selection, or under relaxed selection, and may pinpoint features that play a structural role. Finally, what role does the mating plug play in sperm movement in the female reproductive tract, and does mating plug formation activate reproductive tract stretch receptors? The multitude of molecular and genetic tools available for use in Drosophila will allow researchers to address these, and other, pressing questions about mating plug function and contents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank H. Amrein and B. Rattner for the invitation to write this Extra View, E. Kelleher, D. Duneau, and A. Cohen for useful discussions, an anonymous reviewer for very helpful suggestions.
Funding
The research for this paper was supported by an NSERC Discovery Grant to AW and NIH grant R01-HD038921 to MFW.
References
- 1.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Ann Rev Entomol 2011; 56:21–40; PMID:20868282; http://dx.doi.org/ 10.1146/annurev-ento-120709-144823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poiani A. Complexity of seminal fluid: a review. Behav Ecol Sociobiol 2006; 60:289–310; http://dx.doi.org/ 10.1007/s00265-006-0178-0 [DOI] [Google Scholar]
- 3.Polak M, Starmer WT, Barker JSF. A mating plug and male mate choice in Drosophila hibisci Bock. Anim Behav 1998; 56:919–26; PMID:9790703; http://dx.doi.org/ 10.1006/anbe.1998.0850 [DOI] [PubMed] [Google Scholar]
- 4.Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol 2009; 7:e1000272; PMID:20027206;http://dx.doi.org/ 10.1371/journal.pbio.1000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer B, Morgan ED, Schmid-Hempel P. A nonspecific fatty acid within the bumblebee mating plug prevents females from remating. Proc Natl Acad Sci U S A 2001; 98:3926–8; PMID:11274412; http://dx.doi.org/ 10.1073/pnas.061027998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretman A, Lawniczak MK, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. J Insect Physiol 2010; 56:107–13; PMID:19800888;http://dx.doi.org/ 10.1016/j.jinsphys.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 7.Polak M, Wolf LL, Starmer WT, Barker JSF. Function of the mating plug in Drosophila hibisci Bock. Behav Ecol Sociobiol 2000; 49:196–205; http://dx.doi.org/ 10.1007/s002650000281 [DOI] [Google Scholar]
- 8.Dunham AE, Rudolf VHW. Evolution of sexual size monomorphism: the influence of passive mate guarding. J Evol Biol 2009; 22:1376–86; PMID:19486235; http://dx.doi.org/ 10.1111/j.1420-9101.2009.01768.x [DOI] [PubMed] [Google Scholar]
- 9.Dixson AF, Anderson MJ. Sexual selection, seminal coagulation and copulatory plug formation in primates. Folia Primatologica 2002; 73:63–9; http://dx.doi.org/ 10.1159/000064784 [DOI] [PubMed] [Google Scholar]
- 10.Dorus S, Evans PD, Wyckoff GJ, Choi SS, Lahn BT. Rate of molecular evolution of the seminal protein gene SEMG2 correlates with levels of female promiscuity. Nat Genet 2004; 36:1326–9; PMID:15531881; http://dx.doi.org/ 10.1038/ng1471 [DOI] [PubMed] [Google Scholar]
- 11.Dean MD. Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. Plos Genet 2013; 9:e1003185;http://dx.doi.org/ 10.1371/journal.pgen.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano N, Araki N, Yoshida K, Hibino T, Ohnami N, Makino M, Kanai S, Hasuwa H, Yoshida M, Miyado K, et al.. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci U S A 2014; 111:4145–50; PMID:24591616; http://dx.doi.org/ 10.1073/pnas.1320715111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell SN, Kakani EG, South A, Howell PI, Waterhouse RM, Catteruccia F. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 2015; 347: 985–8; PMID:25722409; http://dx.doi.org/ 10.1126/science.1259435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldini F, Gabrieli P, South A, Valim C, Mancini F, Catteruccia F. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol 2013; 11:e1001695; PMID:24204210;http://dx.doi.org/ 10.1371/journal.pbio.1001695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rono MK, Whitten MM, Oulad-Abdelghani M, Levashina EA, Marois E. The major yolk protein vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol 2010; 8:e1000434; PMID:20652016; http://dx.doi.org/ 10.1371/journal.pbio.1000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso-Pimentel H, Tolbert LP, Heed WB. Ultrastructural examination of the insemination reaction in Drosophila. Cell Tissue Res 1994; 275:467–79; PMID:8137397; http://dx.doi.org/ 10.1007/BF00318816 [DOI] [PubMed] [Google Scholar]
- 17.Bairati A, Perotti ME. Occurrence of a compact mating plug in the genital duct of Drosophila females after mating. Dros Info Serv 1970; 45:67–8 [Google Scholar]
- 18.Avila FW, Cohen AB, Ameerudeen FS, Duneau D, Suresh S, Mattei AL, Wolfner MF. Retention of ejaculate by Drosophila melanogaster females requires the male-derived mating plug protein PEBme. Genetics 2015; 200:1171–9; PMID:26058847;http://dx.doi.org/ 10.1534/genetics.115.176669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markow TA, Ankney PF. Drosophila males contribute to oogenesis in a multiple mating species. Science 1984; 224:302–3; PMID:17734916; http://dx.doi.org/ 10.1126/science.224.4646.302 [DOI] [PubMed] [Google Scholar]
- 20.Knowles LL, Markow TA. Sexually antagonistic coevolution of a postmating-prezygotic reproductive character in desert Drosophila. Proc Natl Acad Sci U S A 2001; 98:8692–6; PMID:11447265; http://dx.doi.org/ 10.1073/pnas.151123998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lung O, Wolfner MF. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol 2001; 31:543–51; PMID:11267893;http://dx.doi.org/ 10.1016/S0965-1748(00)00154-5 [DOI] [PubMed] [Google Scholar]
- 22.Chow CY, Avila FW, Clark AG, Wolfner MF. Induction of excessive endoplasmic reticulum stress in the Drosophila male accessory gland results in infertility. PloS One 2015; 10:e0119386; PMID:25742606; http://dx.doi.org/ 10.1371/journal.pone.0119386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattei AL, Riccio ML, Avila FW, Wolfner MF. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proc Natl Acad Sci U S A 2015; 112:8475–80; PMID:26041806; http://dx.doi.org/ 10.1073/pnas.1505797112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 1999; 153:845–57; PMID:10511562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA 2009; 106:15796–800; PMID:19805225;http://dx.doi.org/ 10.1073/pnas.0904029106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol 2003; 206:3521–8; PMID:12939382; http://dx.doi.org/ 10.1242/jeb.00585 [DOI] [PubMed] [Google Scholar]
- 27.Jenett A, Rubin GM, Ngo TTB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al.. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep 2012; 2:991–1001; PMID:23063364; http://dx.doi.org/ 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al.. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA 2008; 105:9715–20; PMID:18621688;http://dx.doi.org/ 10.1073/pnas.0803697105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig MZ, Uspensky II, Ivanov AI, Kopantseva MR, Dianov CM, Tamarina NA, Korochkin LI. Genetic control and expression of the major ejaculatory bulb protein (PEBme) in Drosophila melanogaster. Biochem Genet 1991; 29:215–39; PMID:1772395; http://dx.doi.org/ 10.1007/BF00590103 [DOI] [PubMed] [Google Scholar]
- 30.Wong A, Turchin MC, Wolfner MF, Aquadro CF. Evidence for positive selection on Drosophila melanogaster seminal fluid protease homologs. Mol Biol Evol 2008; 25:497–506; PMID:18056920; http://dx.doi.org/ 10.1093/molbev/msm270 [DOI] [PubMed] [Google Scholar]
- 31.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 2007; 24:1586–91; PMID:17483113; http://dx.doi.org/ 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- 32.Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM, et al.. Unlocking the secrets of the genome. Nature 2009; 459:927–30; PMID:19536255; http://dx.doi.org/ 10.1038/459927a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green EW, Fedele G, Giorgini F, Kyriacou CP. A Drosophila RNAi collection is subject to dominant phenotypic effects. Nat Methods 2014; 11:222–3; PMID:24577271; http://dx.doi.org/ 10.1038/nmeth.2856 [DOI] [PubMed] [Google Scholar]
- 34.Adams EM, Wolfner MF. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol 2007; 53:319–31; PMID:17276455;http://dx.doi.org/ 10.1016/j.jinsphys.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochando MD, Reyes A, Ayala FJ. Multiple paternity in two natural populations (orchard and vineyard) of Drosophila. Proc Natl Acad Sci U S A 1996; 93:11769–73; PMID:8876212;http://dx.doi.org/ 10.1073/pnas.93.21.11769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garbaczewska M, Billeter JC, Levine JD. Drosophila melanogaster males increase the number of sperm in their ejaculate when perceiving rival males. J Insect Physiol 2013; 59:306–10; PMID:23178803; http://dx.doi.org/ 10.1016/j.jinsphys.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 37.Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FC, Bretman A, Wolfner MF, Chapman T. Seminal fluid protein allocation and male reproductive success. Curr Biol 2009; 19:751–7; PMID:19361995; http://dx.doi.org/ 10.1016/j.cub.2009.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 2010; 328:354–7; PMID:20299550; http://dx.doi.org/ 10.1126/science.1187096 [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Daubnerová I, Isaac RE, Zhang C, Choi S, Chung J, Kim Y. Neuronal pathway that controls sperm ejection and storage in female Drosophila. Curr Biol 2015; 25(6):790–7; PMID: 25702579 [DOI] [PubMed] [Google Scholar]
- 40.Lüpold S, Pitnick S, Berben KS, Blengini CS, Belote JM, Manier MK. Female mediation of competitive fertilization success in Drosophila melanogaster. Proc Natl Acad Sci USA 2013; 110:10693–8;http://dx.doi.org/ 10.1073/pnas.1300954110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol 2005; 15:207–13; PMID:15694303; http://dx.doi.org/ 10.1016/j.cub.2005.01.034 [DOI] [PubMed] [Google Scholar]
- 42.Swanson WJ, Wong A, Wolfner MF, Aquadro CF. Evolutionary expressed sequence tag analysis of Drosophila female reproductive tracts identifies genes subjected to positive selection. Genetics 2004; 168:1457–65; PMID:15579698;http://dx.doi.org/ 10.1534/genetics.104.030478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 2008; 135:311–21; PMID:18077584; http://dx.doi.org/ 10.1242/dev.015156 [DOI] [PubMed] [Google Scholar]
- 44.Prokupek AM, Kachman SD, Ladunga I, Harshman LG. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol 2009; 18:465–75; PMID:19453766; http://dx.doi.org/ 10.1111/j.1365-2583.2009.00887.x [DOI] [PubMed] [Google Scholar]
- 45.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 2009; 61:511–8; PMID:19249272;http://dx.doi.org/ 10.1016/j.neuron.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 46.Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 2009; 61:519–26; PMID:19249273; http://dx.doi.org/ 10.1016/j.neuron.2008.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gou B, Liu Y, Guntur AR, Stern U, Yang CH. Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep 2014; 9:522–30; PMID:25373900; http://dx.doi.org/ 10.1016/j.celrep.2014.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin JS, Ellis SR, Pham HT, Blanksby SJ, Mori K, Koh QL, Etges WJ, Yew JY. Sex-specific triacylglycerides are widely conserved in Drosophila and mediate mating behavior. eLife 2014; 3:e01751; PMID:24618898; http://dx.doi.org/ 10.7554/eLife.01751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guiraudie-Capraz G, Pho DB, Jallon JM. Role of the ejaculatory bulb in biosynthesis of the male pheromone cis-vaccenyl acetate in Drosophila melanogaster. Integr Zool 2007; 2:89–99; PMID:21396023; http://dx.doi.org/ 10.1111/j.1749-4877.2007.00047.x [DOI] [PubMed] [Google Scholar]
- 50.Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol 2007; 17:599–605; PMID:17363250;http://dx.doi.org/ 10.1016/j.cub.2007.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci 2011; 14:757–62; PMID:21516101; http://dx.doi.org/ 10.1038/nn.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelleher ES, Markow TA. Reproductive tract interactions contribute to isolation in Drosophila. Fly 2007; 1:33–7; PMID:18690059;http://dx.doi.org/ 10.4161/fly.3840 [DOI] [PubMed] [Google Scholar]
- 53.Livingstone CD, Barton GJ. Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. Comput Appl Biosci 1993; 9:745–56; PMID:8143162 [DOI] [PubMed] [Google Scholar]
- 54.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009; 25:1189–91; PMID:19151095; http://dx.doi.org/ 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0 http://www.R-project.org/. 2008 [Google Scholar]


