Abstract
Piperine, a natural compound isolated from the fruits of Piper, is known to modulate several neurotransmitter systems such as serotonin, norepinephrine, and GABA, all of which have been linked to the development of convulsions. Fruits of Piper species have been suggested as means for managing seizure disorders. The present study was designed to elucidate the anticonvulsant effect of piperine and its mechanisms of action using in-silico, in-vivo and in-vitro techniques.PASS software was used to determine its possible activity and mechanisms. Furthermore the latency for development of convulsions and mortality rate was recorded in different experimental mouse models of epilepsy (pentylenetetrazole, maximal electroshock, NMDA, picrotoxin, bicuculline, BAYK-8644, strychnine-induced convulsions) after administration of various doses of piperine (5, 10 and 20 mg/kg, i.p.). Finally, the effect of piperine on Na+ and Ca2+ channels were evaluated using the whole cell patch clamp techniqueOur results revealed that piperine decreased mortality in the MES-induced seizure model. Moreover, piperine (10 mg/kg) delayed the onset of tonic clonic convulsions in the pentylenetetrazole test and reduced associated mortality. Furthermore, an anticonvulsant dose of piperine also delayed the onset of tonic clonic seizures in strychnine, picrotoxin and BAY K-8644. Complete protection against mortality was observed in BAYK-8644 induced convulsions. Finally, whole cell patch clamp analysis suggested an inhibitory effect of piperine on Na+ channels. Together, our data suggest Na+ channel antagonist activity as a contributor to the complex anticonvulsant mechanisms of piperine.
Keywords: alkaloids, anticonvulsant activity, anticonvulsant mechanism, Chemoconvulsants, Na+ and Ca2+ channel, piperine
Introduction
Epilepsy is one of the most common neurological conditions, characterized by an enduring predisposition to generate epileptic seizures. It is highly prevalent, affecting more than 70 million people of different age groups worldwide.1 Despite the increasing availability of newer anticonvulsant drugs, epilepsy remains a continuing health concern. Only two-thirds of patients with epilepsy have their seizures controlled with the available antiepileptic drugs, and the remaining patients remain refractory.2 Moreover, the available anti-epileptic drugs are also associated with certain psychiatric side effects.3 Hence, new strategies for managing seizures are desirable. In this regard, medicinal plants can be an important source for the development of new anticonvulsant drugs.4 Since the last decade, a growing body of evidence has established that natural products may be the single most productive source of leads for the development of modern drugs. Additionally, numerous herbal plants and their active phytoconstituents are reported to have anticonvulsant activity.5 Piperine is a major alkaloid obtained from dried ripe fruits of the Piper genus (Piperaceae family). Piperine has undergone a few scattered anticonvulsant pharmacological evaluations owing to its traditional use in epilepsy.6-9 However, the mechanisms that underlie the anticonvulsant effect of piperine are not well understood. Therefore, the present study was designed to explore anticonvulsant mechanisms of piperine using in-silico, in-vivo and in-vitro approaches.
Results
In-silico PASS prediction
In-silico prediction of piperine using PASS suggested its potential as an anticonvulsant (Table 1). The probable mechanism of anticonvulsant activity could be its sodium/calcium channel antagonistic, GABA receptor agonistic, or kainate receptor antagonistic properties. The precise mechanism of anticonvulsant activity was explored in vivo using different chemoconvulsant models of convulsions and in-vitro by using electrophysiology.
Table 1.
PASS predicted activity score of piperine
| Pa | Pi | Activities |
|---|---|---|
| 0.906 | 0.009 | Membrane integrity agonist |
| 0.832 | 0.011 | Neuroprotector |
| 0.814 | 0.004 | Neurotransmitter uptake inhibitor |
| 0.740 | 0.004 | Sigma receptor agonist |
| 0.700 | 0.047 | Depression |
| 0.322 | 0.040 | Sigma 1 receptor agonist |
| 0.423 | 0.203 | Nootropic |
| 0.372 | 0.160 | 5 HT uptake stimulant |
| 0.181 | 0.021 | Nav1.8 sodium channel antagonist |
| 0.281 | 0.132 | GABA transporter 2 inhibitor |
| 0.291 | 0.173 | Dementia treatment |
| 0.179 | 0.078 | Neurodegenerative diseases treatment |
| 0.069 | 0.009 | GABA uptake inhibitor |
| 0.079 | 0.030 | Calcium channel T-type antagonist |
| 0.213 | 0.201 | Anticonvulsant |
| 0.145 | 0.139 | GABA C receptor agonist |
| 0.104 | 0.099 | Calcium channel antagonist |
| 0.038 | 0.034 | Kainate receptor antagonist |
Pentylenetetrazole seizure test
In the present study, a single intra peritoneal injection of pentylenetetrazole (PTZ; 75mg/kg) was found to induce tonic clonic convulsions (onset 1.79 ± 0.32 minutes) which culminated in 100% mortality. Diazepam (5 mg/kg, i.p.) treated animals failed to show any signs of convulsions and were protected fully from mortality. Piperine at tested doses significantly delayed the onset of tonic clonic convulsions (12.35 ± 3.04, 47.6 ± 5.27, 21.71 ± 0.95 at 5, 10 and 20 mg/kg respectively; p < 0.05). Treatment with piperine at 5 and 20 mg/kg doses failed to show protection against mortality; however piperine (10 mg/kg) resulted in 60 % protection against mortality in PTZ induced convulsions (Table 2).
Table 2.
Effects of piperine on pentylenetetrazole induced seizures
| Treatment | Dose (mg/kg) | Onset of myoclonic jerks (min) | Onset of tonic clonic convulsions (min) | % Mortality |
|---|---|---|---|---|
| Vehicle | 10 ml | 1.05 ± 0.21 | 1.79 ± 0.32 | 100 |
| Diazepam | 5 | 60 ± 0 | 60 ± 0 | 0 |
| Piperine | 5 | 4.77 ± 0.82 | 12.35 ± 3.04 | 100 |
| 10 | 18.2 ± 1.35 | 47.6 ± 5.27 | 40 | |
| 20 | 7.992 ± 0.23 | 21.71 ± 0.95 | 100 |
Significant as compared to saline at ** p < 0.01, *** p < 0.001
Maximal electroshock seizure test
A maximal electro shock of 56 mA for 0.2 s induced hind limb extension, and culminated in 100% mortality. Phenytoin (30 mg/kg, i.p.) treated animals failed to show any signs of convulsions and were fully protected against mortality. Piperine at tested doses significantly decreased the duration of hind limb extension (10.25 ± 0.41, 6.43 ± 0.61 at 10 mg/kg and 20 mg/kg respectively) in dose dependent manner. Treatment with piperine (at 5, 10 and 20 mg/kg) also protected the animals against MES induced mortality (Table 3).
Table 3.
Effect of piperine on maximal electroshock-induced seizures
| Treatment | Dose (mg/kg) | Duration of hind limb extension (s) | % mortality |
|---|---|---|---|
| Vehicle | 10 ml | 18.21 ± 0.42 | 100 |
| Phenytoin | 30 | 0 ± 0 | 0 |
| Piperine | 5 | 15.47 ± 2.30 | 20 |
| 10 | 10.25 ± 0.41 | 0 | |
| 20 | 6.43 ± 0.61 | 0 |
Significant as compared to saline at * p < 0.05
N-methyl-D-aspartate test
Treatment with NMDA (0.31µg/mice; i.c.v.) induced turning behavior in mice. The onset of turning behavior was observed at 3.5 ± 0.35 in control. Treatment with piperine (20 mg/kg) did not significantly influence the onset of turning behavior (5.75 ± 1.25) in mice as compared to control (Table 4). This suggests that piperine does not act on glutamatergic pathways.
Table 4.
Effect of piperine in different chemoconvulsant models
| Treatments | Onset of Convulsions (s) | % protection against seizures | % protection against mortality | |
|---|---|---|---|---|
| NMDA(0.31 µg/mouse) | Vehicle | 3.50 ± 0.35 | 0 | 0 |
| PIP 20 mg/kg | 5.75 ± 1.25 | 0 | 0 | |
| Picrotoxin(3.5 mg/kg) | Vehicle | 229.80 ± 10.20 | 0 | 0 |
| PIP 20 mg/kg | 1134.60 ± 147.60 | 0 | 0 | |
| Bicuculline(5 mg/kg) | Vehicle | 132.20 ± 17.40 | 0 | 0 |
| PIP 20 mg/kg | 145.20 ± 4.20 | 0 | 0 | |
| BAY K(37.5 µg/mice) | Vehicle | 6.92 ± 0.25 | 0 | 17 |
| PIP 20 mg/kg | 1800.00 ± 0.00 | 100 | 100 | |
| Strychnine(2 mg/kg) | Vehicle | 182.40 ± 7.80 | 0 | 0 |
| PIP 20 mg/kg | 879.60 ± 25.80 | 0 | 0 | |
Significant as compared to vehicle *** p < 0.001
Strychnine induced seizure test
Treatment with strychnine (2 mg/kg; i.p.) induced tonic clonic convulsions in mice. The onset of tonic clonic convulsions was at 3.04 ± 0.13 (s) and 100% mortality was observed in control. Treatment with piperine at 20 mg/kg dose significantly delayed the onset of tonic clonic convulsion (14.66 ± 0.43; p < 0.001) as compared to control animals. However, no significant difference in percentage mortality, as compared to control, was observed in piperine treated animals (Table 4).
Picrotoxin induced seizure test
Treatment with picrotoxin (3.5 mg/kg; i.p.) induced tonic clonic convulsions in mice. The onset of tonic clonic convulsions was at229.80 ± 10.20(s) and 100 % mortality was observed in the picrotoxin only group. Treatment with piperine at a dose of 20 mg/kg significantly delayed the onset of tonic clonic convulsions (1134.60 ± 147.60; p < 0.001) as compared to control. However, no significant protection against mortality was observed in piperine treated animals compared to control animals (Table 4).
Bicuculline induced seizure test
Delivery of bicuculline (0.5 mg/kg; i.p.) induced tonic clonic convulsions in mice. The average onset of tonic clonic convulsions was at 132.20 ± 17.40 seconds and 100% mortality was observed in control animals. The treatment with piperine at the 20 mg/kg dose did not delay the onset of convulsions (145.20 ± 4.20 s) compared to control. There was no significant difference in percentage mortality in piperine treated group compared to control animals (Table 4).
BAY K-8644 induced seizure test
Delivery of BAY K-8644 (37.5µg/mice; i.c.v.) induced clonic jerks in mice that received vehicle. The onset of tonic clonic convulsions was at 6.92 ± 0.25 (s), followed by 83% mortality in this group. However, no convulsions were observed in piperine (20 mg/kg, i.p.) treated animals, and 100% protection against mortality was observed in BAY K-8644 treated animals (Table 4).
Effect on GABA and serotonin levels
Acute piperine treatment was found to elevate cortical and hippocampal GABA and serotonin levels in a dose dependent manner. Piperine induced changes in GABA levels were more prominent in the cortical region as compared to the hippocampal region. At a dose of 5 mg/kg piperine significantly elevated hippocampal GABA and serotonin levels (p < 0.05), however, it did not significantly alter cortical GABA and serotonin levels. At higher doses (10 and 20 mg/kg) piperine significantly elevated cortical as well as hippocampal GABA and serotonin level in mice (Fig. 1A and B) .
Figure 1.
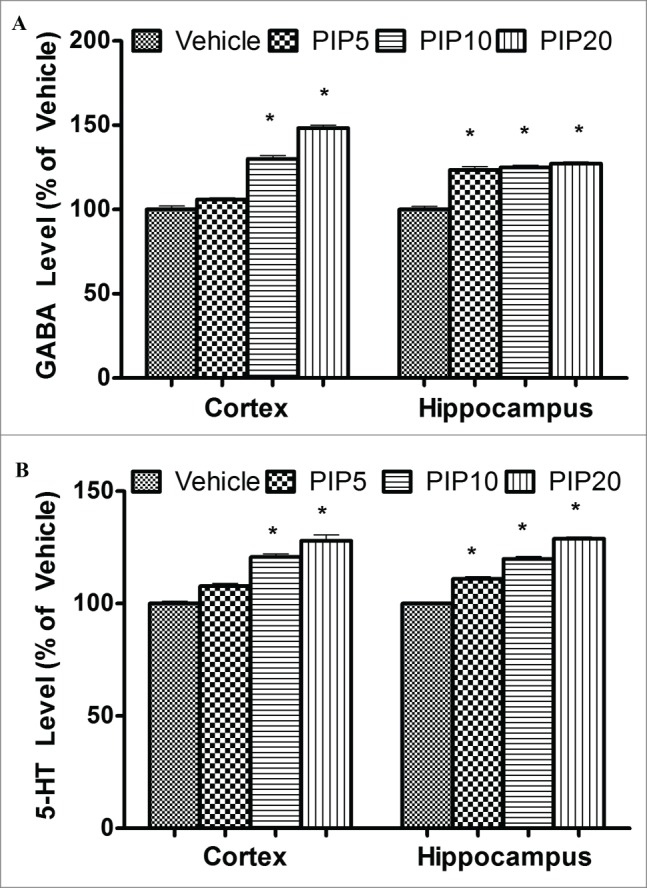
Effect of piperine on GABA and serotonin levels. (A) The bar chart depicts percentage change in GABA level in cortex and hippocampus and vehicle treated animals at different doses (in mg/kg) of piperine. (B) The bar chart depicts percentage change in serotonin levels in cortex and hippocampus at various doses (in mg/kg) of piperine. Error bars are standard errors, the asterisks denote statistical significance (p < 0.05) relative to vehicle.
Electrophysiology
A preliminary screening of 10 µM tonic piperine block of rat Cav1.2, human T-type calcium channels and the rat Nav1.4 sodium channel (rNav1.4) revealed significant block of rNav1.4. Further analysis of the biophysical parameters of rNav1.4 showed that piperine also significantly shifted the half-activation potential of the channel to a more negative voltage (Table 5, Fig. 2A). In contrast, there was no significant effect on half-inactivation voltage (Fig. 2B, Table 5). The effects of piperine on ratNav1.4 channels were dose-dependent, with an IC50 of 10 µM (Fig. 2C). We also tested the effects of piperine on other types of voltage gated channels, including the L-type calcium channel Cav1.2 and the 3 members of the T-type Cav3 calcium channel family. As shown in Figure. 2D, piperine appeared to cause slightly, but significantly greater inhibition of rNav1.4 compared to these voltage gated calcium channels (Fig. 2D).
Table 5.
Biophysical parameters of rNav1.4 sodium channels in the absence and the presence of piperine
| Va (mV) | Vh (mV) | |
|---|---|---|
| rNav1.4 wt | −26.0 | −63.3 |
| 10 µM Pip | −34.0 | −66.2 |
Significant as compared to wt * p<0.05
Figure 2.
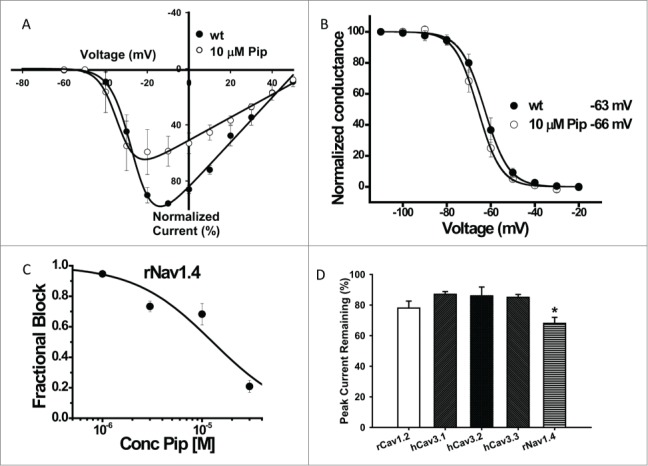
Characterization of electrophysiological effects of piperine. (A) Current voltage relations recorded prior and after application of 10 µM piperine to rNav1.4. Data from multiple paired experiments are included in the figure. Note the negative shift in half activation potential in the presence of piperine. (B) Steady state inactivation curves for rNav1.4 in the absence (wt) and presence of 10 µM piperine. No significant shift was observed. (C) Ensemble dose response curve for tonic rNav1.4 channel inhibition by piperine. The solid line is a fit via the Hill equation (IC50 10 µM ± 4 µM). (D) Effects of 10 µM piperine on rat Cav1.2, human LVA calcium channels and rat Nav1.4, (n = 4-5 per channel). Error bars reflect standard errors. The asterisk denotes that rNav1.4 block was statistically greater relative to the other channels (*p < 0.05).
Discussion
This report investigates the anticonvulsant effect of piperineand provides new insights into its mechanism of action using in silico, in vivo and in vitro approaches. In-silico PASS predictions suggested that an antagonistic effect of piperine on sodium and calcium channels and the enhancement of GABA signaling are likely candidates for explaining its anticonvulsant mechanism. Furthermore, to validate the anticonvulsant effect of piperine, gold standard tests (PTZ and MES induced convulsions) were applied in our study.
Our in-vivo studies suggested that piperine significantly delayed the onset of convulsions in the PTZ-induced convulsion test, suggesting a possible involvement of GABAA receptors. Additionally, delayed onset of convulsions in picrotoxin and strychnine induced convulsion models with piperine treatment, albeit without affecting mortality, also supports the possible involvement of GABAergic and glycinergic signaling pathways, perhaps with a common downstream pathway. Indeed, the acute effect of piperine on cortical and hippocampal GABA levels suggests that piperine treatment increased GABA levels in these area and fits with its anticonvulsant effect in the PTZ test. Previous literature reports support the involvement of GABA transmission in the anti-convulsant effects of piperine in animal models.10 Moreover, the study of da Cruz et al. also supports our findings as it suggested that piperine increases basal GABA and glycine levels in the brain.8 On the other hand, serotonergic modulation may be a possible mechanism for the protective effect in MES induced convulsions.11 Indeed, our acute study suggested that piperine facilitated serotonin release in cortex and hippocampus (in line with previous reports).12 which may result in increased seizure threshold and this facilitatory effect on serotonin release is supported by Pei.13
Interestingly, piperine elicited complete protection in convulsion induced by BAYK-8644 (an L-type voltage dependent calcium channel agonist),14 consistent with the PASS prediction that piperine may affect L-type channels. Ca2+ channel modulatory effects of piperine have previously been reported in in-vitro studies using rat hippocampal neurons.15 but in our present study, piperine only poorly blocked L-type channels in patch clamp experiments. It is possible that piperine allosterically reduces the affinity of BAYK-8644 for L-type calcium channels, thus accounting for the discrepancy between the in-vitro and in-vivo effects.
The dose dependent anticonvulsant effect of piperine in the MES induced convulsions suggested the possibility that piperine might inhibit sodium channel activity. In-silico PASS prediction had in fact predicted this possibility, and our in-vitro results suggest that piperine reduces the peak current of the Na+ channel isoform Nav1.4. We note that Nav1.4 encodes the skeletal muscle isoform of sodium channels, however, many of the antagonist sites are conserved among the sodium channel family and we thus predict that other types of sodium channels, including the neuronal ones, would also be blocked in a similar fashion.16 It is thus possible that piperine may confer its anti-convulsive properties in the animal models tested via inhibiting sodium channel activity. Further experiments on sodium channels associated with epilepsy will need to be carried out in order to test this hypothesis. We also note that the TRPV1 (transient receptor potential vanilloid 1) receptor has recently been reported to be involved in the epileptogenesis process. TRPV1 is a non-selective cation channel with high Ca2+ permeability,17 and activation of this receptor typically promotes glutamate release by increasing the excitability of neurons and synaptiC-terminals.18-21 Piperine has been shown to inhibit TRPV1 receptors, and this activity may thus also contribute to the anti-convulsant action of this compound,22,23 perhaps in conjunction with sodium channel block.
Altogether, our analysis suggests multiple anticonvulsant mechanisms of piperine. The data also highlight the potential utility of piperine and its derivatives as anticonvulsant agents due to its multifaceted mechanism for management of seizures with low chances for development of resistance.
Experimental Procedures
Animals
The studies were carried out on male Swiss Albino mice (22-28g weight) and were obtained from the breeder (Chaudhary Charan Singh Haryana Agricultural University, Hisar, Haryana, India). Swiss Albino mice were housed in standard cages at room temperature (22 ± 2°C), under natural light/dark cycle and had free access to water and food (standard laboratory pellets) before the experiments. The mice were acclimatized at lab conditions for 5 days before the start of the experiment. All the experimental work was carried out from 08:00 to 16:00 h.
Drugs and solutions
All standard chemicals used in this study were of analytical grade. Piperine, N-methyl-D-aspartate (NMDA), pentylenetetrazole (PTZ), picrotoxin, strychnine, +/− BAY K-8644 (Sigma-Aldrich Co, St. Louis, MO, USA), phenytoin and diazepam (Jackson chemicals, Amritsar, India), DMSO (Qualigens Fine Chemicals, Mumbai, India) were used in present study. All the chemicals were dissolved in 0.9% saline except piperine (0.05% DMSO), BAY K-8644 (20% tween 80). Vehicle used in this study was 0.05% DMSO.
In-silico PASS prediction
The in silico predictions were carried out to get biological activity spectra of piperine using a retrained version of a computer program Prediction of Activity Spectra for Substances (PASS; 2012.10.22).24,25 This software estimates the predicted activity spectrum of a compound as probable activity (Pa) and probable inactivity (Pi). Prediction of this spectrum by PASS is based on structure activity relationship analysis of the training set containing more than 205,000 compounds exhibiting more than 3750 kinds of biological activities. Being probabilities, the Pa and Pi values vary from 0.000 to 1.000. The PASS prediction results were interpreted and used in a flexible manner: (i) only activities with Pa > Pi are considered as possible for a particular compound; (ii) ifPa > 0.7, the chance to find activity is experimentally high; (iii) if Pa is >0.5 but less than <0.7, the chance to find activity is experimentally low, but the compound is probably different to known pharmaceutical agents; (iv) if Pa < 0.5, the chance to find activity is experimentally is low, but the chance to find a structurally new chemical compound is high.
Pentylenetetrazole-induced seizure test
Mice were divided into 5 groups each containing 5 animals, and received either vehicle, piperine (5, 10, 20 mg/kg, i.p.) or diazepam (5 mg/kg, i.p.). Thirty minutes later seizures were induced by the pentylenetetrazole (75 mg/kg, i.p.). The animals were observed during the first 60 min for the onset of myoclonic jerks and tonic clonic convulsions and percent protection against mortality.26
Maximal electroshock-induced seizure test
Mice were divided into 5 groups each containing 5 animals, and received either vehicle, piperine (5, 10, 20 mg/kg, i.p.) or phenytoin (30 mg/kg, i.p.). Thirty minutes later seizures were induced by a current stimulus (56 mA, 50 Hz for 0.2 s) delivered by transauricular alligator clip electrodes using Digital Electroconvulsiometer (Rolex, India).The percent protection and onset of hind limb extension (i.e. the hind limbs of animals outstretched at 180° relative to the plane of the body axis) and recovery from seizures was observed for first 60 seconds. Protection was defined as complete protection of hind limb extension.26
N-methyl-D-aspartate test
Mice were divided into 2 groups, each containing 5 animals, and were pretreated with either vehicle or piperine (20 mg/kg, i.p.). Turning behavior was induced in mice by 0.31 µg/10µl i.c.v. injection of NMDA, 30 min after pretreatment each animal was observed for the next 30 minutes. Animals that did not exhibit turning behavior within 30 minutes were declared protected. Turning behavior was characterized as 2 consecutive 360°cycles completed by the same animal.
Strychnine induced seizure test
Mice were divided into 2 groups, each containing 5 animals, and pretreated with either vehicle or piperine (20 mg/kg, i.p.). Tonic clonic convulsions were induced in mice by strychnine (2mg/kg, i.p.), 30 min after pretreatment and mice were observed for next the 30 minutes. Animals that did not exhibit any tonic clonic convulsion within 30 minutes were considered protected.27
Picrotoxin induced seizure test
Mice were divided into 2 groups, each containing 5 animals, and pretreated with either vehicle or piperine (20 mg/kg, i.p.). Tonic clonic convulsions were induced in mice by picrotoxin (5 mg/kg, i.p), 30 min after pretreatment and mice were observed for next the 30 minutes. Animals that did not exhibit any tonic clonic convulsion within 30 minutes were considered protected.28
Bicuculline induced seizure test
Mice were divided into 2 groups, each containing 5 animals, and pretreated with either vehicle or piperine (20 mg/kg, i.p.).Tonic clonic convulsions were induced in mice by bicuculline (0.5mg/kg, i.p), 30 min after pretreatment and mice were observed for next the 30 minutes. Animals that did not exhibit any tonic clonic convulsion within 30 minutes were declared protected.
BAY K-8644 induced seizures test
Mice were divided into 2 groups, each containing 5 animals, and pretreated with either vehicle or piperine (20 mg/kg, i.p.). Clonic jerks were induced in mice by 37.5 µg/10µl i.c.v. injection of +/− BAY k-8644, 30 min after pretreatment and, mice were observed for the next 30 minutes. Animals that did not exhibit any tonic clonic convulsion within 30 minutes were declared protected.29
Effect on GABA and Serotonin levels
To determine the effect of piperine on GABA and serotonin level in cortex and hippocampus, 3 groups of animals (n = 6) were administered with piperine (5, 10 and 20 mg/kg; i.p.). After 30 min all animals were sacrificed to isolate their brain. Cortical and hippocampal GABA and serotonin levels were estimated using HPLC-FD method, previously standardized in our laboratory.30,31,32
cDNA constructs
Human Cav3.2 and rat Cav1.2 cDNA constructs, as well as ancillary calcium channel subunit cDNAs were kindly provided by Dr. Terrance Snutch (University of British Columbia, Vancouver, Canada). Human Cav3.3 was obtained from Dr. Arnaud Monteil (CNRS Montpelllier, France), human Cav3.1 was described previously,33 and rat Nav1.4 was obtained from Dr. Robert French (University of Calgary, Canada). All chemicals were dissolved in dimethyl sulfoxide (DMSO) at the stock concentrations of 10 mM or 30 mM. Dilutions were made in external recording solutions so that the final concentration of DMSO was 0.1% or less. Channel currents were not affected by 0.1% DMSO.
tsA-201 cell culture and transfection
Human embryonic kidney tsA-201 cells were cultured and transfected using the calcium phosphate method as described previously.34 Enhanced green fluorescent protein (EGFP) DNA (0.5 µg of EGFP; Clontech) was transfected as a marker. For experiments involving L-type calcium channels, Cav1.2 α1 subunits (3 µg), were each co-transfected with rat β1b (3 µg) and rat α2δ(3 µg). For experiments involving Nav1.4 and T-type calcium channels, α1subunits were transfected alone (6 µg). Cells were resuspended with 0.25% (w/v) trypsin-EDTA (Invitrogen) and plated on glass coverslips a minimum of 3 to 4 hours before patching and kept at 37°C and 5% CO2.
Electrophysiology
Whole-cell voltage-clamp recordings on tsA- 201 cells were performed at room temperature 2 to 3 days after the transfection. The external recording solution for all calcium channel recordings contained (in mM): 114 CsCl, 20 BaCl2, 1 MgCl2, 10 HEPES, 10 Glucose, adjusted to pH 7.4 with CsOH. The external recording solution for all sodium channel recordings contained (in mM): 137 NaCl, 4 KCl, 1.8 CaCl2,1 MgCl2, 10 HEPES, 10 Glucose, adjusted to pH 7.4 with NaOH. For all recordings, the internal patch pipette solution contained (in mM): 126.5 CsMeSO4, 2 MgCl2, 11 EGTA, 10 HEPES adjusted to pH 7.3 with CsOH. The internal solution was also supplemented with 0.6 mM GTP and 2 mM ATP, which were added directly to the internal solution immediately before use. Liquid junction potentials for the above solutions were left uncorrected.
Drugs were prepared daily in external solution and were applied locally to cells with the use of home built gravity driven microperfusion system that allows rapid solution exchanges.35 Currents were measured by conventional whole-cell patch clamp using an Axopatch 200B amplifier in combination with Clampex 9.2 software (Molecular Devices, Sunnyvale, CA). Series resistance was compensated >85% in all experiments. Any cells exhibiting more than 10% leak current were discarded. For current-voltage relation studies, the membrane potential was held at −110 mV and cells were depolarized from −60 to 50 mV in 10 mV increments. For steady-state inactivation studies, the membrane potential was depolarized by test pulses to 0 mV after 3.6-s conditioning pre-pulses ranging from −110 to −20 mV. Individual sweeps were separated by 12 seconds to allow for complete recovery from inactivation between conditioning pulses. The current amplitude obtained from each test pulse was then normalized to that observed at a holding potential of −110 mV.
Data analysis and statistics
Current-voltage relationships were fitted with the modified Boltzmann equation: I = [Gmax*(V-Erev)]/[1+exp((Va-V)/ka)], where V is the test potential, Vais the half-activation potential, Erev is the reversal potential, Gmax is the maximum slope conductance, and ka reflects the slope of the activation curve. Steady-state inactivation curves were fitted using the equation: I =1/(1 + e(V - Vh)/k), where Vh is the half-inactivation potential and k is the slope factor. Data from concentration-dependence studies were fitted with the equation y = A2 + (A1-A2)/(1 + ([C]/IC50)n) where A1 is initial current amplitude and A2 is the current amplitude at saturating drug concentrations, [C] is the drug concentration and n is the Hill coefficient. Statistical significance was determined by paired or unpaired Student's t-Tests and one-way or repeated measures ANOVA followed by Tukey's Multiple Comparison tests. All data are given as means +/− standard errors.
Funding
Funding for this study was in part provided by DST-RFBR Joint Indian-Russian Project (Grant DST/RFBR No. RUSP-1176/11-04-92713-INDa) and operating grant from the Canadian Institutes of Health Research. Awanish Mishra held a Senior Research Fellowship granted by Indian Council of Medical Research, New Delhi (Grant No. 45/33/2010/PHA-BMS), Jasmine Kaur Punia held research fellowship granted by All India Council for Technical Education, and Chris Bladen held a T. Chen Fong studentship and an AI-HS studentship award.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethics Statement
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) and the care of the animals was carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India vide protocol approval no. 107/99/CPCSEA-2010-21.
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a metaanalytic approach. Epilepsia 2010; 51:883-90; PMID:20067507; http://dx.doi.org/ 10.1111/j.1528-1167.2009.02481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342:314-9; PMID:10660394; http://dx.doi.org/ 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- 3.Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, Gamble C, Jacoby A, Shackley P, Smith DF, et al.. A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial. Health Technol Assess 2007; 11:1-134; http://dx.doi.org/ 10.3310/hta11370 [DOI] [PubMed] [Google Scholar]
- 4.Carlini EA. Plants and the central nervous system. Pharmacol Biochem Behav 2003; 3:501-12; http://dx.doi.org/ 10.1016/S0091-3057(03)00112-6 [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Madaan R, Bansal G, Jamwal A, Sharma A. Plants and plant products with potential anticonvulsant activity – a review. Pharmacog Comm 2012; 2:3-99; http://dx.doi.org/ 10.5530/pc.2012.suppl1.2 [DOI] [Google Scholar]
- 6.Juvekar MR, Kulkarni MP, Juvekar AR, “Anti-stress, nootropic and anticonvulsant potential of fruit extracts of Piper longum L.” Planta Medica 2008; 74:PA244 [Google Scholar]
- 7.Bukhari IA, Pivac N, Alhumayyd MS, Mahesar AL, Gilani AH. The analgesic and anticonvulsant effects of piperine in mice. J Physiol Pharmacol 2013. December; 64(6):789-94; PMID:24388894 [PubMed] [Google Scholar]
- 8.da Cruz GM, Felipe CF, Scorza FA, da Costa MA, Tavares AF, Menezes ML, de Andrade GM, Leal LK, Brito GA, da Graça Naffah-Mazzacoratti M, et al.. Piperine decreases pilocarpine-induced convulsions by GABAergic mechanisms. Pharmacol Biochem Behav 2013; 104:144-53; PMID:23313550; http://dx.doi.org/ 10.1016/j.pbb.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 9.D'Hooge R, Pei YQ, Raes A, Lebrun P, van Bogaert PP, de Deyn PP. Anticonvulsant activity of piperine on seizures induced by excitatory amino acid receptor agonists. Arzneimittelforschung 1996; 46:557-60; PMID:8767343 [PubMed] [Google Scholar]
- 10.Zaugg J, Baburin I, Strommer B, Kim HJ, Hering S, Hamburger M. HPLC-based activity profiling: discovery of piperine as a positive GABA(A) receptor modulator targeting a benzodiazepine-independent binding site. J Nat Prod 2010; 73:185-91; PMID:20085307; http://dx.doi.org/ 10.1021/np900656g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browning RA, Smith JK, Brandon MT. Comparison of regional brain 5-HT and 5-HIAA content in flexor and extensor rats. Pharmacol Biochem Behav 1983; 18:525-8; PMID:6191344; http://dx.doi.org/ 10.1016/0091-3057(83)90275-7 [DOI] [PubMed] [Google Scholar]
- 12.Li S, Wang C, Li W, Koike K, Nikaido T. Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J Asian Nat Prod Res 2007; 9:435-44 [DOI] [PubMed] [Google Scholar]
- 13.Pei YQ. A review of pharmacology and clinical use of piperine and its derivatives. Epilepsia 1983; 24:177-82; PMID:6832079; http://dx.doi.org/ 10.1111/j.1528-1157.1983.tb04877.x [DOI] [PubMed] [Google Scholar]
- 14.Gasior M, Kleinrok Z, Czuczwar SJ. Influence of BAY k-8644, a calcium channel agonist, on the anticonvulsant activity of conventional anti-epileptics against electroconvulsions in mice. Neuropharmacology 1995; 34:433-8; PMID:7566475; http://dx.doi.org/ 10.1016/0028-3908(95)00004-P [DOI] [PubMed] [Google Scholar]
- 15.Fu M, Sun ZH, Zuo HC. Neuroprotective effect of piperine on primarily cultured hippocampal neurons. Biol Pharm Bull 2010; 33:598-603; PMID:20410592; http://dx.doi.org/ 10.1248/bpb.33.598 [DOI] [PubMed] [Google Scholar]
- 16.Fozzard HA, Lee PJ, Lipkind GM. Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr Pharm Des 2005; 11:2671-86; PMID:16101448; http://dx.doi.org/ 10.2174/1381612054546833 [DOI] [PubMed] [Google Scholar]
- 17.Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci 2009; 32:215-24; PMID:19285736; http://dx.doi.org/ 10.1016/j.tins.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 18.Schobel N, Radtke D, Lubbert M, Gisselmann G, Lehmann R, Cichy A, Schreiner BS, Altmuller J, Spector AC, Spehr J, et al.. Trigeminal ganglion neurons of mice show intracellular chloride accumulation and chloride-dependent amplification of capsaicin-induced responses. PLoS One 2012; 7:e48005; PMID:23144843; http://dx.doi.org/ 10.1371/journal.pone.0048005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 2010; 13:1511-8; PMID:21076423; http://dx.doi.org/ 10.1038/nn.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 2008; 57:746-59; PMID:18341994; http://dx.doi.org/ 10.1016/j.neuron.2007.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 2011; 31:14024-31; PMID:21957263; http://dx.doi.org/ 10.1523/JNEUROSCI.2081-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CY, Li W, Qu KP, Chen CR. Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur J Pharmacol. 2013; 714:288-94; PMID:23911889; http://dx.doi.org/ 10.1016/j.ejphar.2013.07.041 [DOI] [PubMed] [Google Scholar]
- 23.McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J Pharmacol 2005; 144:781-90; PMID:15685214; http://dx.doi.org/ 10.1038/sj.bjp.0706040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goel RK, Singh D, Lagunin A, Poroikov V. PASS-assisted exploration of new therapeutic potential of natural products. Med Chem Res 2011; 20: 1509-14; http://dx.doi.org/ 10.1007/s00044-010-9398-y [DOI] [Google Scholar]
- 25.Chaudhary A, Das P, Mishra A, Kaur P, Singh B, Goel RK. Naturally occurring himachalenes to benzocycloheptene amino vinyl bromide derivatives: as antidepressant molecules. Mol Divers 2012; 16:357-66; PMID:22584731; http://dx.doi.org/ 10.1007/s11030-012-9372-3 [DOI] [PubMed] [Google Scholar]
- 26.Singh D, Singh B, Goel RK. Role of saponins for the anticonvulsant effect of adventitious roots of Ficusreligiosa. Pharm Biol 2012; 50:816-22; PMID:22471888; http://dx.doi.org/ 10.3109/13880209.2011.636057 [DOI] [PubMed] [Google Scholar]
- 27.Amabeoku G, Chandomba R. Strychnine-induced seizures in mice: the role of noradrenaline. Prog. Neuropsychopharmacol. Biol Psychiatry 1994; 18:753-63 [DOI] [PubMed] [Google Scholar]
- 28.Singh D, Goel RK. Anticonvulsant effect of Ficus religiosa: Role of serotonergic pathways. J Ethnopharmacol 2009; 123:330-4; PMID:19429380; http://dx.doi.org/ 10.1016/j.jep.2009.02.042 [DOI] [PubMed] [Google Scholar]
- 29.Kaur M, Goel RK. Anticonvulsant activity of Boerhaavia diffusa: Plausible role of calcium channel antagonism. Evid Based Complement Alternat Med 2011:310420(1-7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra A, Goel RK. Psychoneurochemical Investigations to Reveal Neurobiology of Memory Deficit in Epilepsy. Neurochem Res 2013; 38:2503-15; PMID:24100926; http://dx.doi.org/ 10.1007/s11064-013-1163-4 [DOI] [PubMed] [Google Scholar]
- 31.Singh D, Mishra A, Goel RK. Effect of saponin fraction from Ficus religiosa on memory deficit, and behavioral and biochemical impairments in pentylenetetrazol kindled mice. Epilepsy Behav 2013; 27:206-11; PMID:23332444; http://dx.doi.org/ 10.1016/j.yebeh.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Mishra A, Goel RK. Adjuvant anticholinesterase therapy for the management of epilepsy induced memory deficit: A critical preclinical study. Basic Clin Pharmacol Toxicol 2014; 115:512-7; PMID:24890882; http://dx.doi.org/ 10.1111/bcpt.12275 [DOI] [PubMed] [Google Scholar]
- 33.Beedle AM, Hamid J, Zamponi GW. Inhibition of transiently expressed low- and high-voltage-activated calcium channels by trivalent metal cations. J Membr Bio 2002; 187:225-38; http://dx.doi.org/ 10.1007/s00232-001-0166-2 [DOI] [PubMed] [Google Scholar]
- 34.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, et al.. ORL1 receptor-mediated internalization of N-type calciumchannels. Nat Neurosci 2006; 9:31-40; PMID:16311589; http://dx.doi.org/ 10.1038/nn1605 [DOI] [PubMed] [Google Scholar]
- 35.Feng ZP, Doering CJ, Winkfein RJ, Beedle AM, Spafford JD, Zamponi GW. Determinants of inhibition of transiently expressed voltage-gated calcium channels by ω-conotoxins GVIA and MVIIA. J Biol Chem 2003; 278:20171-8; PMID:12654924; http://dx.doi.org/ 10.1074/jbc.M300581200 [DOI] [PubMed] [Google Scholar]


