Abstract
Objectives
Recent research has linked psychological (personality) factors and specific genetic risk polymorphisms to performance on neurocognitive phenotypes. We examined whether episodic or semantic memory performance is associated with (a) three personality traits (i.e., neuroticism, extraversion, openness to experience), (b) two neurodegenerative-related polymorphisms (i.e., Apolipoprotein E (APOE; rs7412; rs429358), Clusterin (CLU; rs11136000)), and (c) cross-domain risk interactions (magnification effects).
Methods
Linear growth models were examined to test independent associations between personality traits and declarative memory performance, and potential interaction effects with APOE and CLU genetic risk. Normal older adults (n = 282) with personality and genetic data from the Victoria Longitudinal Study were included at baseline and for up to 14 years of follow-up.
Results
First, we observed that higher openness to experience levels were associated with better episodic and semantic memory. Second, three significant gene × personality interactions were associated with poorer memory performance at baseline. These synergistic effects are: (a) APOE allelic risk (ε4+) carriers with lower openness to experience levels, (b) CLU (no risk: T/T) homozygotes with higher extraversion levels, and (c) CLU (no risk: T/T) homozygotes with lower neuroticism levels.
Conclusions
Specific neurodegenerative-related genetic polymorphisms (i.e., APOE and CLU) moderate and magnify the risk contributed by selected personality trait levels (i.e., openness to experience, extraversion) on declarative memory performance in non-demented aging. Future research could target interactions of other personality traits and genetic polymorphisms in different clinical populations for predicting other neurocognitive deficits or transitions to cognitive impairment and dementia.
Keywords: Personality traits, Genetic risk, Apolipoprotein E, Clusterin, Memory, Victoria Longitudinal Study
Introduction
The roles of both personality characteristics and genetic influences on non-demented cognitive aging and neurodegenerative diseases have been researched with growing breadth and clarity (Belsky et al, 2009; Harris et al., 2011). Regarding genetic factors, research has linked risk polymorphisms with multifaceted clinical phenotypes (i.e., Alzheimer’s disease (AD)) and neurocognitive performance in normal aging (Deary et al., 2004). Arguably, the magnitude of risk conveyed independently by AD-related single nucleotide polymorphisms (SNPs) may have implications for the timing, trajectories, and potential for interactive or intensification effects in non-demented cognitive aging (Harris et al., 2011; Lindenberger et al., 2008). Specifically, neurodegenerative-related polymorphisms may influence cognitive performance and change in normal aging not only independently but also in particular constellations of interactions, especially those that involve combinations of risk from more than one factor (Belsky et al., 2009; Sapkota et al., 2015). Personality traits constitute an important (but understudied) domain of influence on cognitive performance and decline in non-demented aging. Conceivably, some vulnerability traits may also operate interactively with genetic or environmental factors to magnify the deleterious effects of biological influence in older adults (Eaton et al., 2012; Lindenberger et al. 2008). In the present study, we examine three personality traits and two AD-related SNPs for their independent and interactive effects on declarative memory performance among older adults.
Consistently linked genetic risk factor for sporadic AD is the Apolipoprotein E (APOE; rs7412; rs429358) gene. APOE ε4 allele is connected with increased risk of AD-related dementia (Farrer et al., 1997), but also normal cognitive decline (Small et al., 2004) and mild cognitive impairment (MCI) (Brainerd et al., 2011). We examine a second SNP prominently linked to AD and normal cognitive aging, Clusterin (CLU; rs11136000). Although more commonly associated with AD, in a recent study, cognitive normal CLU C+ carriers showed steeper memory decline than non-carriers who converted to MCI (Thambisetty et al., 2013) and APOE may influence CLU levels in the frontal lobe in AD (Harr et al., 1996; Nuutinen et al., 2009; Wu et al., 2012). Although the mechanisms linking the two genes and cognition remain unclear (Nuutinen et al., 2009), we hope to add to the literature by examining the two genes in the same study and in interaction with personality traits.
Genetic association studies have been useful in identifying the degree of risk associated with some alleles in selected SNPs for cognitive changes with aging (Kremen et al., 2011). However, for many neurocognitive phenotypes, the consideration of personal attributes and lifestyle activities may supplement or modify the observed roles of biological (genetic) factors (Runge et al., 2014; Sachs-Ericsson et al., 2010; Shanahan et al., 2005). Personality traits encompass a wide range of behaviors as typically summarized in a specific set of dimensions or patterns characteristic to given individuals and they are generally stable in adulthood (Soubelet and Salthouse, 2011). Personality trait differences also determine how older adults manage psychological stress (Grant and Langan-Fox, 2007), which is associated with hippocampal atrophy (Gallagher et al., 1996) and accelerated aging due to dysregulation of the hypothalamus-adrenal cortex (McEwen, 1998). Thus, among older adults with personality trait differences similar stressors may result in wide range of behaviors and nervous system changes (Grant and Langan-Fox, 2007). For example, adults with higher neuroticism levels are at increased susceptibility to suicide (Wiktorsson et al., 2013) or depression (Duberstein et al., 2008). Low neuroticism with high extraversion levels has also been linked to lower dementia incidence among some older adults (Wang et al., 2009). A recent study compared cognitive function in older adults with the APOE risk genotype (ε4+) as moderated by personality trait (Dar-Nimrod et al., 2012a). Notably, APOE ε4+ allele carriers with higher neuroticism scores performed lower on the cognitive portion of the AD assessment scale (ADAS) as compared to APOE ε4+ carriers with lower neuroticism scores. In a subsequent study, APOE ε4+ carriers with high levels of neuroticism and extraversion showed worse performance on the ADAS (Dar-Nimrod et al., 2012b). Personality traits linked to clinically significant depression (Duberstein et al., 2008) may be involved in cognitive decline. Examining personality traits by genes may help clinicians identify older adults who are at a magnified risk for both cognitive decline and psychiatric conditions (Byers and Yaffe, 2011).
Three research questions were examined in the present study. First, are levels of neuroticism, extraversion, and openness to experience (openness) associated with baseline performance and longitudinal change in declarative memory? We predicted that (a) higher levels of neuroticism would be associated with poorer memory performance. In contrast, higher levels of (b) extraversion and (c) openness would be associated with better memory performance. Second, do allelic risk carriers for APOE ε4+ and CLU C+ perform poorly on memory tasks more than their lower genetic risk counterparts? Third, will specific gene × personality interactions influence initial and longitudinal memory performance? We expected APOE ε4+ and CLU C+ carriers with higher levels of neuroticism, and lower levels of extraversion and openness to have the worst overall performance compared to their counterparts. To our knowledge, this is the first study to examine personality by genetic interactions for declarative memory performance and change in non-demented older adults.
Method
Participants
Participants from the Victoria Longitudinal Study (VLS), a large-scale and multifactorial investigation of biomedical and neurocognitive aspects of aging were enrolled through advertisements and received a small honorarium for their participation. Written informed consent was obtained from all participants and all VLS data are collected with the approval from the human/institutional research ethics board. Our subsample is comprised of surviving members of two VLS cohorts who were present for the VLS genetic initiative in 2009–2011 (Supplementary Table S1). We linked eligible participants’ newly obtained genetic, existing personality, and five-wave (14-year) longitudinal memory data. Specifically, we combined volunteers from Sample 1 and Sample 2 (n = 282; baseline age: 64.88 (5.45) years; 65.6% female) (Table 1). Mean interval between all waves for Sample 1 was 3.10 years and Sample 2 was 3.92 years. Participants with any missing cognitive data were included and handled using maximum likelihood in Mplus 7 (Muthén and Muthén, 1998–2012). Further information regarding the general VLS participant recruitment and testing procedures (Dixon and de Frias, 2004a) and personality assessment (Small et al., 2003) are available.
Table 1.
Descriptive characteristics (mean and standard deviation) for personality trait and genetic measures.
| Total | |
|---|---|
| n | 282 |
| Age (years) | 64.88 (5.45) |
| Education (years) | 14.89 (3.05) |
| Gender (M/F) | 97/185 |
| Personality | |
| Neuroticism | 77.17 (20.72) |
| Extraversion | 101.25 (16.57) |
| Openness to Experience | 115.50 (17.64) |
| Genes | |
| APOE | ε4− = 202; ε4+ = 66 |
| CLU | T/T = 42; C+ = 239 |
n, Total number; APOE, Apolipoprotein E (ε4− = no risk/ ε4+ = risk); CLU, Clusterin (T/T = no risk/C+ = risk).
DNA Extraction and Genotyping
Saliva was collected according to standard procedures from Oragene-DNA and stored at room temperature in Oragene® disks until DNA extraction. Genotyping was carried out using a PCR-RFLP strategy to analyze the allele status for APOE (rs7412, rs429358) and CLU (rs11136000) (Mcfall et al., 2013; 2015). Both genotype frequencies were in Hardy-Weinberg equilibrium: CLU (χ2 = 0.21, p = 0.6457) and APOE (χ2 = 0.065, p = 0.799). For purposes of analyses, we included two allelic combinations (risk and no risk) with past reports showing that being a carrier of one allelic risk is considered to be at risk (Harold et al., 2009): APOE (risk: ε4+; no risk: ε4−) and CLU (risk: C+; no risk: T/T).
Measures
Episodic Memory: Word List Recall
From a pool of six equivalent lists, two different but comparable lists of 30 English words (Dixon et al., 2004b) were used. Participants were given two minutes to study the list and five minutes to write down their answers. The total numbers of words correctly recalled from each list was averaged and used as the final score.
Semantic Memory: Vocabulary
The total number of correct answers from three 18-items series of tests in the Educational Testing Service kit (Ekstrom et al., 1976) with 54 multiple-choice vocabulary questions was obtained for a final score.
NEO-PI
The NEO-PI (Costa et al., 1985) was used at baseline for all participants to assess the five domains of personality traits: neuroticism, extraversion, openness, conscientiousness, and agreeableness. Based on previous literature (Dar-Nimrod et al., 2012a), we examined neuroticism, extraversion, and openness traits for the present study. The full questionnaire consisted of 181 statements. Participants were required to answer based on how much they agreed with each statement from strongly disagree to strongly agree on a 5-point Likert scale. The present study used a subset of participants from the Small et al. (2003) study, which reported high Cronbach’s alpha for all three traits in both samples.
Statistical Analyses
Descriptive statistics and means were calculated using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA) (Table 1; Table 2). Linear growth models were used to analyze all research questions in Mplus 7. All missing values for cognitive measures were assumed to be missing at random and handled using maximum likelihood. Missing predictor values were handled using list-wise deletion. Only one participant was missing genetic information for CLU and 14 adults with APOE ε2/ε4 alleles were excluded. Risk alleles were coded as −0.56 and no risk alleles as 0.44 to avoid multicollinearity with gene by personality predictors. Age was entered as a continuous variable to rule out differences in cognitive performance and changes associated with baseline age.
Table 2.
Descriptive characteristics (M and SD) for word list recall and vocabulary for all five waves.
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | |
|---|---|---|---|---|---|
| n | 282 | 266 | 255 | 239 | 200 |
| Episodic Memory | |||||
| Word List Free Recall | 18.86 (3.75) | 19.12 (3.82) | 18.64 (3.90) | 17.89 (4.28) | 16.62 (4.78) |
| Semantic Memory | |||||
| Vocabulary | 44.89 (5.78) | 44.96 (5.33) | 44.75 (5.31) | 44.29 (5.18) | 43.90 (5.32) |
n, Total number; M, Mean; SD, Standard deviation.
We established a latent growth model of change over five waves for word recall and vocabulary by examining the best fitting growth models in a recommended order (McFall et al., 2014): (a) a fixed intercept model, (b) a random intercepts model, (c) random intercept, fixed slope model, (d) random intercept, random slope model, and (e) random intercept, random slope, fixed quadratic model. The best fitting baseline change model was determined by examining several fit statistics. The chi-square test of model fit (χ2; p > .05) allowed for an overall indication of good model fit. Additional absolute/comparative fit indices were also examined (Kline, 2011; Little, 2013): root mean square error of approximation ≤ .05, comparative fix index ≥ .95, and standardized root mean square residual ≤ .08. Following the examination of model fit, the χ2 difference statistic was calculated to detect an improvement in fit with the addition of free parameters at each step. The best baseline model of change for word recall and vocabulary was obtained with the random intercept, random slope, and fixed quadratic model (Supplementary Table S2).
Next, each of the three personality traits and two SNPs were added as a time invariant predictor to test the independent effect of personality trait and allelic risk on intercept and slope. Baseline age was added as a covariate on intercept and slope in all analyses. A total of two models for each cognitive domain were examined for personality trait and SNP associations (Research Questions 1 and 2). Intercept and slope were regressed on both SNPs and personality traits. Unstandardized regression coefficients of each predictor were examined. For gene × personality interactions (Research Question 3), we calculated product terms in Mplus 7 to represent the SNP × personality trait interaction. Subsequently, intercept and slope were regressed simultaneously on personality trait, SNP, SNP × personality, and age in each model (Supplementary Table S3c). A total of twelve models were analyzed for Research Question 3.
Results
Mean level memory results for all five waves before any statistical analyses are displayed in Table 2. Age was a significant covariate in all models for Research Questions 1–3. This indicates that we accounted for interindividual differences in declarative memory performance at baseline and any changes as a result of baseline age. For Research Question 1, we observed three significant personality-memory associations. Higher openness scores were significantly associated with higher word recall (β = 0.041; SE = 0.013; p = .002) and vocabulary (β = 0.145; SE = 0.018; p < .001) performance at baseline. Higher extraversion scores predicted lower vocabulary performance (β = −0.099; SE = 0.020; p < .001) at baseline. However, we did not observe any significant associations with neuroticism. We observed stability of personality-declarative memory associations over five waves (Supplementary Table S3a). For Research Question 2, independent effects of APOE and CLU did not significantly predict baseline or change in memory performance (Supplementary Table S3b).
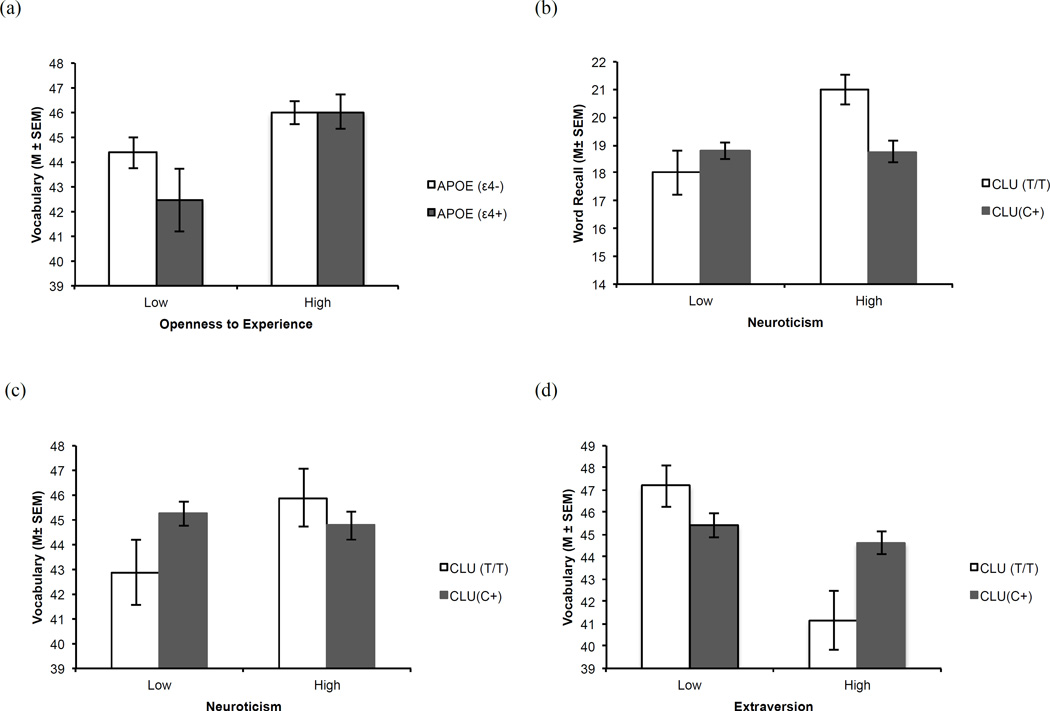
The key analyses were conducted for Research Question 3. We observed four significant gene by personality interactions at baseline. First, a significant interaction between APOE and openness predicted vocabulary performance. Not only did the adults with lower openness levels have poorer vocabulary performance but this effect was magnified for APOE ε4+ carriers (β = −0.088; SE = 0.040; p = .029) (Figure 1a). The remaining significant interactions involved the CLU polymorphism with neuroticism and extraversion. Second, the CLU × neuroticism interaction significantly predicted word recall and vocabulary performance. Specifically, CLU T/T homozygotes with higher neuroticism levels had the best word recall (β = 0.078; SE = 0.028; p = .005) (Figure 1b) and vocabulary performance (β = 0.134; SE = 0.042; p = .001) (Figure 1c). In contrast, CLU homozygotes with lower neuroticism levels had the worst performance. Third, a CLU × extraversion interaction significantly predicted vocabulary performance. In this interaction, CLU T/T homozygotes with lower extraversion levels had the best vocabulary performance. In contrast, CLU homozygotes with higher extraversion levels showed the worst performance (β = −0.118; SE = 0.050; p = .018) (Figure 1d). Longitudinally, we observed stability in SNP × personality trait associations on memory (Supplementary Table S3c).
Figure 1.
Personality by gene interaction effects for baseline declarative memory performance: (a) Adults with low openness to experience levels had poorer vocabulary performance and this effect was magnified for those with APOE allelic risk (ε4+). (b) CLU T/T homozygotes in the low neuroticism group had poorer word recall performance than CLU T/T homozygotes in the high neuroticism group. (c) CLU T/T homozygotes in the low neuroticism group had poorer vocabulary performance than CLU T/T homozygotes in the high neuroticism group. (d) CLU T/T homozygotes in low extraversion group had the best vocabulary performance, whereas those in the high extraversion group had the worst performance.
(Note: High and low represent those above and below the mean personality trait score).
Discussion
We examined interactive associations of three targeted personality traits with APOE and CLU genotypes for baseline performance and change on episodic and semantic memory in non-demented older adults. Although previous studies have observed significant independent associations of personality traits (Grahman et al., 2012) and SNPs (Small et al., 2004; Thambisetty et al., 2013) with memory, we addressed the integration (Dar-Nimrod et al., 2012a) of these two trends. We expected patterns of association that would be consistent with the magnification of vulnerability effects, whereby lower memory performance would be associated with combined risk from both domains. We review the patterns below.
For the three-targeted traits (neuroticism, extraversion, openness), we observed three independent personality-memory associations consistent with the vulnerability hypothesis. Specifically, adults with lower scores on the openness trait had lower baseline word recall and vocabulary performance. Openness-memory associations may also mediated by different cognitive activity levels (Hogan et al., 2012) so lifestyle activities should be examined with the openness trait in future investigations of personality influences on cognitive performance.
Less directly affiliated with the vulnerability hypothesis was the result with extraversion. Higher extraversion levels were associated with worse performance on vocabulary at baseline. Previous studies have reported that older adults with higher extraversion levels have faster performance on speed tasks (Pearman, 2009) but poorer performance on semantic memory (Baker and Bichsel, 2006) or global cognition (Chapman et al., 2012). In the latter two cases, remembering and recall are required, whereas speed is typically a fluid-type reaction time performance. Thus, high extraversion levels may predispose older adults to be more alert in situations requiring quick performance but do not support performance on tasks requiring correct recall of new or cultural information, as observed in our study. Observation of vulnerability effects for extraversion may be dependent on the neurocognitive domain involved or other aspects of the performance situation.
For the neuroticism trait, we observed no significant associations with memory. Past studies with non-demented older adults have reported mixed findings including no associations between neuroticism and crystalized intelligence (Baker and Bichsel, 2006) and three-year global cognitive decline (Jelicic et al., 2003). Conceivably, the range of neuroticism levels in the present study may have been restricted and fairly low, at least compared with the cognitively impaired groups, dementia populations, or groups with existing personality disorder or increased risk for psychiatric conditions (Meins and Dammast, 2000; Wiktorsson et al., 2013). Further, we examined only neuroticism at baseline (Small et al., 2003), so future work could test changes in aging. Although healthy older adults are relatively stable in their personality traits (Soubelet and Salthouse, 2011) and the larger sample from which the present subsample was derived showed relatively little change in personality traits over two waves (Small et al., 2003), longitudinal studies with larger and more diverse samples for personality-cognition associations are encouraged in the future. We acknowledge that our personality trait measure was only tested at baseline and used to predict longitudinal change in memory, which may have limited our results.
We turn now to the key analyses including gene × personality interactions. We observed four interesting and novel cross-domain associations with memory performance at baseline. First, memory performances by adults with higher openness levels at baseline were not affected by APOE genotype. In contrast, APOE ε4+ carriers with lower openness levels showed poorer memory performance than the APOE ε4− group (Figure 1a). Prior research has linked higher hypothalamic-pituitary-axis activity levels to cognitive deficits (Lupien et al., 1998) in APOE ε4+ carriers (Peskind et al., 2001) and lower openness levels to poor cognitive performance (Grahman et al., 2012). This linkage directly supports our vulnerability-related interpretation. Positive correlations between openness levels and memory even in the presence of genetic vulnerability (APOE ε4+) suggest that higher openness levels may serve as a potential protective factor for cognitive decline. Adults with higher openness levels have been associated with more social lifestyle and cognitive engagement (Grahman et al., 2012). Therefore, the protective effects observed for adults with higher openness levels may be modified through their social and cognitive lifestyle.
Second, CLU T/T homozygotes were protected from the vulnerability associated with high neuroticism levels on word recall and vocabulary at baseline. Adults with CLU T/T and high neuroticism levels had the best word recall (Figure 1b) and vocabulary (Figure 1c) performance. Previous reports have shown that neuroticism levels discriminate between healthy aging and early-stage AD (Duchek et al., 2007), and an active lifestyle may act as a buffer against the negative effects of high neuroticism in at-risk adults (Wang et al., 2009). The present sample may have benefited from both healthy aging, with lower neuroticism levels, and a relatively advantaged socially engaged lifestyle (Runge et al., 2014). Whether lifestyle activities play a specific role in this gene-personality dynamic should be tested in future studies.
Third, CLU T/T homozygotes with lower extraversion levels had better vocabulary performance than those with higher extraversion levels. In contrast, extraversion levels did not influence vocabulary performance for CLU C+ carriers (Figure 1d). Similarly, previous studies have linked high extraversion levels to poorer memory performance (Baker and Bichsel, 2006; Luchetti et al., 2015). Adults with CLU allelic risk may already be at a disadvantage on memory tasks (Braskie et al, 2011) but CLU T/T homozygotes in our sample were at a magnified risk for poor vocabulary performance if they had higher extraversion levels.
We note briefly that we observed no significant independent gene-memory associations at baseline or longitudinally. Past studies are mixed but some have also shown no cognitive associations with APOE ε4+ risk carriers in non-demented populations (Bunce et al., 2014; Jorm et al., 2007; Juva et al., 2000). Similarly, cognitive decline has only been observed among CLU C+ risk carriers who eventually reached MCI status (Thambisetty et al., 2013). Different health and environmental risk factors may influence APOE and CLU-memory associations in older adults. Previous studies have reported moderation effects with gender (Mortensen and Høgh, 2001), vascular health (McFall et al., 2015), gene synergistic effects and effect modifications (Sapkota et al., 2015), or more prevalence in the dementia population (Elias-Sonnenschein et al., 2008). We conducted post-hoc analyses (a) to examine the remaining two personality traits (conscientiousness; agreeableness) independently and in interaction with APOE and CLU, and (b) re-analyze our results to test for any differences with the inclusion of APOE ε2/ε4 as risk, and gender and baseline education as covariates. We observed three significant findings, and no difference in our result with APOE ε2/ε4, gender, and education. First, high conscientiousness levels were associated with poor baseline word recall performance (β = −0.055; SE = 0.026; p = .035). Second, higher agreeableness scores were associated with steeper 12-year decline in vocabulary (β = −0.019; SE = 0.009; p = .035). Third, APOE risk carriers with high agreeableness scores had the worst word recall performance at baseline (β = 0.177; SE = 0.080; p = .028).
Several limitations of the present study should be mentioned. First, we included a non-demented homogenous group of older Caucasians from Canada. Personality traits and heritability of genes may differ among nationalities (Allik and McCrae, 2004). Future studies should test differences in socioeconomic backgrounds (Costa et al., 2001) and compare clinical populations and different ethnicities. Second, we observed a potential limitation of power as our post-hoc power analyses (Preacher and Coffman, 2006) revealed a medium effect size for all models (Supplementary Table S4). Third, although the presence of up to 14 years of longitudinal data was a design strength, the stability associated with personality and semantic memory during this period may have restricted variability in change. Conceivably, a larger sample could provide significant longitudinal results and should be examined.
Selective personality trait levels independently affected cognitive performance in non-demented aging. Moreover, cross-domain interactions showed that personality trait effects on declarative memory were moderated by APOE and CLU allelic risk. Ongoing efforts to better evaluate the potential synergistic effects of non-clinical personality characteristics and genetic risk conveyed from leading AD-related genotypes may lead to improved understanding not only of non-demented cognitive aging but also potentially earlier detection of individuals at risk for exacerbated memory decline.
Supplementary Material
Key Points.
In non-demented older adults, lower openness to experience is associated with worse episodic and semantic memory.
APOE ε4 allelic risk magnifies this association.
Acknowledgments
We thank the volunteer participants and the VLS staff for their many contributions. More information about the VLS may be found at: http://www.ualberta.ca/~vlslab/.
Financial support
This work was supported by grants from (a) the National Institutes of Health (National Institute on Aging; R01 AG008235) to Roger A. Dixon, and (b) Alberta Health Services (University Hospital Foundation) to David Westaway, Jack Jhamandas, and Roger A. Dixon. Dr. Dixon is also supported by the Canada Research Chairs program. The funding source did not have a role in the study design, data collection, statistical analysis, results interpretation, report writing, or submission decisions.
Footnotes
Conflict of Interest
All authors confirm that there is no actual or potential conflict of interest.
References
- Allik J, McCrae RR. Toward a geography of personality traits: Patterns of profiles across 36 cultures. J Cross Cult Psychol. 2004;35:13–28. [Google Scholar]
- Baker T, Bichsel J. Personality predictors of intelligence: Differences between young and cognitively healthy older adults. Pers Individ Dif. 2006;41:861–871. [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, et al. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Petersen RC, Smith GE, Taub ES. Is the apolipoprotein e genotype a biomarker for mild cognitive impairment? Findings from a nationally representative study. Neuropsychology. 2011;25:679–689. doi: 10.1037/a0024483. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Jahanshad N, Stein JL, et al. Common Alzheimer’s disease risk variant within the CLU gene affects white matter microstructure in young adults. J Neurosci. 2011;31:6764–6770. doi: 10.1523/JNEUROSCI.5794-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D, Bielak AMA, Anstey KJ, et al. APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J Gerontol B Psychol Sci Soc Sci. 2014;69:379–386. doi: 10.1093/gerona/glt103. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Duberstein P, Tindle HA, et al. Personality predicts cognitive function over 7 years in older persons. Am J Geriatr Psychiatry. 2012;20:612–621. doi: 10.1097/JGP.0b013e31822cc9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. The NEO Personality Inventory manual. Odessa, FL: Psychological Assessment Resources; 1985. [Google Scholar]
- Costa P, Terracciano A, McCrae RR. Gender differences in personality traits across cultures: Robust and surprising findings. J Pers Soc Psychol. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Dar-Nimrod I, Chapman BP, Robbins JA, et al. Gene by neuroticism interaction and cognitive function among older adults. Int J Geriatr Psychiatry. 2012a;11:1147–1154. doi: 10.1002/gps.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar-Nimrod I, Chapman BP, Franks P, et al. Personality factors moderate the associations between Apolipoprotein genotype and cognitive function as well as late onset Alzheimer’s disease. Am J Geriatr Psychiatry. 2012b;20:1026–1035. doi: 10.1097/JGP.0b013e318267016b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Wright AF, Harris SE, Whalley LJ, Starr JM. Searching for genetic influences on normal cognitive ageing. Trends Cogn Sci. 2004;8:178–184. doi: 10.1016/j.tics.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. Victoria Longitudinal Study: From cognitively aging to illustrating changes in memory compensation. Aging Neuropsychol Cogn. 2004a;11:346–376. [Google Scholar]
- Dixon RA, Wahlin Å, Maitland SB, et al. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Mem Cognit. 2004b;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Palsson SP, Waern M, Skoog I. Personality and risk for depression in a birth cohort of 70-year olds followed by 15 years. Psychol Med. 2008;38:663–672. doi: 10.1017/S0033291707002620. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Storandt M, Larsen R. The power of personality in discriminating between healthy aging and early-stage Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2007;62:353–361. doi: 10.1093/geronb/62.6.p353. [DOI] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, South SC, et al. Genes, environments, personality, and successful aging: towards a comprehensive developmental model in later life. J Gerontol A Biol Sci Med Sci. 2012;67:480–488. doi: 10.1093/gerona/gls090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JEW, Harman HH, Dermen D. Manual for the kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, et al. Predictive value of the APOE-epsilon4 allele for progression from MCI to AD-type dementia: A meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1149–1156. doi: 10.1136/jnnp.2010.231555. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease - A meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Gallaher M, Landfield PW, McEwen B, et al. Hippocampal neurodegeneration in aging. Science. 1996;274:484–485. doi: 10.1126/science.274.5287.484. [DOI] [PubMed] [Google Scholar]
- Grahman EK, Lachman ME. Personality stability is associated with better cognitive performance in adulthood: Are the stable more able? J Gerontol B Psychol Sci Soc Sci. 2012;67:545–554. doi: 10.1093/geronb/gbr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Langan-Fox J. Personality and the occupational stressor-strain relationship: the role of the Big Five. J Occup Health Psychol. 2007;12:20–33. doi: 10.1037/1076-8998.12.1.20. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1998;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease and shows evidence for additional susceptibility genes. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of Apolipoproteins E, J, and A-I in Alzheimer’s disease. J. Neurochem. 1996;66:2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn Sci. 2011;15:388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Deary IJ, Whalley LJ. Openness to experience and activity engagement facilitate the maintenance of verbal ability in older adults. Psychol Aging. 2012;27:849–854. doi: 10.1037/a0029066. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Bosma H, Ponds RWHM, et al. Neuroticism does not affect cognitive functioning in later life. Exp Aging Res. 2003;29:73–78. doi: 10.1080/03610730303704. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, et al. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Juva K, Verkkoniemi A, Viramo P, et al. APOE ε4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology. 2000;54:412–415. doi: 10.1212/wnl.54.2.412. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling, 3rd. New York, NY: Guilford; 2011. [Google Scholar]
- Kremen WS, Lyons MJ. Behavior genetics of aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7th. Boston, MA: Elsevier/Academic Press; 2011. pp. 93–107. [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, et al. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2:234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD. Longitudinal structural equation modeling. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Luchetti M, Terracciano A, Stephan Y, Sutin AR. Personality and cognitive decline in older adults: Data from a longitudinal sample and meta-analysis. J Gerontol B Psychol Sci. 2015 doi: 10.1093/geronb/gbu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Meins W, Dammast J. Do personality traits predict the occurence of Alzheimer’s disease? Int J Geriatr Psychiatry. 2000;15:120–124. doi: 10.1002/(sici)1099-1166(200002)15:2<120::aid-gps84>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- McFall GM, Wiebe SA, Vergote D, et al. ApoE and pulse pressure interactively influence level and change in the aging of episodic memory: Protective effects among ε2 carriers. Neuropsychology. doi: 10.1037/neu0000150. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GM, Wiebe SA, Vergote D, et al. IDE (rs6583817) polymorphism and pulse pressure are independently and interactively associated with level and change in executive function in older adults. Psychol Aging. 2014;29:418–430. doi: 10.1037/a0034656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EL, Høgh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57:89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7th. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: A forgotten player in Alzheimer’s disease. Brain Res Rev. 2009;61:89–104. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Pearman A. Basic cognition in adulthood: Combined effects of sex and personality. Pers Individ Dif. 2009;47:357–362. [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie C, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56:1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Coffman DL. Computing power and minimum sample size for RMSEA [Computer software] [Accessed 23 December 23, 2014];2006 May; http://quantpsy.org/ [Google Scholar]
- Runge SK, Small BJ, McFall GP, Dixon RA. APOE moderates the association between lifestyle activities and cognitive performance: Evidence of genetic plasticity in aging. J Int Neuropsychol Soc. 2014;20:478–486. doi: 10.1017/S1355617714000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs-Ericsson NJ, Sawyer KA, Corsentino EA, Collins NA, Blazer DG. APOE epsilon4 allele carriers: Biological, psychological, and social variables associated with cognitive impairment. Aging Ment Health. 2010;14:679–691. doi: 10.1080/13607860903292594. [DOI] [PubMed] [Google Scholar]
- Sapkota S, Vergote D, Westaway D, Jhamandas J, Dixon RA. Synergistic associations of catecho-O-methyltransferase and brain-derived neurotrophic factor with executive function in aging are selective and modified by apolipoprotein E. Neurobiol Aging. 2015;36:249–256. doi: 10.1016/j.neurobiolaging.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Small BJ, Hertzog C, Hultsch DF, Dixon RA. Stability and change in adult personality over 6 years: Findings from the Victoria Longitudinal Study. J Gerontol B Psychol Sci Soc Sci. 2003;58:166–176. doi: 10.1093/geronb/58.3.p166. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;1:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Soubelet A, Salthouse TA. Personality-cognition relations across adulthood. Dev Psychol. 2011;47:303–310. doi: 10.1037/a0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held LL, An Y, Kraut M, et al. Alzheimer risk variant CLU and brain function during aging. Biol Psychiatry. 2013;73:399–405. doi: 10.1016/j.biopsych.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Karp A, Herlitz A, et al. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72:253–259. doi: 10.1212/01.wnl.0000339485.39246.87. [DOI] [PubMed] [Google Scholar]
- Wiktorsson S, Berg Al, Billstedt E, et al. Neuroticism and extroversion in suicide attempters aged 75 and above and a general population comparison group. Aging Ment Health. 2013;17:479–488. doi: 10.1080/13607863.2012.749835. [DOI] [PubMed] [Google Scholar]
- Wu Z, Yu J, Li Y, Tan L. Clusterin in Alzheimer’s disease. Adv Clin Chem. 2012;56:155–173. doi: 10.1016/b978-0-12-394317-0.00011-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.