Abstract
Despite the immune reconstitution promoted by combined antiretroviral therapy (cART), lymphomas still represent the most common type of cancer in HIV-infected individuals. Cofactors related to immunodeficiency such as oncogenic viruses, chronic antigenic stimulation, and cytokine overproduction are thought to be the main drivers of HIV lymphomagenesis, although the current scenario does not convincingly explain the still-high incidence of lymphomas and the occurrence of peculiar lymphoma histotypes in HIV-infected patients under cART. Recent findings are challenging the current view of a mainly indirect role of HIV in lymphoma development and support the possibility that HIV may directly contribute to lymphomagenesis. In fact, mechanisms other than immune suppression involve biologic effects mediated by HIV products that are secreted and accumulate in lymphoid tissues, mainly within lymph node germinal centers. Notably, HIV-infected patients with lymphomas, but not those not affected by these tumors, were recently shown to carry HIV p17 protein variants with enhanced B-cell clonogenic activity. HIV p17 protein variants were characterized by the presence of distinct insertions at the C-terminal region of the protein responsible for a structural destabilization and the acquisition of novel biologic properties. These data are changing the current paradigm assuming that HIV is only indirectly related to lymphomagenesis. Furthermore, these recent findings are consistent with a role of HIV as a critical microenvironmental factor promoting lymphoma development and pave the way for further studies that may lead to the design of more effective strategies for an early identification and improved control of lymphomas in the HIV setting.
Introduction
Although the risk for AIDS-defining cancers declined dramatically after the introduction of combined antiretroviral therapy (cART), the occurrence of lymphomas in the HIV setting has not decreased to the same extent. In fact, lymphomas still represent the most common type of cancer in HIV-infected individuals, comprising more than 50% of all AIDS-defining cancers,1,2 and are the most frequent cause of death in these patients.3,4 Characteristically, these lymphomas have high-grade features, and usually present at an advanced stage, often with extranodal involvement, as shown by the marked propensity to involve the gastrointestinal tract, central nervous system (less frequently after cART), liver, bone marrow, and perinodal soft tissues.
To a certain extent, the development of lymphomas in HIV-infected patients is similar to the pathogenesis of lymphoproliferations associated with other immunodeficiency disorders, including congenital or posttransplant immunodeficiency.5 In the HIV setting, cofactors indirectly related to immunodeficiency such as oncogenic viruses, chronic antigenic stimulation, and cytokine overproduction probably play a more pronounced pathogenic role.6 Nevertheless, the picture emerging from the complex interactions of these factors is still unsatisfactory, and is unable to explain why HIV-infected patients continue to show a relatively high incidence of lymphomas and the development of histotypes with peculiar cyto-histopathological features, despite the immune reconstitution promoted by cART. Possible insights in this respect may come from recent observations that may challenge the current view, according to which HIV is mainly indirectly related to lymphoma development.
Novel findings support the possibility that HIV may directly contribute to lymphomagenesis through mechanisms other than immune suppression, involving biologic effects mediated by HIV products. In fact, recent evidence indicates that HIV-encoded proteins endowed with peculiar biologic effects on B lymphocytes are secreted and accumulate in lymphoid tissues, mainly within lymph node germinal centers, where they may act as critical microenvironmental factors promoting lymphoma development.7,8 Moreover, recent studies have shown that HIV viremia during highly active antiretroviral therapy is a predictor of HIV-associated lymphomas. Overall, these findings add further strength to the argument that HIV may directly contribute to lymphomagenesis.9,10 These data are in line with a more general relevance of microenvironment in lymphomagenesis11-13 and are opening a new field of investigation in the HIV setting with a possibly significant effect in terms of treatment/prevention of HIV-related lymphomas.
The multifaceted landscape of HIV-associated lymphomas
HIV-associated lymphomas can be grouped as those occurring also in the general population of patients (most cases), and more specifically in HIV-infected patients (approximately 5% of cases) and in other immunodeficiency disorders (<5% of cases).4,14 Table 1 lists the different types of HIV-associated lymphomas, as defined by World Health Organization (WHO), together with the major categories of lymphoid proliferations occurring in HIV-infected patients, including multicentric Castleman disease (MCD).15
Table 1.
Categories of HIV-associated lymphomas
| BL-plasmacytoid |
| Primary central nervous system lymphoma |
| DLBCL, IB-plasmacytoid |
| DLBCL, CB |
| Plasmablastic lymphoma of the oral cavity type |
| PEL and its solid variant |
| Classic PEL in the absence of tumor masses |
| Solid PEL with serous effusion |
| Solid PEL without serous effusion |
| MCD-associated large cell lymphoma |
| Hodgkin lymphoma |
| Other histotypes (rare) |
| Lymphomas of the marginal zone |
| Unclassifiable lymphomas with features intermediate between BL and DLBCL |
| Polymorphic B-cell lymphoma (PTLD-like) |
PTLD, posttransplant lymphoproliferative disorder.
The most common lymphomas arising in HIV-infected individuals include Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL). Classic Hodgkin lymphoma (cHL) is also common in the HIV setting, although the global incidence of this malignancy is lower than that of BL and DLBCL. Other lymphomas occurring in HIV-infected patients include primary effusion lymphoma (PEL) and its solid variants, lymphoma associated with Kaposi sarcoma herpesvirus (KSHV)-related MCD, and plasmablastic lymphoma (PBL) of the oral cavity type (Table 1). These 3 types of lymphomas rarely occur in the general population. These lymphomas are more specific to HIV infection,14,16 suggesting they are characterized by special pathogenic features that make them more likely to develop in the HIV setting.
Intriguingly, even lymphomas also occurring in the general population frequently show distinct cytological and histological characteristics when they arise in HIV-infected patients.4,15 In fact, HIV-associated BL may show plasmacytoid features that are only rarely displayed by HIV-unrelated cases. Moreover, the different morphological variants of DLBCL include a variant composed of centroblasts with multiple nucleoli (the centroblastic variant); a more frequent variant composed of immunoblasts with a single, prominent nucleolus (the immunoblastic variant); and a third variant with large tumor cells having anaplastic nucleus (the anaplastic variant). Immunoblastic lymphoma tends to occur in HIV-infected patients with more advanced HIV disease compared with centroblastic lymphoma. DLBCL primarily involving the central nervous system commonly belongs to the immunoblastic variant and represents the DLBCL variant associated with HIV infection, the incidence of which showed the most important decrease after the introduction of cART.
Although the incidence of HIV-associated DLBCL declined after the introduction of cART, DLBCL remains the main type of cancer in HIV-infected people.17,18 Curiously, the incidence of cHL has increased and the incidence of other HIV-associated lymphomas such as BL, PEL, and PBL of the oral cavity type remains stable. The still high incidence of lymphomas despite the immunoreconstitution promoted by cART strongly suggests that factors other than HIV-related immunosuppression are probably still acting as lymphomagenic factors in the HIV setting.4
Well-recognized pathogenic pathways
Immunodeficiency states usually increase susceptibility to cancer as a result of reduced immune surveillance and enhanced chances for virus-driven oncogenesis.19,20 The viral contribution to the development of HIV-associated malignancies has been extensively studied, but only 2 oncogenic viruses (ie, Epstein Barr virus [EBV] and KSHV) have been pathogenically associated with specific lymphoma types occurring in the HIV setting.4,21,22 Table 2 lists the lymphoid proliferations occurring in HIV-infected patients that carry infection by EBV and/or KSHV. Importantly, however, HIV-associated lymphomas are frequently associated with single or multiple genetic lesions,21,23 as shown by Table 3.24,25 Moreover, HIV is thought also to contribute to lymphomagenesis through induction of chronic B-cell activation, as a result of HIV-mediated immune dysfunction.21 Therefore, the pathogenesis of HIV-associated lymphomas is considered the result of the concerted action of different factors, mainly including impaired immune surveillance, genetic alterations, viral infection, and chronic B-cell activation.
Table 2.
Lymphoid proliferations in people with HIV/AIDS associated with infection by other viral agents
| HIV | EBV (Latency) | KSHV | |
|---|---|---|---|
| Hodgkin lymphoma | + | + (II) | — |
| BL-plasmacytoid | + | −/+ (I) | — |
| DLBCL-IBL plasmacytoid | + | + (II) | — |
| PEL and its solid variants | + | + (I) | + |
| PBL of the oral cavity type | + | +/− (I) | — |
| MCD-associated LBCL | + | — | + |
| MCD | + | — | + |
+, positive in 100% of cases; −, negative in 100% of cases; −/+, positive in less than 50% of cases; +/−, positive in more than 50% of cases; IBL, immunoblastic lymphoma.
Table 3.
Germinal center- or postgerminal center-derived lymphomas in HIV-infected individuals: immunodeficiency level, phenotypic features, and genetic abnormalities
| Germinal center | Postgerminal center | ||||
|---|---|---|---|---|---|
| Germinal center B-cell type | Activated B-cell type | Plasmablastic type/plasma cell type | |||
| Histotype | BL | DLBCL-CB | DLBCL-IB | PBL | PEL |
| Phenotypic features | |||||
| BCL-6 | + | + | — | — | — |
| MUM1 | — | — | + | + | + |
| CD138 | — | — | −/+ | +/− | + |
| Genetic abnormalities | |||||
| BCL-2 | — | — | 30% | 20% | — |
| BCL-6 | 100% | >75% | — | <10% | — |
| TP53 | 50-60% | Rare | — | — | — |
| MYC | 100% | 0-50% | — | 40% | — |
Immunodeficiency increases from mild (left) to severe (right) across the table.
−, absent; +, present; +/−, usually present; −/+, usually absent.
Regarding the relationship of pathogenic factors and specific types of HIV-associated lymphomas, there are no significant differences at the genetic level, according to the involvement of the c-MYC oncogene and other genetic abnormalities within the morphological spectrum of BL.26-28 In contrast, EBV is present in 50% to 70% of plasmacytoid variant BL, but only in 30% of classic BL variants.16 Interestingly, studies of HIV-associated BL cases suggest that microRNAs may be involved in the pathogenesis of some of the EBV-infected BLs lacking MYC translocation.29,30 Also, DLBCL in HIV-infected patients is quite different from DLBCL occurring in the general population, because HIV-associated DLBCL displays more frequent association with EBV, along with expression of EBV-encoded latent membrane protein-1 (LMP-1).4 DLBCL can be divided into germinal center (GC) (mainly belonging to the centroblastic variant) and post-GC center (mainly belonging to the immunoblastic variant) subtypes, although the clinical significance of this subclassification in HIV setting remains to be investigated.31 An immunoblastic/plasmablastic derivation seems to be common to several DLBCLs displaying a variable expression of plasmacytoid differentiation markers and post-GC phenotypes, as shown by occurrence of immunoglobulin heavy chain variable region gene mutations and lack of BCL6 protein expression.16 PELs are consistently associated with KSHV infection and clonal immunoglobulin gene rearrangements, which often contain somatic hypermutations. However, PEL tumor cells do not carry recurrent alterations in oncogenes or tumor suppressor genes.32-34 Gene expression profiling studies comparing EBV-infected and EBV-uninfected PELs have found differences in a significant number of genes in the MAPK pathway, suggesting pathogenic differences according to the EBV status.35 In plasmablastic lymphomas of the oral cavity type, typical surface markers of mature B cells are usually downregulated, whereas those associated with plasma cells are upregulated. Similarly, transcription factors associated with B cells are downregulated, whereas the terminal differentiation program is consistently expressed. EBV appears to be highly associated (60%-75% of cases) with this lymphoma.36-38 A consistent association with KSHV has been found in almost all HIV-infected patients with MCD.15,39 The cells harboring KSHV in MCD have been called plasmablasts. These cells may be found in large sheets thought to represent frank plasmablastic lymphomas.40 In HIV-infected patients, nearly all cases of cHL are associated with EBV infection and express a type II latency (Table 2). HIV-associated cHL displays biological peculiarities when compared with cHL occurring in the general population. In particular, HIV-associated cHL is characterized by an unusually large proportion of tumor cells, the so-called Reed-Sternberg cells, infected by EBV. LMP-1 is expressed in virtually all HIV-associated cHL cases, suggesting EBV has an etiologic role in their pathogenesis.4
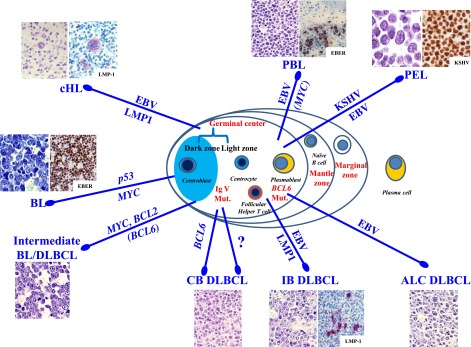
Figure 1 summarizes the association of the different types of HIV-associated lymphomas with known genetic lesions and/or oncogenic viruses. In this scenario, different, but not mutually exclusive, pathogenic pathways might occur (Figure 1). A distinct pathway involves BL and is associated with mild immunodeficiency of the host and multiple genetic lesions of the tumor. Other pathways involve HIV-associated centroblastic lymphoma and immunoblastic lymphoma, both being associated with marked disruption of the immune function of the host. Most of these DLBCL variants carry EBV infection; however, only immunoblastic lymphomas express LMP-1, the major viral oncoprotein. Therefore, expression of BCL6 and EBV infection without LMP-1 expression do not sharply separate the centroblastic from immunoblastic subtypes of DLBCL (Figure 1). Another pathway is associated with HIV-associated PEL. This specific subtype consistently harbors infection by KSHV and, in the HIV setting, by EBV (Figure 1).
Figure 1.
Main viral and molecular pathogenic pathways. Lymphomas in patients infected with HIV are heterogeneous, not only pathologically but also in terms of pathogenetic pathways and cellular derivation. The molecular pathway in HIV-associated BL involves activation of MYC (100% of cases), inactivation of p53 (50%-60% of cases), and infection by EBV (30%-50% of cases). Unclassifiable lymphomas with features intermediate between BL and DLBCL may occur. Molecular studies have shown rearrangements in BCL2 (and BCL6) and MYC genes. The molecular pathogenesis of HIV-associated centroblastic (CB) and immunoblastic (IB) DLBCL is complex and more heterogeneous. Infection with EBV occurs in 30% of DLBCL with CB morphology and 90% of DLBCL with IB and anaplastic (ALCL) morphology. Many EBV-positive IB DLBCLs express the EBV-encoded transforming protein LMP-1. There is an association between molecular changes in the BCL6 proto-oncogene and 20% of HIV-associated CB DLBCLs. Molecular studies of cells in PEL have shown no rearrangements in BCL1, BCL2, BCL6, and MYC genes. However, mutations in the BCL6 5′ noncoding region are common in PEL. In addition to consistent infection by KSHV, PELs are also commonly infected by EBV (80%). Regarding the molecular pathogenesis of PBL of the oral cavity type, EBV infection of the neoplastic clone has been frequently reported. In HIV-infected patients, nearly all cases of cHL are associated with EBV infection and express LMP-1.
A lymphomagenic role for HIV beyond immunosuppression
HIV does not directly infect lymphoma cells, and according to the current view, this virus is believed to contribute to lymphomagenesis through 2 main indirect mechanisms: induction of a chronic B-cell activation, favored by HIV-mediated immune dysfunction, and loss of immunoregulatory control of oncogenic herpesviruses, such as EBV and KSHV (Table 2). Recent evidence, however, indicates that now is probably the time to revisit this assumption in light of data suggesting that HIV may contribute to lymphomagenesis by acting directly on B lymphocytes as a critical microenvironmental factor. Indeed, HIV itself and various HIV-encoded proteins, including gp120, may trigger and sustain the aberrant activation of B lymphocytes that characterizes HIV+ patients. The underlying chronic antigenic stimulation and inflammation may lead to a poly- or oligo-clonal expansion of dysregulated B lymphocytes, which is also favored by the abnormal production of B-cell growth-promoting cytokines such as IL-6 and IL-10.41 Uncontrolled persistent stimulation of B lymphocytes may favor the emergence of monoclonal B-cell proliferations that are at increased risk of acquiring critical genetic alterations, ultimately leading to lymphoma development. Notably, CD40 ligand (CD40L), a costimulatory molecule expressed by activated T lymphocytes, can be inserted at the surface of HIV-1 particles when budding from activated CD4+ T cells,42 and CD40L-bearing HIV virions were shown to strongly activate B cells, thus mimicking a physiological stimulation.42,43 As part of CD40L-triggered events, hyperactivated B cells express activation-induced cytidine deaminase (AID), a DNA editing enzyme mediating immunoglobulin class switch recombination and somatic hypermutation.44 In addition to introducing point mutations in both immunoglobulin and nonimmunoglobulin genes (ie, BCL-6), AID can also induce chromosome translocations involving oncogenes that are critical for the development of B-cell lymphomas.44,45 In particular, aberrant AID expression is thought to be responsible for the c-MYC/immunoglobulin heavy chain recombination observed in GC-derived lymphomas, such as BL.45
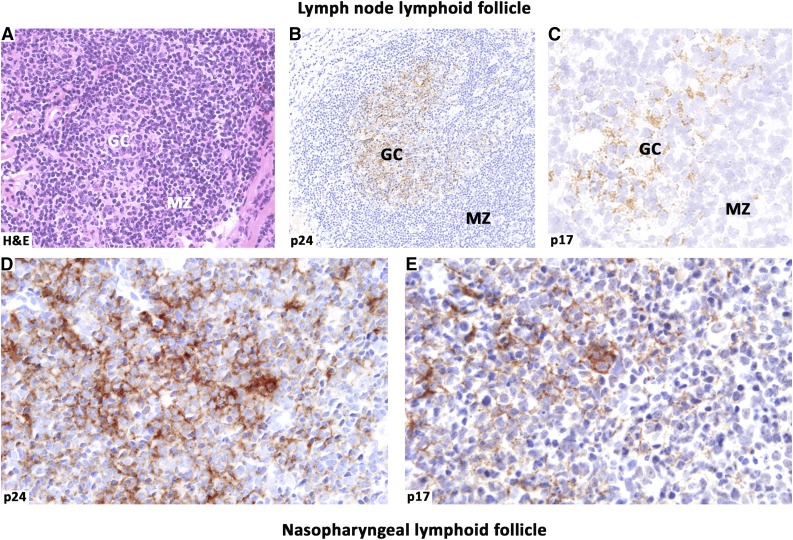
Intriguingly, mice transgenic for a defective HIV-1 provirus lacking part of the gag-pol region showed overexpression of the HIV proteins p17, gp120, and nef and development of B-cell lymphomas,46 supporting a pathogenic role for aberrant expression of HIV proteins and B-cell stimulatory cytokines in lymphomagenesis. Moreover, about 30% of mice transgenic for the Tat (transactivator of transcription) protein of HIV were shown to develop lymphomas of B-cell origin.47 Being released by HIV-infected cells within lymphoid tissues, Tat may act as a microenvironmental factor able to functionally interact with and enter into B lymphocytes, leading to deregulation of the pRb2/p130 oncosuppressor protein.48 Of particular pathogenic relevance is the ability of Tat to enhance the production of the growth-promoting IL-6 and IL-10 cytokines,49,50 to upregulate the expression levels of the DNA repair β-polymerase,51 and to promote angiogenesis.52 Notably, HIV gp120 and matrix protein p17 accumulate and persist in the lymph nodes of HIV-1-infected patients, even under cART, in the absence of any detectable HIV replication.53 Figure 2 shows the persistence of HIV-encoded p24 and p17 proteins in the germinal centers of lymphoid follicles in HIV-infected patients under cART. These findings are consistent with the possibility that HIV may act as a local lymphomagenic factor through the effects mediated by different proteins secreted within lymphoid tissues, or even persisting at extranodal sites.
Figure 2.
HIV-released proteins within lymphoid follicles. Persistence of HIV-encoded proteins in the GC of lymphoid follicles in patients under cART. Histological sections from lymph node (A-C) and nasopharynx (D-E) were immunolabeled for the capsid p24 (B and D) and the matrix p17 (C,E) proteins. HIV p24 and p17 deposited in the GCs show similar distribution in the biopsy sites and display a dendritic pattern. Matrix p17 accumulates and persists within lymphoid tissues of HIV-infected patients in both lymph nodes and extranodal sites, where this protein may exert its pathogenic effects on B cells. Images were acquired with the Olympus Dot.Slide Virtual microscopy system, using an Olympus BX51 microscopy equipped with PLAN APO 2×/0.08 and UPLAN SApo 40×/0.95 objectives. Images were assembled using Adobe Photoshop 6 (Adobe Systems, San Jose, CA).
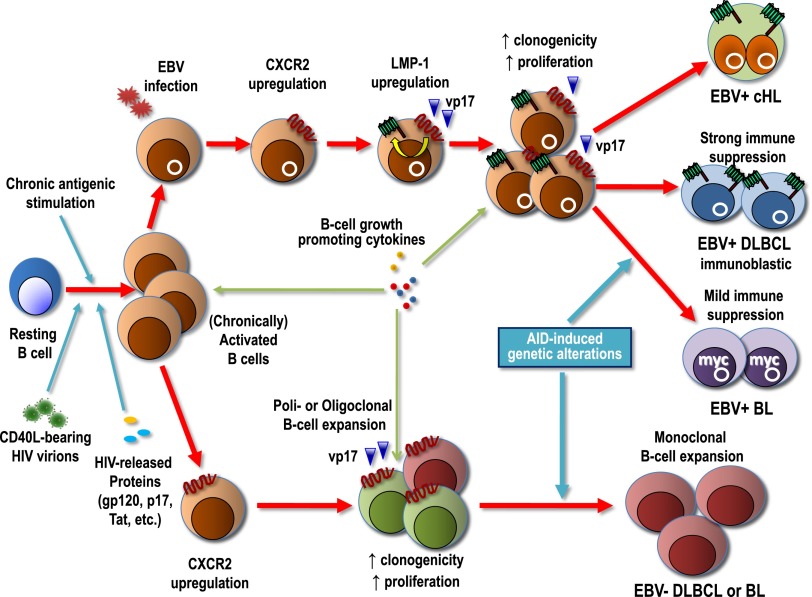
Nevertheless, lymphomas occur in a relatively limited proportion of HIV-infected patients, indicating that the presence of gp120, p17, and Tat per se is not sufficient to drive lymphomagenesis, being that these proteins probably are expressed in all patients. Recent evidence has, however, suggested a new possible scenario in which the well-known HIV genome hypervariability could favor the generation of HIV-encoded protein variants. Distinct variants with enhanced B-cell activating and growth-promoting properties are potentially able to increase the risk of developing lymphoma. In particular, it has been demonstrated that, unlike the prototype clade B p17 and p17 derived from HIV-infected individuals without lymphoma, p17 variants derived from an Ugandan HIV-1 strain A1 or from HIV-related lymphoma were shown to activate the PI3K/Akt signaling pathway and increase the proliferation and clonogenicity of B cells.8,54 These effects were mainly mediated by interaction with CXCR2, a functional cellular receptor for p17.7,55 Notably, stimuli physiologically found within germinal center microenvironment, such as soluble or cell-bound CD40L, as well as EBV infection or expression of the LMP-1 EBV oncoprotein, were shown to upregulate CXCR2 expression,7,55 making B lymphocytes permissive to p17-mediated effects. In turn, clonogenic p17 variants may upregulate LMP-1 expression, thus creating a pathogenic loop (Figure 3). This mechanism is highly relevant for EBV-driven lymphomagenesis in the HIV setting, considering that LMP-1 prevents apoptosis of B lymphocytes that are unable to produce functional B-cell receptors or that present a high load of prelymphomatous genetic alterations in their genome.56 This possible scenario is particularly intriguing for the pathogenesis of HIV-associated cHL (Figure 3), which, for still obscure reasons, is invariably EBV-infected and LMP-1 positive, whereas only 40% of HIV-unrelated cases are EBV-infected.
Figure 3.
Proposed model of the pathogenesis of EBV-associated and EBV-unrelated lymphomas of HIV-infected patients. In a systemic background of immune suppression variably mitigated by cART, B lymphocytes are chronically activated by persistent antigenic stimulation, HIV virions bearing CD40L, HIV-released proteins (gp120, p17, Tat, etc), and various cytokines. Uncontrolled B-cell activation or EBV infection may upregulate CXCR2, an IL-8 receptor that may also serve as cellular receptor for HIV p17 and its variants. Triggering of CXCR2 by distinct p17 protein variants accumulated within nodal or extranodal lymphoid tissues may enhance B-cell clonogenicity and growth, thus increasing the likelihood of critical genetic alterations (chromosomal translocations involving c-MYC, Bcl-6 mutations, etc), which are also promoted by AID expression. In EBV-infected B cells, p17 variants may upregulate LMP-1, the major EBV oncoprotein, which further contributes to the development of EBV-associated lymphomas in this setting. In conditions of profound immune suppression, LMP-1 can be expressed by lymphoma cells, as it is the case of immunoblastic DLBCL, whereas the expression of this immunogenic EBV protein is silenced as immune escape mechanism in lymphomas associated with mild immunosuppression such as EBV-associated BL. HIV p17-mediated upregulation of LMP-1 may also contribute to the development of cHL in this setting.
p17 variants with enhanced clonogenic activity for both EBV+ and EBV− B-cells were recently shown to carry insertions at the C-terminal region, which were shown to specifically induce a structural destabilization of the protein.8 As a consequence, these particular p17 variants may acquire peculiar biologic properties more conducive to lymphomagenesis. The generation of these variants may occur more frequently in HIV-infected patients with lymphoma, who show an higher intrapatient p17 sequence diversity compared with HIV-infected individuals without lymphoma.8 The possibility that p17 variants with enhanced lymphomagenic potential may be selected within lymphoid tissues of HIV+ patients with lymphoma constitutes an attractive hypothesis that deserves to be investigated.
Conclusion and perspectives
Recent data are changing the current paradigm assuming that HIV is only indirectly related to lymphomagenesis. The emerging scenario from these new data suggests that HIV may promote lymphomagenesis not only as a consequence of its ability to sustain a chronic B-cell activation and to impair immune control of oncogenic herpesviruses but also through a direct contribution, possibly mediated by HIV-encoded proteins, particularly p17 variants. Available findings stimulate further studies aimed at assessing whether distinct molecular signatures within lymphoma-derived p17 gene sequences may serve as biomarkers potentially useful to more precisely define HIV-infected individuals at increased risk of developing lymphomas, including those associated with EBV. From a therapeutic point of view, strategies aiming at neutralizing lymphomagenic p17 proteins may be useful for the prevention of lymphoma development in at-risk patients, and may be also beneficial for the treatment of overt lymphomas in the HIV setting.
Acknowledgments
R.D. is supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (contract 14287).
This work was supported in part by an institutional grant from Centro di Riferimento Oncologico Aviano for an intramural project “Infectious agents and cancer” (A. Carbone). A. Carbone is a member of the WHO IARC Monograph Working Group on Biological Agents, Lyon, 2009.
Authorship
Contribution: A. Carbone and R. Dolcetti designed the review. All authors contributed to the writing and proofreading of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonino Carbone, Centro di Riferimento Oncologico Aviano, Istituto Nazionale Tumori, IRCCS, Via F Gallini 2, 33081 Aviano, Italy; e-mail: acarbone@cro.it.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117(5):1089–1096. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenhende MA, Roussillon C, Henard S, et al. doi: 10.1371/journal.pone.0129550. ANRS EN20 Mortalité 2010 study group. Cancer-related causes of death among HIV-infected patients in France in 2010: evolution since 2000. PLoS One. 2015;10(6):e0129550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol. 2014;11(4):223–238. doi: 10.1038/nrclinonc.2014.31. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol. 2015;42(2):247–257. doi: 10.1053/j.seminoncol.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Pantanowitz L, Carbone A, Dolcetti R. Microenvironment and HIV-related lymphomagenesis. Semin Cancer Biol. 2015;34:52–57. doi: 10.1016/j.semcancer.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Martorelli D, Muraro E, Mastorci K, et al. A natural HIV p17 protein variant up-regulates the LMP-1 EBV oncoprotein and promotes the growth of EBV-infected B-lymphocytes: implications for EBV-driven lymphomagenesis in the HIV setting. Int J Cancer. 2015;137(6):1374–1385. doi: 10.1002/ijc.29494. [DOI] [PubMed] [Google Scholar]
- 8.Dolcetti R, Giagulli C, He W, et al. Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis. Proc Natl Acad Sci USA. 2015;112(46):14331–14336. doi: 10.1073/pnas.1514748112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoufaly A, Stellbrink HJ, Heiden MA, et al. ClinSurv Study Group. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200(1):79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach CJ, Buchanan AL, Cole SR, et al. Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) HIV viremia and incidence of non-Hodgkin lymphoma in patients successfully treated with antiretroviral therapy. Clin Infect Dis. 2014;58(11):1599–1606. doi: 10.1093/cid/ciu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 12.Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34(36):4673–4682. doi: 10.1038/onc.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone A, Younes A. Lymphomas and microenvironment: Impact on lymphomagenesis. Foreword. Semin Cancer Biol. 2015;34:1–2. doi: 10.1016/j.semcancer.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Raphael M, Said J, Borish B, Cesarman E, Harris NL. Lymphomas associated with HIV infection. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008. pp. 340–342. [Google Scholar]
- 15.Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113(6):1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 16.Gloghini A, Dolcetti R, Carbone A. Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin Cancer Biol. 2013;23(6):457–467. doi: 10.1016/j.semcancer.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Shiels MS, Engels EA, Linet MS, et al. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992-2009. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1069–1078. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS. 2014;28(15):2313–2318. doi: 10.1097/QAD.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvey A, Ojesina AI, Pedamallu CS, et al. The tumor virus landscape of AIDS-related lymphomas. Blood. 2015;125(20):e14–e22. doi: 10.1182/blood-2014-11-599951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chadburn A, Abdul-Nabi AM, Teruya BS, Lo AA. Lymphoid proliferations associated with human immunodeficiency virus infection. Arch Pathol Lab Med. 2013;137(3):360–370. doi: 10.5858/arpa.2012-0095-RA. [DOI] [PubMed] [Google Scholar]
- 21.IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens. Part B: biological agents. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Vol. 100, Lyon: 2012. [Google Scholar]
- 22.De Paoli P, Carbone A. Microenvironmental abnormalities induced by viral cooperation: Impact on lymphomagenesis. Semin Cancer Biol. 2015;34:70–80. doi: 10.1016/j.semcancer.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Carbone A. Emerging pathways in the development of AIDS-related lymphomas. Lancet Oncol. 2003;4(1):22–29. doi: 10.1016/s1470-2045(03)00957-4. [DOI] [PubMed] [Google Scholar]
- 24.Morton LM, Kim CJ, Weiss LM, et al. Molecular characteristics of diffuse large B-cell lymphoma in human immunodeficiency virus-infected and -uninfected patients in the pre-highly active antiretroviral therapy and pre-rituximab era. Leuk Lymphoma. 2014;55(3):551–557. doi: 10.3109/10428194.2013.813499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedel DJ, Rositch AF, Redfield RR, Blattner WA. HIV-associated lymphoma sub-type distribution, immunophenotypes and survival in an urban clinic population [published online ahead of print June 19, 2015]. Blood. doi: 10.3109/10428194.2015.1055483. [DOI] [PubMed] [Google Scholar]
- 26.Ferry JA. Burkitt’s lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11(4):375–383. doi: 10.1634/theoncologist.11-4-375. [DOI] [PubMed] [Google Scholar]
- 27.Carbone A, Gloghini A, Larocca LM, et al. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood. 2001;97(3):744–751. doi: 10.1182/blood.v97.3.744. [DOI] [PubMed] [Google Scholar]
- 28.Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13(5):577–585. doi: 10.1634/theoncologist.2008-0036. [DOI] [PubMed] [Google Scholar]
- 29.Leucci E, Cocco M, Onnis A, et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol. 2008;216(4):440–450. doi: 10.1002/path.2410. [DOI] [PubMed] [Google Scholar]
- 30.Leucci E, Onnis A, Cocco M, et al. B-cell differentiation in EBV-positive Burkitt lymphoma is impaired at posttranscriptional level by miRNA-altered expression. Int J Cancer. 2010;126(6):1316–1326. doi: 10.1002/ijc.24655. [DOI] [PubMed] [Google Scholar]
- 31.Carbone A, Gloghini A, Kwong YL, Younes A. Diffuse large B cell lymphoma: using pathologic and molecular biomarkers to define subgroups for novel therapy. Ann Hematol. 2014;93(8):1263–1277. doi: 10.1007/s00277-014-2116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 33.Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88(2):645–656. [PubMed] [Google Scholar]
- 34.Carbone A, Cesarman E, Gloghini A, Drexler HG. Understanding pathogenetic aspects and clinical presentation of primary effusion lymphoma through its derived cell lines. AIDS. 2010;24(4):479–490. doi: 10.1097/QAD.0b013e3283365395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan W, Bubman D, Chadburn A, Harrington WJ, Jr, Cesarman E, Knowles DM. Distinct subsets of primary effusion lymphoma can be identified based on their cellular gene expression profile and viral association. J Virol. 2005;79(2):1244–1251. doi: 10.1128/JVI.79.2.1244-1251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein H, Harris NL, Campo E. Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 256–257. [Google Scholar]
- 37.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323–2330. doi: 10.1182/blood-2014-10-567479. [DOI] [PubMed] [Google Scholar]
- 38.Wang H-Y, Wong-Sefidan I, Reid E. Plasmablastic lymphoma. In: Yarchoan E, editor. Cancers in People with HIV and AIDS. New York: Springer; 2014. pp. 223–234. [Google Scholar]
- 39.Carbone A, De Paoli P, Gloghini A, Vaccher E. KSHV-associated multicentric Castleman disease: A tangle of different entities requiring multitarget treatment strategies. Int J Cancer. 2015;137(2):251–261. doi: 10.1002/ijc.28923. [DOI] [PubMed] [Google Scholar]
- 40.Polizzotto MN, Uldrick TS, Yarchoan R. Multicentric Castleman disease. In: Yarchoan E, editor. Cancers in People with HIV and AIDS. New York: Springer; 2014. pp. 245–260. [Google Scholar]
- 41.Vendrame E, Hussain SK, Breen EC, et al. Serum levels of cytokines and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol Biomarkers Prev. 2014;23(2):343–349. doi: 10.1158/1055-9965.EPI-13-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin G, Roy J, Barat C, Ouellet M, Gilbert C, Tremblay MJ. Human immunodeficiency virus type 1-associated CD40 ligand transactivates B lymphocytes and promotes infection of CD4+ T cells. J Virol. 2007;81(11):5872–5881. doi: 10.1128/JVI.02542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imbeault M, Ouellet M, Giguère K, et al. Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J Virol. 2011;85(5):2189–2200. doi: 10.1128/JVI.01993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol Rev. 2010;237(1):226–248. doi: 10.1111/j.1600-065X.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- 45.Robbiani DF, Bunting S, Feldhahn N, et al. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol Cell. 2009;36(4):631–641. doi: 10.1016/j.molcel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curreli S, Krishnan S, Reitz M, et al. B cell lymphoma in HIV transgenic mice. Retrovirology. 2013;10:92. doi: 10.1186/1742-4690-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kundu RK, Sangiorgi F, Wu LY, et al. Expression of the human immunodeficiency virus-Tat gene in lymphoid tissues of transgenic mice is associated with B-cell lymphoma. Blood. 1999;94(1):275–282. [PubMed] [Google Scholar]
- 48.Lazzi S, Bellan C, De Falco G, et al. Expression of RB2/p130 tumor-suppressor gene in AIDS-related non-Hodgkin’s lymphomas: implications for disease pathogenesis. Hum Pathol. 2002;33(7):723–731. doi: 10.1053/hupa.2002.125372. [DOI] [PubMed] [Google Scholar]
- 49.Scala G, Ruocco MR, Ambrosino C, et al. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med. 1994;179(3):961–971. doi: 10.1084/jem.179.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blazevic V, Heino M, Lagerstedt A, Ranki A, Krohn KJ. Interleukin-10 gene expression induced by HIV-1 Tat and Rev in the cells of HIV-1 infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(3):208–214. doi: 10.1097/00042560-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava DK, Tendler CL, Milani D, English MA, Licht JD, Wilson SH. The HIV-1 transactivator protein Tat is a potent inducer of the human DNA repair enzyme beta-polymerase. AIDS. 2001;15(4):433–440. doi: 10.1097/00002030-200103090-00001. [DOI] [PubMed] [Google Scholar]
- 52.Rusnati M, Presta M. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis. 2002;5(3):141–151. doi: 10.1023/a:1023892223074. [DOI] [PubMed] [Google Scholar]
- 53.Popovic M, Tenner-Racz K, Pelser C, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2005;102(41):14807–14812. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giagulli C, Marsico S, Magiera AK, et al. Opposite effects of HIV-1 p17 variants on PTEN activation and cell growth in B cells. PLoS One. 2011;6(3):e17831. doi: 10.1371/journal.pone.0017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caccuri F, Giagulli C, Reichelt J, et al. Simian immunodeficiency virus and human immunodeficiency virus type 1 matrix proteins specify different capabilities to modulate B cell growth. J Virol. 2014;88(10):5706–5717. doi: 10.1128/JVI.03142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Küppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clin Invest. 2012;122(10):3439–3447. doi: 10.1172/JCI61245. [DOI] [PMC free article] [PubMed] [Google Scholar]