Abstract
Nearly half a century of neurobiological research using the nematode Caenorahbitis elegans has produced a remarkably detailed understanding of how genotype controls behavioral phenotype. However, the role of simple physical forces in regulating behavior has been understudied. Here, we review our recent observations of 3 behaviors of C. elegans suspended in solution that can be fully explained by the laws of mechanics. These behaviors are bordertaxis, the attraction toward solid surfaces; positive rheotaxis, the propensity to swim against the flow; and synchrophilia, the tendency of animals when close to each other to synchronize their gaits. Although these 3 behaviors are not directly regulated by the animal's nervous system, bordertaxis and rheotaxis require the animal to have an undulating gait. We conjecture that these behaviors are advantageous to the animals, and thus evolution may have favored microorganism that swim with an undulating gait.
Introduction
In 1974, Sidney Brenner1 introduced the use of the simple nematode Caenorhabditis elegans (C. elegans) for modern biological research. He argued, “In principle, it should be possible to dissect the genetic specification of a nervous system in much the same way as was done for biosynthetic pathways in bacteria or for bacteriophage assembly.” The general sentiment among Brenner and his contemporaries (e.g., Seymour Benzer, one of the founders of the field of neurogenetics) was that, while biological processes are governed by the laws of physics and chemistry, behavior arises from the activity of genes functioning in the nervous system. Brenner stated, “Behavior is the result of a complex and ill-understood set of computations performed by nervous systems and it seems essential to decompose the problem into 2: one concerned with the question of the genetic specification of nervous systems and the other with the way nervous systems work to produce behavior.”1 Indeed, close to a half century of research, following Brenner's famous paper, has produced an amazingly detailed understanding of how the nervous system develops and how it controls behavior.
The implicit assumption of this approach is that behavior is neurally-mediated: the nervous system digests the sum of environmental sensory information as well as its internal states, and then controls the animal's behavior. For example, gently touching the anterior end of the worm's body with an eye lash is sensed by mechanoreceptor neurons (MRN), which communicate to interneurons, which then communicate with motor neurons, which then execute a reversal of the animal's locomotion.2 But are all behaviors neurally-mediated?
We began our research with simple observations: watching worms swim in buffered water. While most C. elegans behavioral research to date has been carried out by monitoring animals cultivated on an agar surface, the liquid environment is likely highly relevant to free-living terrestrial nematodes found in nature. In addition, with the proliferation of microfluidic devices for C. elegans research,3 recent experimental approaches have made increasing use of animals in liquid environments. Therefore, careful descriptions and understanding of the behavior of animals suspended in liquids are crucial for the design of microfluidic experiments and for the correct interpretation of experimental data obtained with these devices.
C. elegans worms swim by undulatory movements comprised of stiff, dorsoventral body waves that propagate from the head of the animal to its tail and exert a force on the environment. Fluid mechanicians characterize the flow regime associated with the animal's swimming by the magnitude of the Reynolds number Re = ρUa/μ, the ratio of the inertial force (ρU2) to the viscous force (μU/a). When Re is small, viscous effects dominate and the flow field induced by the swimmer adjusts instantaneously to its body movement. The wild-type adult C. elegans swims at an approximate velocity U∼300μm/s and has a body radius a~40 μm. The aqueous liquid has a density ρ ∼ 103kg/m3 and dynamic viscosity μ ∼ 10−3 Ns/m2. Hence, for C. elegans adults, Re ∼ 0.01, a value much smaller than 1 and the swimmer's thrust is balanced by viscous drag.
Low Reynolds number flows in Newtonian fluids, such as water, are time-reversible. Purcell stated4: “At low Reynolds number, everything reverses just fine. Time, in fact, makes no difference— only configuration. If I change quickly or slowly, the pattern of motion is exactly the same.” We refer the reader to Purcell's celebrated article4 for a lucid exposition of low Reynolds number swimming and to the video5 for a striking demonstration of low Reynolds number time-reversibility. Due to this time reversibility at low Reynolds numbers, swimming is challenging. Any reciprocal motion, such as non-propagating, periodic body bending, would result in no net advancement. That is, body displacement gained during the first half of the bending period would be perfectly negated during the second half, causing the swimmer to retract its trajectory and end up back where it has started. To break this symmetry and generate forward propulsion, low Reynolds number swimmers such as C. elegans must have evolved a motion that is non-reciprocal—a bending wave that propagates along the animal's body from anterior-to-posterior.
We observed the behavior of C. elegans adult animals swimming in solution and asked whether they exhibit behaviors that can be fully explained by the laws of mechanics. Although the focus of our work is C. elegans, our observations are applicable to all low Reynolds number, undulatory swimmers.
Observations
Bordertaxis
We monitored nematodes swimming in a microfluidic conduit in the presence and absence of external flow. We observed that, in dilute suspension, the worms aggregated next to the conduit's side walls and were not uniformly distributed along the conduit's width (Fig. 1A).6 The worms exhibited a tendency to accumulate next (be “attracted”) to solid surfaces. We dub this trait bordertaxis. Here, we understand taxis to imply the motion or orientation of the organism in response to an external stimulus or force. We observed bordertaxis behavior in wild-type animals as well as in animals deficient in mechanosensory neuron (MRN) function,6 suggesting that the well-studied MRNs required for the response to light body touch are not required for bordertaxis.
Figure 1.
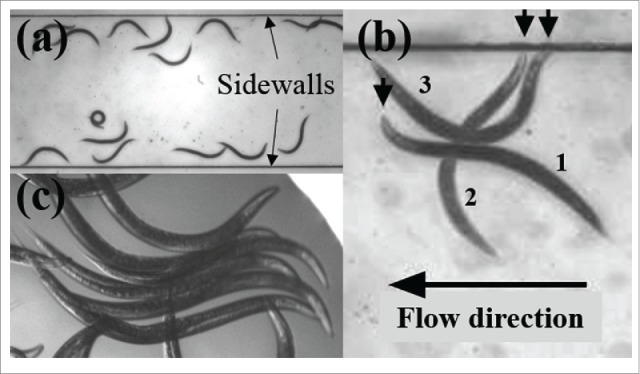
(A) In a conduit, the nematodes aggregate next to the side walls (bordertaxis). (B) In the presence of external flow, the nematode rotates to align itself against the flow (positive rheotaxis). The downward arrows from left to right indicate the head of the same animal at times = 0 s (1), 0.75s (2), and 1.5s (3). The fluid flow is directed to the left. The various video frames were shifted in space to fix the horizontal position of the animal's center of mass. (C) When in tight quarters, the nematodes synchronize their gait to avoid jamming (synchrophilia).
Positive rheotaxis
In addition to accumulating close to surfaces, we noted that, in the presence of externally applied fluid flow along the conduit's axis, the majority of the animals oriented with their heads facing into the flow. That is, the animals were swimming upstream.7 Animals that were initially far from the conduit's side walls and oriented with their heads facing in the same direction as the flow moved toward one of the side walls (i.e., they engaged in bordertaxis). When close to the wall, these animals rotated to align against the flow. Figure 1B depicts a superposition of a sequence of video frames of an animal, initially facing with the flow, undergoing rotation, to end facing against the flow. Figure 1B was constructed by combining a few video frames taken at different times and longitudinally translating the animal's positions to align their center of mass. Swimming against the flow is a behavior common to many species and is known as positive rheotaxis.
Positive rheotaxis behavior has been reported in several other nematodes, including the rice-eater Aphelenchoides besseyi8; the potato-eaters Meloidogyne (M.) chitwoodi and M. hapla9; the root-eater Meloidogyne incognita10,11; the banana-eater Radopholus similis12; and the human-parasites Ancylostoma duodenale and Strongyloides stercoralis.13 On occasion, rheotaxis was observed to overcome other stimuli such as chemotaxis.9,14
Synchrophilia
In yet another set of experiments, we observed that when animals were confined in space, they tended to synchronize their gaits to avoid jamming and to optimize their use of space (Fig. 1C).15 We dub this phenomenon synchrophilia.
We then sought to determine whether these 3 traits– bordertaxis, positive rheotaxis, and synchrophilia–are an involuntary consequence of physical forces. We began our studies with the simplest hypothesis that the observed traits are controlled by the laws of physics. We reasoned that a failure to explain the behavior using physics principles would suggest more complex biological controls.
Mechanisms
We first examine the mechanism of attraction to solid surfaces. We computed the flow field next to a boundary that is perpendicular to the plane of the swimmer's motion (Fig. 1A). We found that the interaction between the swimmer–induced flow field and the nearby boundary generates a torque that steers the swimmer toward the boundary. As a result, the swimmer swims toward the wall until its head collides with the wall. Due to the collision, the animal rotates away from the wall. As the swimmer bounces away from the wall, the hydrodynamic torque steers it back toward the boundary. The combined actions of attraction (hydrodynamic rotation toward the wall) and steric hindrance (repulsion due to collisions) cause the animal to swim along the wall for prolonged time intervals and thus provide a mechanism for bordertaxis. The technical details are elucidated in our paper.6
To convince ourselves that only laws of physics are involved in bordertaxis, we carried out fluid mechanics numerical simulations in which we implemented a swimmer's gait similar to the one observed in the experiments and tracked the motion of the swimmer. The computer animations were strikingly similar to the experimental videos.6 The similarity between the computer simulations and what we have observed in experiments supports the notion that surface attraction is, indeed, the result of the combined effects of hydrodynamic and steric forces and does not require neural involvement.
Since we anticipate no involvement of the nervous system in bordertaxis, this trait should not require sensory input. To partially test for the role of sensory input, we focused on the role of the mechanosensory neurons (MRNs), which are involved in the response to a light touch to the body.2 We experimented with touch-insensitive mutants, lacking mec-3 or mec-4 gene function. The mec-4 null mutants are insensitive to weak mechanical stimuli to the body,16 whereas mec-3 null mutants are insensitive to both weak and harsh mechanical stimuli to the body.17 Both genes are required for the function of the 6 mechanoreceptor neurons (MRNs) that sense gentle touch along the animal's body.18 The mec-3 and mec-4 null mutants behaved similar to wild-type animals, indicating that MRN function is not required for bordertaxis. While we cannot yet exclude a role for other sensory neurons (such as, for example, ciliated mechanosensory neurons), the combination of a plausible mechanism based on mechanics, detailed computer simulations, and absence of a role for MRNs, suggests that our simplest hypothesis is correct. That is, the animals' behavior is controlled by physical forces.
Can physical interactions also explain positive rheotaxis behavior? The answer is yes and the explanation turns out to be remarkably simple. Next to walls, bordertaxis behavior results in the animal being inclined toward the wall with its head closer to the wall than its tail. In the presence of fluid flow through the conduit, the velocity at the stationary wall is zero and increases as the distance from the wall increases. That is, the fluid flow results in a velocity gradient next to the stationary wall. As a result, the animal's tail is located in a region of higher velocity than its head. This difference in the velocities between the locations of the head and tail rotates the animal to face against the flow (Fig. 1B). To further convince ourselves that the phenomenon of rheotaxis can be explained by mechanical interactions, we carried out fluid mechanics numerical simulations.7 Also here, the computer animations were strikingly similar to the experimental videos,7 suggesting that rheotaxis does not require neural involvement.
Our theory allows for simple predictions. Since our proposed mechanism of rheotaxis requires a velocity gradient, one would predict that far from the boundary, where there is no velocity gradient, animals would not demonstrate a propensity to swim against the flow. Our observations matched this prediction. We observed positive rheotaxis only in animals close to the wall; animals located near the midwidth of the conduit, approximately equidistant from each side wall, showed random orientations.7 A second prediction made by our model is that animals confined in a narrow conduit, whose width is similar to the animal's length, will not exhibit rheotaxis. This is because the animal's head and tail are exposed to velocities of similar magnitude and there is no mechanism to rotate the animal. This prediction was also confirmed in experiments.7 The requirement for a velocity gradient to achieve positive rheotaxis may explain why rheotaxis in nematodes has not been universally observed.19-21
Finally, can physical interactions also explain the synchronization behavior that we observed in C. elegans (Fig. 1C)? To answer this question, we devised an experiment that allowed us to closely examine pair interactions to determine whether synchronization results from deliberate sensory action, long-range hydrodynamic forces, or short-range collisions.15 To quantify the phenomenon, we used the average phase shift between the 2 animals' gaits as the metric of synchronization. We found that animals synchronized their swimming gaits only when they were in close proximity. In other words, the average phase shift between closely-positioned animals was nearly zero. As the distance between the animals increased, the phase shift between pairs of animals' gaits exhibited random behavior. Thus, synchronization appears to be caused primarily by short-range, steric interactions and likely does not require mechanosensation. Indeed, touch-insensitive mutants, lacking mec-3 or mec-4 gene function exhibited synchronization behavior similar to wild-type animals.
Our experimental data for the phase shift between the gaits of pairs of swimmers as a function of the distance between the swimmers are in striking agreement with the predictions of Monte Carlo, volume exclusion (hard sphere-like) computer simulations that account only for steric interactions. Briefly, sinusoid-like, rigid objects, mimicking swimmers with randomly-selected phases, were placed at random in a confined space without infringing on a sinusoid object already located in that space. The phase difference between the newly inserted objects and the fixed object were documented as a function of the distance between the objects. Our simulations are described in detail in our paper.15 The agreement between our simulations and experimental data further supports the notion that no active neural response or hydrodynamic forces are involved in the synchronization process. The experimental data reveal that animals synchronize their gait simply through a sequence of collisions. This type of synchronization is reminiscent of the alignment of nanorods dispersed in liquid. In the case of the nanorods, the alignment is driven by thermal fluctuations. In the case of the much larger nematodes, in which thermal fluctuations are insignificant, the source of the fluctuations that leads to collisions is the animal's natural gait. Since the animals are relatively rigid,15 their gait is not significantly affected by proximity to other animals or surfaces. Thus, in the case of worms, muscle energy appears to play a similar role to that of thermal energy in colloids. The collisions between animals shift the relative positions of their centers of mass to bring the animals into synch. As in colloidal systems, the actions of independent agents (worms, in our case) lead to a collective, synchronized behavior (Fig. 1C).
Conclusion
Living species vary widely in the complexity of their nervous system. Organisms lacking a nervous system, such as single cells and bacteria, must rely on physiochemical interactions to survive, carry out their diverse functions, and maintain their life cycles. Animals equipped with a nervous system can rely to varying degrees on sensory inputs and neural processing to control their activities and responses to environmental stimuli. It would perhaps be fair to state that the response of all animals is a combination of involuntary actions regulated by physiochemical forces and neurally-mediated responses. As the neural complexity of the animal increases so does the role of the animal's neurvous system in controlling behavior. Animals with relatively simple nervous systems, such as C. elegans and other nematodes, may take advantage of traits that do not require nervous system involvement to support their life cycles.
Although traits such as bordertaxis, rheotaxis, and synchrophilia are not controlled by the nervous system, they are almost certainly beneficial to the animals. Maintaining proximity to solid surfaces places the animals in regions that are often rich in bacteria,22 a major food source for free-living nematodes.23,24 Animal aggregation near solid surfaces could favor mate finding. Movement close to a wall may assist in navigation, such as the migration of the hookworm through the host's blood stream.24 Regions close to a solid surface are subject to slower fluid velocities, enabling upstream swimming. For example, the plant pathogenic nematode Aphelenchoides ritzema-bosi swims along the surface of a stem, against the current, to invade the host.20 Proximity to epidermis increases the probability of host-penetration by parasitic nematodes. When propelling themselves along the host epidermis, both Aphelenchus avenae and Meloidogyne javanica's heads undergo frequent collisions with the epidermis, probing for penetration sites.25,26 Likewise, the ability of undulatory swimmers to align against the flow (rheotaxis) enables animals to navigate their environment and to maintain their positions in the presence of adverse flows such as next to plants in the presence of rain, in the hosts' guts and blood vessels. The ability to synchronize enables animals to navigate tight spaces without jamming and to make better use of limited space.
As first proposed by Brenner, much of the motivation for studying animals with simple nervous systems, such as C. elegans, is to understand the molecular and computational principles by which the nervous system controls behavior. But perhaps somewhat surprisingly, over the course of nearly a half-century of research, the role of simple physical forces in the control of behavior has been understudied. Here, we have described 3 behavioral traits controlled by the laws of physics.
Are we proposing that genes and neurons do not play any role at all in these behaviors? Clearly, this is not the case. The neuromuscular system controls the animal's swimming gait, which in turn, supports the aforementioned traits. One may speculate that at earlier stages of evolution, worms with various gaits may have existed. Natural selection may have favored animals with swimming gaits that support traits beneficial to the animals such as bordertaxis, rheotaxis, and synchrophilia. For example, an animal that executes a periodic motion like a scallop4 or an animal that bends its body periodically in a sinusoidal fashion without propagating bending waves along its body would be unable to propel in a Newtonian liquid, and would not exhibit bordertaxis and rheotaxis. Worms with swimming gaits that did not support these traits, such as non-undulatory swimmers, may have perished.
A good understanding of the traits described here is important when one desires to control the nematodes' life cycles. For example, in the case of parasitic nematodes that reside in the blood stream, it may suffice to administer minimally toxic drugs that interfere with the animal's motility, without actually directly killing the animal, to disturb the animal's life cycle and keep it from progressing upstream, against the blood flow. Indeed, the most widely used anti-helminthics, including those recognized by the 2015 Nobel committee,27 act by impairing undulatory movements of the nematodes.
When microfluidic systems are used in nematode research, one can take advantage of the aforedescribed traits for various useful purposes. For example, we took advantage of bordertaxis to skim nematodes out of a flow stream and to sort nematodes based on their level of activity.6 In another application, we took advantage of the animals' tendency to go against the flow to sort nematodes based on their motility, and identify a gene, previously unknown in C. elegans, that suppresses quiescent behavior.28 A good understanding of the physically-induced traits is also essential when designing mircofluidic systems that utilize other taxis mechanisms for sorting animals since the physically-induced traits may overwhelm other responses and bias device performance.14
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported in part by National Institutes of Health (NIH) NIA Grant 5R03AG042690-02 and by National Science Foundation NSEC Grant DMR08-32802 through the University of Pennsylvania Nano-Bio Interface Center (NBIC). D.M.R. was supported by NIH Grant R01NS064030.
References
- 1.Brenner S., The genetics of Caenorhabditis elegans. Genetics 1974; 77:71–94; PMID:4366476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S., The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 1985; 5:956–64; PMID:3981252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San-Miguel A., Lu H., 2013, Microfluidics as a tool for C. elegans research. WormBook: Sept 24: 1–19, http://dx.doi.org/ 10.1895/wormbook.1.162.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purcell E. M. Life at low Reynolds-number. Am J Phys 1977; 45, 4748-11; http://dx.doi.org/ 10.1119/1.10903 [DOI] [Google Scholar]
- 5.https://www.youtube.com/watch?v=p08_KlTKP50. Last visited: 10/31/2015. [Google Scholar]
- 6.Yuan J, Raizen DM, Bau HH. A hydrodynamic mechanism for attraction of undulatory microswimmers to surfaces (bordertaxis). J R Soc, Interf 2015; 12:20150227; http://dx.doi.org/ 10.1098/rsif.2015.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, Raizen DM, Bau HH. Propensity of undulatory swimmers, such as worms, to go against the flow Proc Natl Acad Sci U S A 2015; 112:3606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamo JA, Madamba CP, Chen TA. Vertical migration of the rice white-tip nematode, Aphelenchoides besseyi. J Nematol 1976; 8:146–52; PMID:19308213 [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkerton JN, Mojtahedi H, Santo GS, O'Bannon JH. Vertical Migration of Meloidogyne chitwoodi and M. hapla under Controlled Temperature. J Nematol 1987; 19:152–7; PMID:19290123 [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto T, Hasegawa S, Otobe K, Mizukubo T. The effect of soil water flow and soil properties on the motility of second-stage juveniles of the root-knot nematode (Meloidogyne incognita). Soil Biol Biochem 2010; 42:1065–72; http://dx.doi.org/ 10.1016/j.soilbio.2010.03.003 [DOI] [Google Scholar]
- 11.Fujimoto T, Hasegawa S, Otobe K, Mizukubo T. Effect of water flow on the mobility of the root-knot nematode Meloidogyne incognita in columns filled with glass beads, sand or andisol. Appl Soil Ecol 2009; 43:200–5; http://dx.doi.org/ 10.1016/j.apsoil.2009.07.006 [DOI] [Google Scholar]
- 12.Chabrier C, Carles C, Quénéhervé P, Cabidoche Y-M. Nematode dissemination by water leached in soil: Case study of Radopholus similis (Cobb) Thorne on nitisol under simulated rainfall. Appl Soil Ecol 2008; 40:299–308; http://dx.doi.org/ 10.1016/j.apsoil.2008.05.004 [DOI] [Google Scholar]
- 13.Wallace HR. The Biology of Plant Parasitic Nematodes. Edward Arnold Ltd, London, 1963. ISBN-10: 071312167X, ISBN-13: 978-0713121674 [Google Scholar]
- 14.Solvas XCi, Geier FM, Leroi AM, Bundy JG, Edel JB, deMello AJ.. High-throughput age synchronisation of Caenorhabditis elegans. Chem Communicat 2011; 47:9801–3; PMID:21818494; http://dx.doi.org/ 10.1039/c1cc14076k [DOI] [PubMed] [Google Scholar]
- 15.Yuan J, Raizen DM, Bau HH. Gait synchronization in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2014; 111:6865–70; PMID:24778261; http://dx.doi.org/ 10.1073/pnas.1401828111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 1989; 243:1027–33; PMID:2646709; http://dx.doi.org/ 10.1126/science.2646709 [DOI] [PubMed] [Google Scholar]
- 17.Way JC, Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Gen Dev 1989; 3:1823–33; PMID:2576011; http://dx.doi.org/ 10.1101/gad.3.12a.1823 [DOI] [PubMed] [Google Scholar]
- 18.Goodman MB. Mechanosensation. WormBook, ed. The C. elegans Research Community, WormBook, http://www.wormbook.org/chapters/www_mechanosensation/mechanosensation.html [Google Scholar]
- 19.Lane C. Behaviour of infective hookworm larvae. Ann Trop Med Parasit 1930; 24:411–21 [Google Scholar]
- 20.Wallace HR. Movement of Eelworms: V. Observations on Aphelenchoides Ritzema-Bosi (SCHWARTZ, 1912) Steiner, 1932 on Florists‘ Chrysanthemums. Annal Appl Biol 1959; 47:350–60; http://dx.doi.org/ 10.1111/j.1744-7348.1959.tb02550.x [DOI] [Google Scholar]
- 21.Wallace HR. The Orientation of Ditylenchus Dipsaci To Physical Stimuli. Nematologica 1961; 6:222–36; http://dx.doi.org/ 10.1163/187529261X00063 [DOI] [Google Scholar]
- 22.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004; 2:95–108; PMID:15040259; http://dx.doi.org/ 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- 23.Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol 2006; 209:89–102; PMID:16354781; http://dx.doi.org/ 10.1242/jeb.01955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol 2004; 58:197–288; PMID:15603764; http://dx.doi.org/ 10.1016/S0065-308X(04)58004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace HR. Undulatory locomotion of the plant parasitic nematode Meloidogyne javanica. Parasitology 1968; 58:377-91. [Google Scholar]
- 26.Fisher JM, Evans AAF. Penetration and Feeding By Aphelenchus Avenae. Nematologica 1967; 13:425–8; http://dx.doi.org/ 10.1163/187529267X00661 [DOI] [Google Scholar]
- 27.http://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/ (visited October 12, 2015) [Google Scholar]
- 28.Yuan J, Zhou J, Raizen DM, Bau HH. High-throughput, motility-based sorter for microswimmers such as C. elegans. Lab Chip 2015; 15:2790–8; PMID:26008643; http://dx.doi.org/ 10.1039/C5LC00305A [DOI] [PMC free article] [PubMed] [Google Scholar]


