Abstract
Preventing attrition is a major concern in behavioral weight loss intervention studies. The purpose of this analysis was to identify baseline and six-month predictors associated with participant attrition across three independent clinical trials of behavioral weight loss interventions (PREFER, SELF, and SMART) that were conducted over 10 years. Baseline measures included body mass index, Barriers to Healthy Eating, Beck Depression Inventory-II (BDI), Hunger Satiety Scale (HSS), Binge Eating Scale (BES), Medical Outcome Study Short Form (MOS SF-36 v2) and Weight Efficacy Lifestyle Questionnaire (WEL). We also examined early weight loss and attendance at group sessions during the first 6 months. Attrition was recorded at the end of the trials. Participants included 504 overweight and obese adults seeking weight loss treatment. The sample was 84.92% female and 73.61% white, with a mean (±SD) age of 47.35±9.75 years. After controlling for the specific trial, for every one unit increase in BMI, the odds of attrition increased by 11%. For every year increase in education, the odds of attrition decreased by 10%. Additional predictors of attrition included previous attempts to lose 50–79 pounds, age, not possessing health insurance, and BES, BDI, and HSS scores. At 6-months, the odds of attrition increased by 10% with reduced group session attendance. There was also an interaction between percent weight change and trial (p<.001). Multivariate analysis of the three trials showed education, age, BMI, and BES scores were independently associated with attrition (ps ≤.01). These findings may inform the development of more robust strategies for reducing attrition.
Keywords: attrition, weight loss, binge eating
1. INTRODUCTION
Obesity and its related co-morbidities remain a significant public health concern (1). Randomized clinical trials (RCTs) testing weight loss interventions have resulted in the identification of efficacious strategies to treat overweight and obesity (2, 3). However, participant attrition reduces the effectiveness of weight-loss RCTs. Premature withdrawal from weight loss trials can prevent participants from adopting healthful behaviors that support long-term weight loss (4). Additionally, important research information is lost, which not only can reduce internal and external validity, but also bias trial outcomes (5).
Highly variable attrition rates ranging from 10–80% have been reported in RCTs characterized by varying trial designs, interventions, and study duration (6–9). For example, in a 12-month RCT for weight loss, an attrition rate of 42% among overweight and obese participants was reported (10). Similarly, in shorter weight-loss RCTs lasting 12–16 weeks, attrition rates ranging from 20% to 50% were reported (8). Recognizing participant characteristics associated with attrition may enhance retention and the subsequent development of effective weight loss interventions (4).
Obesity researchers have identified factors linked to attrition in weight loss trials; inconsistent associations have been reported between baseline factors (e.g., depression, body mass index (BMI)) and attrition (7, 9, 11–16). An association between reported binge eating and rates of attrition has been published; however, the relationship has not been reliable (17, 18). A recent systematic review reveals there are no significant associations between pre-treatment weight-loss expectations among participants and attrition (19). Notably, the majority of studies described in the systematic review had methodological limitations such as a brief intervention duration and/or short follow-up period (9, 13, 14, 20, 21). Additionally, none of the studies reported attrition rates across multiple clinical trials for durations exceeding 12 months.
The purpose of this study was to conduct a secondary analysis of data from three RCTs of behavioral weight loss interventions, ranging from 18 to 24 months in duration, to identify socio-demographic, anthropometric, and psychosocial factors associated with participant attrition at baseline. Additionally, we also examined percent weight change and attendance to group sessions at six months as early predictors of attrition. For the purposes of this study, attrition was defined as non-completion of the final end-of-trial assessment, which is commensurate with current literature (8, 21) on the topic.
This study is unique in that it analyzes data from three RCTs (i.e., PREFER, SELF, and SMART) that were conducted over a 10-year period and included a diverse, pooled sample of 504 adults. Variable selection included those drawn from more recently developed measures which assess not only participants’ self-efficacy and hunger and satiety, but also important factors inconsistently associated with attrition in smaller trials such as health-related quality of life, depressive symptoms, and binge eating (7, 22). The rationale for including variables previously associated with attrition is to generate new evidence surrounding psychosocial factors and eating behaviors that may be related to attrition. Additionally, this study provides researchers with information to assist in identifying participants who may be at risk for RCT withdrawal.
2. RESEARCH METHODS AND PROCEDURES
2.1 Trial Design and Participants
PREFER, SMART, and SELF were RCTs targeting weight loss over an extended period that featured a standard behavioral intervention. The design, recruitment, and randomization procedures of PREFER, SMART, and SELF have been described in detail (23–25).
Individuals were eligible for RCT enrollment across all studies if they met the following criteria: (1) over 18 years of age, (2) BMI between 27 and 43 kg/m2, (3) successfully completed a 5-day food diary at screening, (4) agreed to be randomly assigned to a treatment group, and (5) willing to provide informed consent. Individuals were ineligible if they met any of the following exclusion criteria: (1) has a medical condition requiring physician supervision of diet and/or physical activity, (2) is undergoing current pharmacological treatment that might affect weight, (3) has a physical limitation that restricted exercise ability, (4) current alcohol consumption of four or more drinks/day, (5) is participating in a weight-loss program or has used weight loss medication within the last 6 months, (6) is pregnant or intends to become pregnant during the trial period, (7) has a serious mental illness (e.g., schizophrenia), and (8) has a fasting plasma glucose level greater than 125 mg/dl at baseline.
Details of each trial are listed in Table 1. PREFER (Paving the Road to Everlasting Food and Exercise Regimes) was an 18-month trial (2002–2004) that examined the effect of dietary approaches and preferences using a 2×2 factorial design, which allowed participants to indicate their preference for one of two dietary options: a calorie-restricted, lacto-ovo-vegetarian diet or a standard calorie- and fat-restricted diet (n = 176) (25). Individuals first were randomized to their choice of treatment (yes/no) and subsequently to one of the two diets. The SMART (Self-monitoring and Recording Using Technology) study was a 24-month trial (2005–2009) that examined the effect of three self-monitoring methods on weight loss (n = 210) (23). Participants were randomized to use one of three strategies for self-monitoring their diet and physical activity: use of a paper diary, use of a personal digital assistant (PDA), or use of PDA + daily dietary feedback messages (PDA + FB). SELF (Self-Efficacy Lifestyle Focus) was an 18-month clinical trial (2008–2013) that examined the effect of a self-efficacy enhancement intervention (SE) on weight loss (n = 130). Participants were randomized to standard behavioral treatment (SBT) or to a SBT + SE weight loss intervention group; SBT + SE included one-to-one sessions that augmented the standard group sessions and targeted enhanced self-efficacy (24). The University of Pittsburgh, Institutional Review Board approved each trial.
Table 1.
Description of Baseline Socio-Demographic Characteristics of Each Trial
| Trial Description | Intervention | BMI(±SD) | Age*(±SD) | Years of Education (±SD) | Race | Marital Status | Income |
|---|---|---|---|---|---|---|---|
| PREFER (N=176, 2002–2004): Weekly group sessions for first 6 months; biweekly for months 7–9; monthly for months 10–12 | SBT, Treatment Preference Yes vs. Treatment Preference No; LOV vs. Standard Diet | 34.02±4.09 | 44.03±8.76 | 15.20±2.53 | White: 70.45% | Never Married: 19.54% | <$10– 30,000: 16.67% |
| Non- White: 29.56% | Married: 63.79% | $30– 50,000: 26.44% | |||||
| Other: 16.67% | >$50,000: 56.90% | ||||||
| SMART (N=210; 2005–2009): Weekly for the first four months, every 2 weeks for months 5–12; monthly for months 13–18, 21 | SBT, Self- Monitoring: Paper Diary vs. Electronic Diary | 33.44±3.87 | 52.97±9.59 | 15.91±3.06 | White: 77.89% | Never Married: 14.57% | <$10– 30,000: 16.49% |
| Non- White: 22.81% | Married: 67.84% | $30– 50,000: 23.20% | |||||
| Other: 17.59% | >$50,000: 60.61% | ||||||
| SELF (N=130, 2008–2013): Weekly for the first month, every 2 weeks in the second month, monthly for months 3–12; every 6 weeks for months 13–18 | SBT vs. SBT + Self-Efficacy Enhancement | 33.91±4.45 | 46.63±9.14 | 15.67±3.02 | White: 71.32% | Never Married: 12.40% | <$10– 30,000: 13.39% |
| Non- White: 28.67% | Married: 64.34% | $30– 50,000: 22.05% | |||||
| Other: 23.26% | >$50,000: 64.07% |
significantly different by trial, p<.001
Note: SD: standard deviation; LOV: Lacto-Ovo-Vegetarian; Standard Diet: Calorie and Fat Control; SBT: Standard Behavioral Treatment
2.2 Justification for Combining the Three Trials
Table 1 presents the participants’ sociodemographic profiles in the three studies. While the three trials featured differences, all three delivered SBT for weight loss and were conducted in Greater Pittsburgh. In each study, all participants were given calorie goals that were determined by their weight and gender (i.e., at < 200 lb, women were prescribed a 1,200 kcal diet and men 1,500 kcal; at > 200 lb, women were prescribed a 1,500 kcal diet and men 1,800 kcal). Participants were also instructed to reduce fat consumption to less than 25% of their daily intake and participate in 150 minutes of physical activity weekly.
With the aim of understanding the factors affecting attrition, we completed analyses at 18 and 24 months—the time points indicating the end-of-study. The PREFER and SELF trials were conducted over 18-months, while SMART was conducted over 24 months. Although some may question the use of varying time points, to understand attrition, we needed to follow the original design of each study and measure attrition at the final assessment. Moreover, there were no significant differences in attrition across the three studies (p = .06). With the exception of age, sociodemographic and anthropometric factors did not differ by trial. Finally, our analyses controlled for study (PREFER, SELF, or SMART) in each model, and tested for interactions between study and each predictor.
2.3 Baseline and 6-Month Measures
Table 2 presents the baseline measures used across the three studies. With the exception of two scales, measures were the same across the three studies. The Beck Depression Inventory and the Hunger Satiety Scale were not used in SMART. Additionally, only two of the four cohorts in SMART completed the Weight Efficacy Lifestyle Questionnaire (WEL).
Table 2.
Measures Used in the PREFER, SMART, AND SELF Trials
| Measures | PREFER | SMART | SELF |
|---|---|---|---|
| Socio-demographic Characteristics | X | X | X |
| Medical History | X | X | X |
| Binge Eating Scale | X | X | X |
| Barriers to Healthy Eating | X | X | X |
| Beck Depression Index-II | X | X | |
| Hunger Satiety Scale | X | X | |
| Medical Outcomes Study - Short Form 36 | X | X | X |
| Weight Lifestyle Self-Efficacy | X | Xa | X |
| Body Mass Index | X | X | X |
| Waist Circumference (cm) | X | X | X |
Only 2 of the 4 cohorts completed the Weight Lifestyle Self-Efficacy
2.3.1 Socio-Demographic and Anthropometric Data
Baseline socio-demographic characteristics were obtained via a self-administered, standardized questionnaire. Trained staff performed the anthropometric measures (e.g., BMI and waist circumference). A Tanita Digital Scale (Tanita Corporation of America, Inc., IL) was used to record weight with the participant wearing light clothing and no shoes; height was recorded using a wall-mounted stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Percent weight change was defined as the percentage of change from the baseline weight. A Gullick II measuring tape evaluated waist circumference.
2.3.2 Psychosocial Data
Barriers to Healthy Eating (BHE) is a 22-item questionnaire that assesses participants’ perceived barriers to healthy eating. The Likert response scale ranges from 1 (no problem) to 5 (a significant problem) to rate various situations or conditions (e.g., complexity of the regimen, and cost of foods) that can interfere with following the diet. Higher scores indicated the participants reported more diet-related barriers. The BHE scale, which was used in a previous weight loss study (26), has an internal consistency reliability (Cronbach’s alpha) of 0.86 in our studies (27).
The Beck Depression Inventory-II (BDI) is a 21-item scale is used to assess participants’ self-reported depressive symptoms (28). Higher scores indicate more severe depressive symptoms. The recommended score cut points for classifying depressive symptoms are as follows: minimal (0–13), mild (14–19), moderate (20–28), and severe (≥29) (29). The BDI has high internal consistency; Cronbach’s alpha coefficients are 0.81 for non-psychiatric populations (30).
The Hunger Satiety Scale (HSS), a 6-item scale, measures an individual’s level of hunger and satiety. It consists of three components: hunger (i.e., How hungry are you after meals?), satiety (i.e., How full are you after meals?), and taste (i.e., How tasty is your diet?). A higher score reflects less hunger, greater satiety, and better taste. Cronbach’s alpha coefficients for each subscale in our trials are as follows: taste0.76, hunger 0.60, and satiety 0.78 (31).
The Weight Efficacy Life-Style Questionnaire (WEL) assesses participants’ level of confidence in resisting eating in varied situations and emotional states, with higher scores indicating more confidence. Responses to the 20 items on this questionnaire correspond to a 10-point Likert scale from 0 (i.e., not confident) to 9 (i.e., very confident) (32). The WEL contains five components: (1) negative emotions (i.e., I can resist eating when I am angry), (2) availability (i.e., I can control my eating on the weekends), (3) social pressure (i.e., I can resist eating even when I have to say no to others), (4) physical discomfort (i.e., I can resist eating when I feel physically run down), and (5) positive activities (i.e., I can resist eating when I am watching TV). Reported Cronbach’s alpha coefficients from the literature range from 0.70 to 0.90 (32, 33).
The Binge Eating Scale (BES) is a 16-item multiple-choice instrument used to identify non-binge, moderate binge, or severe binge eating patterns among individuals. The total score range is 0–46, with a higher score (i.e., > 27) indicating more severe binging (34). This instrument was used in the screening phase of all three trials. Cronbach’s alpha has been reported at .87–.88 among various samples of those who are obese (35, 36).
The Medical Outcomes Study, Short-Form Survey (MOS SF-36v2) is used to measure general health-related quality of life (HRQoL) and has domain scores ranging from 0 to 100. Higher values indicate a better state of health. The MOS SF-36v2 has two component summary scores: mental health and physical health (37). The MOS SF-36v2 has been used in several weight loss trials and has well-established reliability and validity (38, 39). Cronbach’s alpha has been reported at >0.70 (39).
2.3.3 Adherence to Attending Group Sessions
Adherence to attending group sessions was defined as the number of sessions actually attended in the first 6 months/total number of sessions)*100.
3. ANALYSIS
Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC). To achieve trial independence, we excluded 11 SMART trial subjects and one SELF trial subject from the analyses because they were also participants in the earlier PREFER trial. The outcome variable was binary: participants who completed the final trial assessment were considered completers, and those who failed to attend the final assessment were non-completers. Descriptive statistics were computed as (1) means and standard deviations for continuous variables and (2) frequencies and percentages for categorical variables. The significance level was set at 0.05 for two-sided hypothesis testing. Baseline characteristics were compared among trials using ANOVA for continuous variables and Chi-square tests for categorical variables. To examine the effect of each baseline predictor, percent weight change at 6 month and proportion of group sessions attended at 6 month on the probability of not completing the trial, logistic regression was used, with the model controlling for participation in a specific trial. We assumed no weight change at 6 month if subjects' weights at 6 month were missing. Baseline predictors that had [A] either p-values less than 0.20 or [B] significant interactions with the trial in these analyses were considered in the multivariate analysis. Results were expressed as odds ratios (ORs) and their corresponding 95% confidence intervals (CI). Sensitivity analyses were conducted for influential data points. The Hosmer Lemeshow test was used to test the goodness of fit of the multivariate model.
4. RESULTS
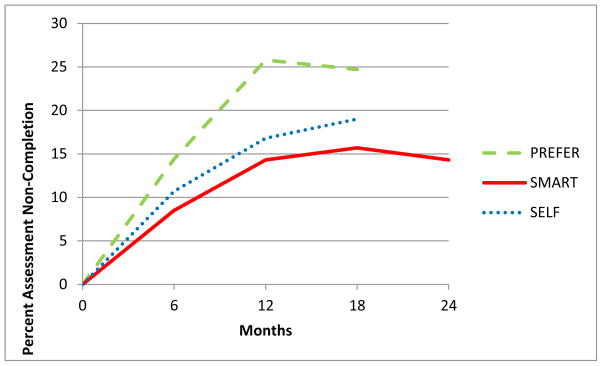
The pooled sample of the three trials (N = 504) was predominantly female (84.92%), white (73.61%), employed full-time (77.69%) and, on average, completed 15.57 years of formal education. The mean (± SD) age was 47.35 ± 9.75 years, with a mean BMI of 33.83 ± 4.18 kg/m2. Of the 504 participants who provided the baseline measures and participated in the intervention, 100 (19.84%) did not complete the final 18- or 24-month assessment, making them non-completers. See Figure 1.
Figure 1.
Non-Completion of Assessment Over Time by Study.
Table 3 details differences between the completers and non-completers of the behavioral weight-loss trials at baseline. Controlling for trial, for every one unit increase in BMI, the odds of attrition increased by 11% (OR=1.11, 95% CI: 1.06, 1.18). However, for every year increase in education, the odds of attrition decreased by 10% (OR = 0.90, 95% CI: 0.82, 0.98). Individuals who did not have health insurance had 3.125 times the odds of attrition compared to people with health insurance (OR = 0.32, 95% CI: 0.11, 0.96). Moreover, those with a history of intentionally losing 50–79 pounds had 1.92 times the odds of attrition compared to those with no history of such large weight loss (OR = 0.52, 95% CI: 0.29, 0.95). For every one unit increase in waist circumference, the odds of attrition increased by 2% (OR=1.02, 95% CI: 1.00, 1.04). Additionally, for every one unit increase in BDI (OR=1.05, 95% CI: 1.01, 1.09) and BES scores (OR=1.05, 95% CI: 1.02, 1.08), the odds of attrition increased by 5%. Furthermore, for every one unit increase in age, the odds of attrition decreased by 5% (OR=0.95, 95% CI: 0.93, 0.98). And finally, for every one unit increase in HSS score, the odds of attrition decreased by 7% (OR=0.93, 95% CI: 0.89, 0.98). Sex, race, employment status, and marital status were not significantly associated with attrition. No interactions were observed between these variables and a given trial.
Table 3.
Baseline Predictors of Study Attrition
| Characteristics | Total Sample (N=504) M±SD or n (%) |
Completers (n=404) M±SD or n (%) |
Non- Completers (n=100) M±SD or n (%) |
OR (95% CI) |
|---|---|---|---|---|
| Socio-demographic | ||||
| Age (years) | 47.35±9.75 | 48.20±9.23 | 43.89±11.04 | 0.95 (0.93, 0.98) |
| Education (years) | 15.57±2.88 | 15.74±2.90 | 14.88±2.70 | 0.90 (0.82, 0.98) |
| Employment Status | 390 (77.69%) | 318 (79.10%) | 72 (72.00%) | 0.67 (0.40, 1.12) |
| Full-time (ref= part- time) | ||||
| Ethnicity | 371 (73.61%) | 301 (74.50%) | 70 (70.00%) | 0.83 (0.51, 1.35) |
| White (ref =Non- white) | ||||
| Gender | 428 (84.92%) | 338 (83.66%) | 90 (90.00%) | 1.73 (0.85, 3.51) |
| Female (ref= male) | ||||
| Health Insurance | 488 (97.21%) | 394 (98.01%) | 94 (94.00%) | 0.32 (0.11, 0.96) |
| Yes (ref= no) | ||||
| Marital Statusa | 329 (65.54%) | 262 (65.01%) | 67 (67.68%) | 1.36 (0.73, 2.52) |
| Married (ref=other) | ||||
| Marital Statusa | 79 (15.74%) | 62 (15.38%) | 17(17.17%) | 1.40 (0.64, 3.05) |
| Never Married (ref =other) | ||||
| Anthropometric | ||||
| BMI(kg/m2) | 33.83±4.18 | 33.46±4.15 | 35.30±4.01 | 1.11 (1.06, 1.18) |
| Waist Circumference (cm) | 105.26±12.80 | 104.61±12.81 | 107.89±12.48 | 1.02 (1.00, 1.04) |
| Psychosocial | ||||
| BHE | 61.47±14.00 | 61.21±14.18 | 62.52±13.25 | 1.01 (0.99, 1.02) |
| BES | 15.58±7.67 | 15.04±7.53 | 17.73±7.89 | 1.05 (1.02, 1.08) |
| BDI | 7.68±7.00 | 7.13±6.99 | 9.54±6.75 | 1.05 (1.01, 1.09) |
| HSS | 28.78±5.39 | 29.23±5.22 | 27.30±5.71 | 0.93 (0.89, 0.98) |
| MOS SF36-Mental | 49.00±9.96 | 49.45±9.84 | 47.18±10.28 | 0.97 (0.95, 1.00) |
| MOS SF36-Physical | 51.74±7.02 | 51.87±6.94 | 51.24±7.32 | b |
|
| ||||
| WEL | ||||
| Availability | 16.68±8.35 | 16.66±8.48 | 16.77±7.93 | 1.00 (0.97, 1.03) |
| Negative Emotions | 18.81±9.47 | 19.22±9.34 | 17.33±9.86 | 0.98 (0.95, 1.01) |
| Physical Discomfort | 24.92±7.54 | 25.11±7.40 | 24.23±8.06 | 0.98 (0.95, 1.02) |
| Positive Activities | 24.31±7.34 | 24.58±7.10 | 23.36±8.12 | b |
| Social Pressure | 22.18±8.58 | 22.00±8.63 | 22.81±8.42 | 1.01 (0.98, 1.04) |
| WEL Total | 106.90±33.37 | 107.57±33.17 | 104.51±34.21 | b |
| History of Weight Loss/Gain | ||||
| Intentionally lost 50–79 lbs. | 422 (86.48%) | 347 (88.07%) | 75 (79.79%) | 0.52 (0.29, 0.95) |
| Never (ref=1 or more times) | ||||
Missing data reported: completers (n=403); non-completers (n=99)
This variable interacted with trial (p<.05).
Note: SD: Standard deviation; CI: Confidence Interval; BES: Binge Eating Scale; HSS: Hunger Satiety Scale; WEL: Weight Efficacy Lifestyle; MOS-SF-36v2: Medical Outcomes Survey-Short Form36; BHE: Barriers to Health Eating BDI: Beck Depression Inventory-II
There was a significant interaction between total WEL score and trial (p = 0.04) and between WEL positive activities and trial (p =0 .01). There also was a significant interaction between health-related quality of life (physical component) and trial (p = 0.04). That is, the association between total WEL score, the WEL positive activities subscale, health-related quality of life, and the probability of attrition was dependent on the specific trial.
At 6 months, for every one percent increase in adherence to attending group sessions, the odds of attrition decreased by 10% (OR=0.90; 95% CI: 0.89, 0.92). There was a significant interaction between trial and percent weight change at 6 months (p<.001). That is, the non-completers who were enrolled in the SMART trial were able to achieve a larger weight loss (−2.58%) than the non-completers who were enrolled in PREFER (−1.81%) and SELF (−0.83%).
To further examine the association between baseline factors and attrition, two multivariate logistic regression models were used to overcome the problem of measures not being present in all three trials (see Table 3). Multivariate analysis of the three trials showed that age (p < 0.001), education (p < 0.01), BMI (p < 0.01), and binge eating scores (p = 0.01) were significantly associated with attrition. Multivariate analysis of the PREFER and SELF trials showed that BMI (p = 0.04), the binge eating score (p = 0.03), and the history of losing 50–79 lb (p = 0.01) were independently associated with attrition. In this model, there were significant interactions between not only the HSS score and trial (p = 0.02), but also HSS score and age (p = 0.02).
4. DISCUSSION
This is the first study to investigate attrition across three RCTs that examined the effects of behavioral interventions for weight loss that were conducted over a 10-year period. Results revealed the baseline factors related to the highest odds of attrition in these trials were having a higher BMI, fewer years of education, a history of previous attempts to lose a large amount of weight (50–79 pounds), and no health insurance. Reduced group session attendance at 6 months was also related to increased odds of attrition. Across all of our three trials, multivariate results indicated younger age, fewer years of education, a higher binge eating score, and, a higher BMI were associated with attrition. This study also examined newer measures of hunger, satiety, and self-efficacy and confirmed several factors previously reported to be associated with attrition, such as higher BMI and binge eating behaviors.
Younger age has consistently been reported as a factor associated with attrition (9, 14, 40, 41) and an increased likelihood of missing consecutive data-collection visits (42). The relationship between age and attrition may be explained by the competing reponsibilities that are typically associated with young adulthood, such as the demands of parenting and familial responsibilities. Recently, to offset participant burden, investigators have used web-based interventions or have conducted trials in primary care offices; these approaches likely have been helpful, with documented rates of attrition between 5% and 15% (43–45). Older adults may be more able to commit to the requirements of a trial due to retirement and less demanding schedules (46). Moreover, older adults are more likely to have health insurance and engage in more frequent health service utilization, which may prompt a provider to encourage weight loss (47).
Consistent with other trials (6, 48), participants in this study who had a higher BMI were more likely to not complete the trial. Possible reasons may include (1) increased difficulty adhering to calorie- and fat-restricted diets, (2) being discouraged about needing to lose a significant amount of weight, and (3) possibly seeing the success of others in the trial that began the study with a lower BMI. These findings are important as they can be applied during initial screening to identify these attrition risk factors and implement specific strategies to mitigate them.
The estimated prevalence of binge eating behaviors is as high as 23–55% among individuals seeking weight loss treatment (34, 49). Moreover, binge eating symptoms have been associated with trial attrition (17) and with poorer weight loss outcomes (50). Although this study excluded participants with evidence of a binge eating disorder (i.e., score > 27), baseline scores were significantly higher among non-completers than completers. This finding suggests a need to not only be alert for unreported binge eating, but also to provide additional support to participants who score in the upper quartile of the acceptable range of BES scores during screening.
Within our studies, those with the highest risk of attrition were those who had fewer years of education, no health insurance, higher BMI, attended fewer group sessions during the first 6 months, and had a history of previous attempts of losing 50–79 pounds. Socio-economic status, having a higher weight or BMI, and a history of more weight loss attempts have been established as recurrent challenges in successful weight loss (16, 51, 52). Reduced attendance has been found to be associated with attrition previously (16); stronger strategies may be needed to prevent excessive absenteeism in the early phase of the intervention (52). Additionally, providing supplementary support to those who exhibit the signs suggesting higher risk for withdrawal may prove useful (53–55).
This study had a few limitations. Similar to other behavioral intervention studies is its reliance on self-report psychosocial measures; moreover, some measures were not included in all three trials, and the length of follow-up varied. Although the generalizability of our overall findings may be limited due to the relatively homogenous trial population, there was 26% minority representation in the trials. Strengths of this study included an extensive battery of baseline measures, and a profile of participants in three weight loss intervention trials spanning over 10 years. Moreover, the trials on which we reported achieved a mean retention rate of 80% at 18 months and 85% at 24 months (SMART), which matched or exceeded rates reported for other weight-loss studies (54, 56). In addition, these results may be generalized to other RCTs targeting lifestyle changes, and can (1) enable researchers to identify attrition “red flags” among participants and (2) encourage the development of strategies to reduce early trial withdrawal (Table 4).
Table 4.
Red Flags for Potential Attrition of Participants in Behavioral Weight Loss Trials
|
In summary, participant characteristics may provide clues about potential risk factors for non-completion in a weight loss intervention trial. Future attrition research should assess the relationship between the timing of participant dropout and interactions among participant socio-demographic profile, treatment, and trial length (4). In addition, future interventions should include strategies to reduce attrition in weight loss intervention trials.
Highlights.
Participant characteristics provide clues for attrition risk in three weight loss trials.
Fewer years of education and not having health insurance were associated with highest risk for attrition.
Attending fewer group sessions in the first 6 months was associated with risk for attrition.
History of previous attempts to lose 50–79 pounds was associated with risk for attrition.
Footnotes
Trial registration: these studies are registered at clinialtrial.gov. PREFER: NCT00330629; SMART: NCT00277771; and SELF: NCT00896194.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96(9):3248–50. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- 2.Burke LE, Wang J. Treatment Strategies for Overweight and Obesity. Journal of nursing scholarship : an official publication of Sigma Theta Tau International Honor Society of Nursing /Sigma Theta Tau. 2011 Oct 20; doi: 10.1111/j.1547-5069.2011.01424.x. Epub 2011/10/25. Eng. [DOI] [PubMed] [Google Scholar]
- 3.Hamman R, Wing RR, Edelstein S, Lachin J, Bray G, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011 Nov;12(11):912–34. doi: 10.1111/j.1467-789X.2011.00915.x. Epub 2011/08/06. eng. [DOI] [PubMed] [Google Scholar]
- 5.Anderson J, Konz E, Frederick R, Wood C. Longterm weight-loss maintenance: a meta-analysis of US studies. American Journal of Clinical Nutrition. 2001;74:579–84. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 6.Grossi E, Dalle Grave R, Mannucci E, Molinari E, Compare A, Cuzzolaro M, et al. Complexity of attrition in the treatment of obesity: clues from a structured telephone interview. International Journal of Obesity. 2006 Jul;30(7):1132–7. doi: 10.1038/sj.ijo.0803244. Epub 2006/01/25. eng. [DOI] [PubMed] [Google Scholar]
- 7.Inelmen EM, Toffanello ED, Enzi G, Gasparini G, Miotto F, Sergi G, et al. Predictors of drop-out in overweight and obese outpatients. International Journal of Obesity. 2005 Jan;29(1):122–8. doi: 10.1038/sj.ijo.0802846. Epub 2004/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Blew RM, et al. Pretreatment predictors of attrition and successful weight management in women. International Journal of Obesity and Related Metabolic Disorders. 2004;28(9):1124–33. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 9.Honas JJ, Early JL, Frederickson DD, O'Brien MS. Predictors of attrition in a large clinic-based weight-loss program. Obesity Research. 2003 Jul;11(7):888–94. doi: 10.1038/oby.2003.122. Epub 2003/07/12. eng. [DOI] [PubMed] [Google Scholar]
- 10.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. Journal of the American Medical Association. 2005 Jan 5;293(1):43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 11.Niedhammer I, Bugel I, Bonenfant S, Goldberg M, Leclerc A. Validity of self-reported weight and height in the French GAZEL cohort. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000 Sep;24(9):1111–8. doi: 10.1038/sj.ijo.0801375. [DOI] [PubMed] [Google Scholar]
- 12.Kong A, Beresford SA, Imayama I, Duggan C, Alfano CM, Foster-Schubert KE, et al. Adoption of diet-related self-monitoring behaviors varies by race/ethnicity, education, and baseline binge eating score among overweight-to-obese postmenopausal women in a 12-month dietary weight loss intervention. Nutr Res. 2012 Apr;32(4):260–5. doi: 10.1016/j.nutres.2012.03.001. Epub 2012/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler JL, Follick MJ, Abrams DB, Rickard-Figueroa K. Participant characteristics as predictors of attrition in worksite weight loss. Addictive Behaviors. 1985;10(4):445–8. doi: 10.1016/0306-4603(85)90044-9. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell C, Stuart RB. Effect of self-efficacy on dropout from obesity treatment. Journal of consulting and clinical psychology. 1984 Dec;52(6):1100–1. doi: 10.1037//0022-006x.52.6.1100. Epub 1984/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 15.Clark MM, Guise BJ, Niaura RS. Obesity level and attrition: support for patient-treatment matching in obesity treatment. Obesity Research. 1995 Jan;3(1):63–4. doi: 10.1002/j.1550-8528.1995.tb00122.x. Epub 1995/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Heymsfield SB, Nguyen AM. Predictors of attrition and weight loss success: Results from a randomized controlled trial. Behaviour research and therapy. 2009 Aug;47(8):685–91. doi: 10.1016/j.brat.2009.05.004. Epub 2009/06/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus MD, Wing RR, Hopkins J. Obese binge eaters: affect, cognitions, and response to behavioural weight control. J Consult Clin Psychol. 1988 Jun;56(3):433–9. doi: 10.1037//0022-006x.56.3.433. Epub 1988/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Ho KS, Nichaman MZ, Taylor WC, Lee ES, Foreyt JP. Binge eating disorder, retention, and dropout in an adult obesity program. The International Journal of Eating Disorders. 1995 Nov;18(3):291–4. doi: 10.1002/1098-108x(199511)18:3<291::aid-eat2260180312>3.0.co;2-y. Epub 1995/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2005 Feb;6(1):43–65. doi: 10.1111/j.1467-789X.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 20.Clark MM, Cargill BR, Medeiros ML, Pera V. Changes in self-efficacy following obesity treatment. Obesity Research. 1996;4(2):179–81. doi: 10.1002/j.1550-8528.1996.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 21.Jelalian E, Hart CN, Mehlenbeck RS, Lloyd-Richardson EE, Kaplan JD, Flynn-O'Brien KT, et al. Predictors of attrition and weight loss in an adolescent weight control program. Obesity. 2008 Jun;16(6):1318–23. doi: 10.1038/oby.2008.51. Epub 2008/03/22. eng. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obes Rev. 2005;6(1):43–65. doi: 10.1111/j.1467-789X.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 23.Burke LE, Styn MA, Glanz K, Ewing LJ, Elci OU, Conroy MB, et al. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemporary clinical trials. 2009 Nov;30(6):540–51. doi: 10.1016/j.cct.2009.07.003. Epub 2009/08/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke LE, Styn MA, Ye L, Sereika SM, Ewing LJ. The SELF behavioral weight loss treatment trial: design and baseline characteristics. Annals of Behavioral Medicine. 2012;43(Suppl 1):s240. [Google Scholar]
- 25.Burke LE, Warziski M, Acharya S, Styn MA, Steenkiste A, Music E, et al. PREFER Trial: A randomized clinical trial testing treatment preference and two dietary options combined with behavioral weight management. Obesity. 2006;14(Suppl):A32. doi: 10.1038/oby.2006.235. [DOI] [PubMed] [Google Scholar]
- 26.Wing RR, Jeffery RW, Pronk N, Hellerstedt WL, et al. Effects of frequent phone contacts and optional food provision on maintenance of weight loss. Annals of Behavioral Medicine. 1996;18:172–6. doi: 10.1007/BF02883394. [DOI] [PubMed] [Google Scholar]
- 27.Burke LE, Kim Y, Music E. The barriers to healthy eating scale: Psychometric report. Annals of Behavioral Medicine. 2004;27( Supp):S101. [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961 Jun;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT. The Beck Depression Inventory II. San Antonio: Harcourt Brace & Co; 1996. [Google Scholar]
- 30.Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 31.Goode RW, Zheng Y, Sereika S, Styn M, Burke LE. Examination of the Psychometric Properties of the Hunger Satiety Scale. Annals of Behavioral Medicine. 2013;45(Suppl):s43. [Google Scholar]
- 32.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. Journal of consulting and clinical psychology. 1991;59:739–44. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 33.Turk MW, Sereika SM, Yang K, Ewing LJ, Hravnak M, Burke LE. Psychosocial correlates of weight maintenance among black & white adults. American journal of health behavior. 2012 Mar;36(3):395–407. doi: 10.5993/AJHB.36.3.10. Epub 2012/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behaviors. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 35.Grupski AE, Hood MM, Hall BJ, Azarbad L, Fitzpatrick SL, Corsica JA. Examining the Binge Eating Scale in screening for binge eating disorder in bariatric surgery candidates. Obesity surgery. 2013 Jan;23(1):1–6. doi: 10.1007/s11695-011-0537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freitas SR, Lopes CS, Appolinario JC, Coutinho W. The assessment of binge eating disorder in obese women: a comparison of the binge eating scale with the structured clinical interview for the DSM–IV. Eating behaviors. 2006 Aug;7(3):282–9. doi: 10.1016/j.eatbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 37.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. Journal of clinical epidemiology. 2005 Jun;58(6):568–78. doi: 10.1016/j.jclinepi.2004.10.015. Epub 2005/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 39.Kosinski M, Keller SD, Ware JE, Jr, Hatoum HT, Kong SX. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: relative validity of scales in relation to clinical measures of arthritis severity. Med Care. 1999 May;37(5 Suppl):MS23-39. doi: 10.1097/00005650-199905001-00003. [DOI] [PubMed] [Google Scholar]
- 40.Dalle Grave R, Calugi S, Molinari E, Petroni ML, Bondi M, Compare A, et al. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obesity Research. 2005 Nov;13(11):1961–9. doi: 10.1038/oby.2005.241. Epub 2005/12/13. eng. [DOI] [PubMed] [Google Scholar]
- 41.Reid RJ, Coleman K, Johnson EA, Fishman PA, Hsu C, Soman MP, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health affairs (Project Hope) 2010 May;29(5):835–43. doi: 10.1377/hlthaff.2010.0158. Epub 2010/05/05. eng. [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick SL, Jeffery R, Johnson KC, Roche CC, Van Dorsten B, Gee M, et al. Baseline predictors of missed visits in the look AHEAD study. Obesity (Silver Spring) 2013 Sep 2; doi: 10.1002/oby.20613. Epub 2013/09/03. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. The New England journal of medicine. 2011 Nov 24;365(21):1959–68. doi: 10.1056/NEJMoa1108660. Epub 2011/11/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennet G, Foley P, Levine E, Whiteley JA, Askew S, Steinberg D, et al. Behavioral treatment for weight grain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med. 2013;173(19):1770–7. doi: 10.1001/jamainternmed.2013.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett G, Warner E, Glasgow R, Askew S, Goldman J, Ritzwoller D, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172(7):565–74. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diabetes Prevention Program Research G. Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. The journals of gerontology Series A, Biological sciences and medical sciences. 2006 Oct;61(10):1075–81. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill RS, Karmali S, Hadi G, Al-Adra DP, Shi X, Birch DW. Predictors of attrition in a multidisciplinary adult weight management clinic. Canadian journal of surgery Journal canadien de chirurgie. 2012 Aug;55(4):239–43. doi: 10.1503/cjs.035710. Epub 2012/05/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farley RL, Wade TD, Birchmore L. Factors influencing attendance at cardiac rehabilitation among coronary heart disease patients. European Journal of Cardiovascular Nursing. 2003 Sep;2(3):205–12. doi: 10.1016/S1474-5151(03)00060-4. Epub 2003/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 49.Mazzeo SE, Saunders R, Mitchell KS. Binge eating among African American and Caucasian bariatric surgery candidates. Eating behaviors. 2005 Jun;6(3):189–96. doi: 10.1016/j.eatbeh.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Meyer C, McPartlan L, Sines J, Waller G. Accuracy of self-reported weight and height: relationship with eating psychopathology among young women. The International Journal of Eating Disorders. 2009 May;42(4):379–81. doi: 10.1002/eat.20618. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Blew RM, et al. Pretreatment predictors of attrition and successful weight management in women. International Journal of Obesity and Related Metabolic Disorders. 2004 Sep;28(9):1124–33. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 52.Colombo O, Ferretti VV, Ferraris C, Trentani C, Vinai P, Villani S, et al. Is dropout from obesity a predictable and preventable event? Nutrition Journal. 2014;13(13):1–7. doi: 10.1186/1475-2891-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: a randomized controlled trial. Obesity (Silver Spring) 2010 Feb;18(2):308–13. doi: 10.1038/oby.2009.242. Epub 2009/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagoto S, Schneider KL, Whited MC, Oleski JL, Merriam P, Appelhans B, et al. Randomized controlled trial of behavioral treatment for comorbid obesity and depression in women: the Be Active Trial. Int J Obes (Lond) 2013 Nov;37(11):1427–34. doi: 10.1038/ijo.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djuric Z, Mirasolo J, Kimbrough L, Brown DR, Heilbrun LK, Canar L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. Journal of the National Medical Association. 2009 Jun;101(6):552–64. doi: 10.1016/s0027-9684(15)30940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stahre L, Tarnell B, Hakanson CE, Hallstrom T. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. International journal of behavioral medicine. 2007;14(1):48–55. doi: 10.1007/BF02999227. [DOI] [PubMed] [Google Scholar]