Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option for progressive marrow failure, myelodysplastic syndrome, or leukemia associated with dyskeratosis congenita (DC). HSCT for DC is limited by a high incidence of treatment-related mortality, thought to be related to underlying chromosomal instability and sensitivity to chemotherapy and radiation. We report our experience in 7 patients with DC who underwent allogeneic transplantation using a reduced-intensity conditioning (RIC) preparative regimen that contained chemotherapy only (no radiation). This RIC regimen, designed specifically for patients with DC, contained alemtuzumab, fludarabine, and melphalan (with melphalan at 50% reduced dosing), with the goal of decreasing toxicity and improving outcome. All 7 patients engrafted, with none developing mixed chimerism or rejection. Two patients experienced acute graft-versus-host disease (GVHD) and 1 went on to develop limited chronic GVHD of the skin. Five patients remain alive and well at a median follow-up of 44 months (range, 14 to 57 months). We conclude that a radiation-free RIC regimen results in durable engraftment, acceptable toxicity, and improved overall survival in patients with DC undergoing allogeneic HSCT.
Keywords: Dyskeratosis congenita, Hematopoietic stem cell, transplantation, Reduced-intensity conditioning
INTRODUCTION
Dyskeratosis congenita (DC) is a rare genetic telomeropathy resulting in bone marrow failure (BMF) and cancer predisposition. The classic triad of dystrophic nails, oral leukoplakia, and skin hyperpigmentation is found in the majority of patients with DC, though these features may be subtle and present at different later ages [1]. Patients with DC have very short telomeres (< first percentile for their age) resulting from mutations in telomere biology genes, including DKC1, TERC (hTR), TERT, NOP10, NOLA2 (NHP2), NOLA3 (NOP10), TCAB1 (WDR79, WRAP53), RTEL1, and TINF2 [2-6].
Patients with DC are at risk of BMF, myelodysplastic syndrome (MDS)/leukemia, and other cancers [7,8]. Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment for severe BMF or MDS/leukemia in these patients. Allogeneic HSCT corrects the underlying defect in hematopoietic precursors that led to BMF and/or MDS. However, it does not alter other features of the disease, such as pulmonary fibrosis, liver cirrhosis and fibrosis, or proliferative retinopathy [3,9-16]. Long-term HSCT outcomes for patients with DC have been poor, especially after myeloablative HSCT [17,18]. Reported complications include graft rejection and failure, graft-versus-host disease (GVHD), sepsis, pulmonary fibrosis, hepatic cirrhosis, and veno-occlusive disease [12,19]. Some of these are, in part, related to the underlying telomere biology disorder and its associated pulmonary or liver disease [12,17,18,20-23].
Reduced-intensity conditioning (RIC) regimens reported by a handful of institutions have shown improvement in overall HSCT outcomes for DC [24-26]. Some of these studies, however, incorporate radiation-containing conditioning regimens [18,24,27], which may promote engraftment but may also contribute to organ toxicity (particularly, pulmonary and hepatic fibrosis) due to the underlying telomere dysfunction [28,29]. We report our outcomes in patients with DC undergoing allogeneic HSCT using a nonradiation-containing RIC preparative regimen with alemtuzumab, fludarabine, and melphalan (with melphalan at reduced dosing of 70 mg/m2).
METHODS
Patients
Seven children with DC underwent RIC allogeneic HSCT between September 2010 and April 2014; 6 at Cincinnati Children’s Hospital Medical Center and 1 child at Sydney Children’s Hospital, Australia. Patient charts were reviewed retrospectively after obtaining approval from the institutional review boards.
Conditioning Regimens and GVHD Prophylaxis
Six of 7 patients received alemtuzumab over days −22 to −18 (n = 1), −14 to −10 (n = 4), or −6 to −2 (n = 1), per institutional standard at the time. One patient with both MDS (with monosomy 7) and DC did not receive alemtuzumab to avoid mixed chimerism in the presence of a malignant clone. Alemtuzumab dosing for the majority of the patients (n = 5) consisted of 10 mg, 15 mg, and 20 mg on consecutive days, after a test dose of 3 mg on day −22 or −14. Two patients less than 10 kg in weight received .2 mg/kg/dose × 5 days. All patients received fludarabine at 30 mg/m2/dose to 40 mg/m2/dose (5 mg/kg/dose if < 10 kg) on days −8 to −4 (higher dose fludarabine was used in the patient not receiving alemtuzumab and another as per institutional practice). The melphalan dose was reduced by 50% to 70 mg/m2 to avoid excessive toxicity related to baseline chemo-sensitivity.
All patients received GVHD prophylaxis consisting of cyclosporine and either prednisone (n = 4) or mycophenolate mofetil (n = 3). Cyclosporine trough levels were maintained between 250 ng/mL and 350 ng/mL. Diagnoses of acute GCHD (aGVHD) and chronic GVHD were based on published criteria [30-32].
Supportive Care
All patients were isolated in high-efficiency particulate air filtered rooms from admission to day of discharge. All patients received intravenous immunoglobulin replacement to maintain levels within normal range (or higher in the presence of active viral infection). Filgrastim was started at 5 mcg/kg/dose on day +1 (excluding the patient treated for MDS) and continued until engraftment. All patients received antiviral, antifungal, and anti-pneumocystis jiroveci prophylaxis per institutional standards. Cytomegalovirus, Epstein-Barr virus, and adenovirus surveillance PCRs were monitored weekly and viral reactivations were treated according to standard institutional guidelines.
Engraftment and Donor Chimerism
Outcomes studied included neutrophil engraftment, defined as achieving an absolute neutrophil count ≥ .5 × 109/L for 3 consecutive days, and platelet engraftment, defined as platelet count ≥ 20 × 109/L without transfusion for 7 days. Engraftment studies were performed using XY fluorescence in situ hybridization in the case of opposite sex donors and short tandem repeat analysis in the case of same sex donors. Engraftment was followed weekly until day 100 and as needed thereafter. Sorted engraftment study was performed as necessary. Surviving patients were censored at last follow-up and death from any cause was considered an event.
RESULTS
Patients, Diseases, and Transplantation Characteristics
Patient and disease characteristics of the 7 patients who underwent transplantation using RIC are shown in Table 1. There were 4 males and 3 female patients with the median age at allogeneic HSCT of 6.75 years (range, 1.3 to 12.5 years). All patients had telomere lengths < first percentile for their age, and 6 patients also had an identified genetic mutation in the DC complex (Table 1).
Table 1.
Patient and Disease Characteristics
| Patient | Sex | Age at Diagnosis, yr |
Gene Mutation |
Telomere Length < First Percentile |
Pre-HSCT BM Cellularity |
Pre-HSCT PFTs | Pre-HSCT Parenchymal Lung Disease |
Pre-HSCT Liver Abnormalities* |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 6.75 | Unknown | Yes | 60% with MDS | Normal | Small airway disease | Nil |
| 2 | Female | 7 | TINF2 | Yes | 5% | Mild decreased DLCO | Normal | Nil |
| 3 | Female | 1.3 | RTEL1 | Yes | 30% | Normal | Normal | Nil |
| 4 | Male | 3.1 | DKC1 | Yes | 5% | Normal | Normal | Nil |
| 5 | Male | 12.5 | TERT | Yes | 10% | Mild restrictive disease | Diffuse lung disease | Nil |
| 6 | Female | 11 | TERT | Yes | 5% | Normal | Normal | Nil |
| 7 | Male | 3.2 | TINF2 | Yes | 20% | Normal | Normal | Nil |
BM indicates bone marrow; PFT, pulmonary function test; DLCO, carbon monoxide diffusing capacity.
Including abdominal ultrasonography, computed tomography scan, and hepatic profile.
Indications for transplantation included transfusion-dependent BMF in 6 patients (including 1 patient with Hoyeraal-Hreidarsson variant with severe colitis) and MDS with monosomy 7 (refractory cytopenia with multilineage dysplasia) in 1 patient.
There were 6 unrelated donors and 1 matched sibling donor (who did not carry the TINF2 mutation identified in the patient). Five patients received stem cells from a fully matched unrelated donor, 1 patient received stem cells from a matched sibling donor, and 1 patient received stem cells from a 1 antigen–mismatch unrelated donor. Bone marrow was used as the allogeneic stem cell source in all patients. The median infused total nucleated cell count was 8.8 × 108/kg (range, 5.5 to 10 × 108/kg).
Engraftment
Median time to neutrophil engraftment for all patients was 12 days (range, 10 to 16 days) (Table 2). The median time to platelet engraftment for all was 20 days (range, 14 to 35 days). None of the patients experienced mixed chimerism and none developed graft failure at a median follow-up of 44 months (range, 14 to 57 months).
Table 2.
Transplantation Characteristics and Outcomes
| Patient | Age at HSCT |
Donor Source and Match |
TNC Dose/ kg, × 108/kg |
Timing of Campath, d |
Neutrophil Engraftment, d |
Platelet Engraftment, d |
Mixed Chimerism |
Acute GVHD |
Chronic GVHD |
Viral Reactivation |
Follow-Up, mo, and Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.1 | URD, 8/8 | 9.2 | N/A | +14 | +20 | No | No | No | EBV | 48, alive |
| 2 | 9.3 | URD, 8/8 | 5.5 | −22 to −19 | +12 | +17 | No | Grade II skin |
Limited skin |
CMV, BK | 28, dead |
| 3 | 1.7 | URD, 8/8 | 10 | −14 to −10 | +10 | +26 | No | Grade III GI | No | No | 5, dead |
| 4 | 3.5 | URD, 7/8 | 10 | −14 to −10 | +10 | +14 | No | No | No | BK, Adenovirus | 34, alive |
| 5 | 13.2 | URD, 10/10 | 8.8 | −14 to −10 | +16 | +35 | No | No | No | CMV, EBV | 11, alive |
| 6 | 11.9 | URD, 10/10 | 5.7 | −14 to −10 | +10 | * | No | No | No | CMV, BK, EBV | 5, alive |
| 7 | 3.8 | MSD, 6/6 | 7.2 | −6 to −2 | +14 | +22 | No | No | No | CMV | 37, alive |
URD indicates unrelated donor; N/A, not available; EBV, Epstein-Barr virus; CMV, cytomegalovirus; GI, gastrointestinal; MSD matched sibling donor.
Platelet count did not drop below 20 × 109/L.
GVHD
Two patients experienced aGVHD. One patient developed grade II skin GVHD, which progressed to limited chronic GVHD of the skin (identifiable as they did not have cutaneous manifestations of DC before HSCT). Grade III gastrointestinal GVHD was observed in 1 patient who had pre-existing colitis before HSCT (confirmed on gastrointestinal biopsy). No patients experienced grade IV aGVHD.
Infections
Two patients experienced bacteremia with Staphylococcus aureus. Cytomegalovirus was the most frequent viral reactivation, occurring in 4 patients, and was successfully treated with antiviral and supportive medications. Other viral reactivations included adenoviremia (n 1), Epstein-Barr viremia (n = 3), and BK viremia (n = 3). No patients died as the result of viral reactivations. No patients experienced fungal infections.
Survival
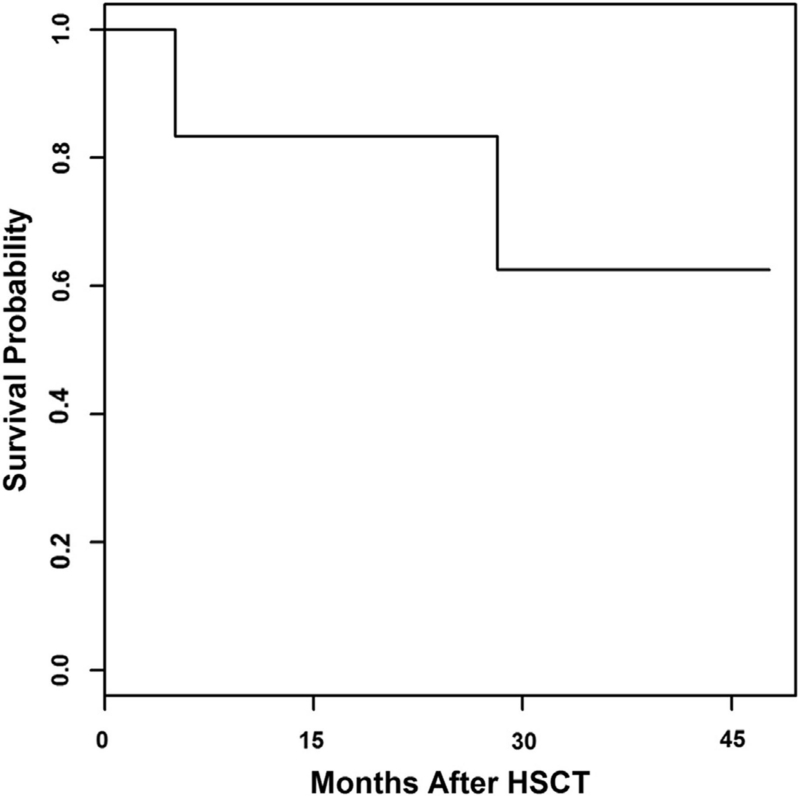
Five patients (71%) remain alive and well at a median follow-up of 44 months (range, 14 to 57 months) (Figure 1). Cause of death for 2 patients included multiorgan failure at 28 months after HSCT secondary to methicillin-resistant Staphylococcus aureus sepsis and idiopathic pneumonia syndrome at 5 months after HSCT.
Figure 1.
Overall survival.
Discussion
Allogeneic HSCT for DC remains challenging because of the inherent chemo-sensitivity and related increased toxicity, along with increased potential for late effects related to the natural history of the disease. To our knowledge, this is the largest series of pediatric patients who underwent transplantation for DC using a uniform RIC regimen containing alemtuzumab, fludarabine, and melphalan. We report an overall survival of 71%, which is comparable to other recent reports of HSCT with RIC for DC without exposure to potentially damaging radiation [18,24].
Traditionally, myeloablative transplantations for DC have resulted in poor outcomes, with toxicity and infections contributing to increased mortality [17,33]. Myeloablative grafts also resulted in long-term toxicities, including pulmonary fibrosis, which likely develops from the combination of the chemotherapy and the inherent pulmonary complications of DC. Similarly, a Center for International Blood and Marrow Transplant Research analysis of 34 patients with DC also confirmed increased mortality associated with myeloablative therapy during unrelated donor transplantation [18]. Although the Center for International Blood and Marrow Transplant Research analysis included heterogeneous chemotherapy regimens, more recent trends towards RIC-based therapy may lead to decreased acute and chronic toxicity in this fragile population.
Complications after HSCT can at times add to the natural history of the disease or can be difficult to distinguish from it. Vascular complications affecting the renal, pulmonary, and hepatic systems have been described in patients with DC, causing death up to 5 years after HSCT [34]. Similarly, pulmonary disease can manifest with symptoms that mimic chronic GVHD, making diagnosis and treatment difficult, and contributing to morbidity [20]. We have not observed late effects in any of the patients treated using our RIC regimen, though we realize that we need longer follow-up to monitor for these complications.
RIC regimens can be associated with a higher incidence of mixed chimerism and graft failure [35]. Previous reports of transplantation in DC have shown varying levels of mixed chimerism and rates of both primary and secondary graft failure [18,24,36,37]. In contrast, none of our patients developed mixed chimerism. Additionally, there was no early or late graft failure or graft rejection seen in our cohort, which is quite encouraging. Use of fludarabine in our conditioning regimen may have provided the needed potent immunosuppression to facilitate full donor cell engraftment. The use of alemtuzumab in an intermediate schedule from day -14 has been shown to reduce the incidence of both mixed chimerism and GVHD compared with proximal and distal schedules [38] and may have benefited our patients. Encouragingly, despite the use of alemtuzumab, viremias were seen; however, none of our patients experienced significant morbidity or mortality associated with viral infections, which has been a concern with the use of alemtuzumab [39].
Complications related to DC, in addition to long-term effects of HSCT, remain an important post-transplantation problem for this vulnerable population. Late death after HSCT has been reported to occur because of gastrointestinal and pulmonary complications, as well as development of second cancers [22,40,41]. The risk of pulmonary complications in DC patients both before and after transplantation has led to careful use of conditioning agents, with an effort to avoid both busulfan- and radiation-based conditioning regimens [8,18].
An additional important issue related to HSCT for DC is the wide range of clinical phenotypes seen in DC and the possibility of nonmanifesting or very mildly affected individuals within families, complicating the selection of related HSCT donors [42]. Potential related HSCT donors should be tested either for the mutation present in the proband or, if the mutation is not known, for telomere length. The matched sibling donor available for 1 of our patients was tested for the TINF2 mutation observed in the recipient and was confirmed to be negative for the same before HSCT. Similarly, children who present with marrow failure should be screened for telomeropathy as well as Fanconi anemia because both these diseases may occur without syndromic features.
Our study is limited by its retrospective nature and a relatively small number of patients, making evaluation of risk factors for outcomes quite challenging. Larger, prospective collaborative studies are needed to have a meaningful pre-HSCT risk factor assessment for children with DC undergoing HSCT and to identify the best conditioning regimen for these high-risk patients. Similarly, long-term follow-up is essential to see if the benefits of RIC indeed translate into improved long-term HSCT outcomes. In summary, our results show that patients with DC can successfully undergo HSCT using a radiation-free RIC regimen with superior overall survival, engraftment, and minimal toxicity. It is possible that successful outcomes could be achieved with even lower doses of melphalan or elimination of the alkylating agent entirely in future studies. Regular follow-up for the complications that are not corrected by HSCT is essential in this group including oral, pulmonary, hepatic, ophthalmological, and dermatological examination or evaluation, along with genetic counseling for at-risk family members.
Footnotes
Financial disclosure statement: There is nothing to disclose.
Conflict of Interest statement: The authors declare no conflict of interest.
Authorship statement: A.S.N, R.A.M, K.M, S.M.D, S.J, T.A.O, and P.A.M collected and analyzed the data. P.A.M and S.M.D designed the study. A.S.N. and P.A.M. wrote the manuscript. All authors reviewed the paper and approved the final version.
REFERENCES
- 1.Womer R, Clark JE, Wood P, et al. Dyskeratosis congenita: two examples of this multisystem disorder. Pediatrics. 1983;71:603–609. [PubMed] [Google Scholar]
- 2.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Myers K, Ye Z, D’Orazio J. Dyskeratosis congenita caused by a novel TERT point mutation in siblings with pancytopenia and exudative retinopathy. Pediatr Blood Cancer. 2014;61:2302–2304. doi: 10.1002/pbc.25161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason PJ, Bessler M. The genetics of dyskeratosis congenita. Cancer Genet. 2011;204:635–645. doi: 10.1016/j.cancergen.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JW. Telomere shortening by mutations in the RTEL1 helicase cause severe form of dyskeratosis congenita, Hoyerall-Hreidarsson syndrome. Clin Genet. 2013;84:210. doi: 10.1111/cge.12175. [DOI] [PubMed] [Google Scholar]
- 6.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 7.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:79–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 9.Ballew BJ, Joseph V, De S, et al. A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome. PLoS Genet. 2013;9:e1003695. doi: 10.1371/journal.pgen.1003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz de Leon A, Cronkhite JT, Katzenstein AL, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du HY, Pumbo E, Ivanovich J, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr Transplant. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Brown TC, Juhlin CC, et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr Relat Cancer. 2014;21:427–434. doi: 10.1530/ERC-14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon M, Hosen I, Gousias K, et al. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17:45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vulliamy TJ, Marrone A, Knight SW, et al. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Liu T, Sofiadis A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014;120:2965–2979. doi: 10.1002/cncr.28800. [DOI] [PubMed] [Google Scholar]
- 17.Langston AA, Sanders JE, Deeg HJ, et al. Allogeneic marrow transplantation for aplastic anaemia associated with dyskeratosis congenita. Br J Haematol. 1996;92:758–765. doi: 10.1046/j.1365-2141.1996.424984.x. [DOI] [PubMed] [Google Scholar]
- 18.Gadalla SM, Sales-Bonfim C, Carreras J, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biol Blood Marrow Transplant. 2013;19:1238–1243. doi: 10.1016/j.bbmt.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthou C, Devergie A, D’Agay MF, et al. Late vascular complications after bone marrow transplantation for dyskeratosis congenita. Br J Haematol. 1991;79:335–336. doi: 10.1111/j.1365-2141.1991.tb04543.x. [DOI] [PubMed] [Google Scholar]
- 20.Yabe M, Yabe H, Hattori K, et al. Fatal interstitial pulmonary disease in a patient with dyskeratosis congenita after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19:389–392. doi: 10.1038/sj.bmt.1700674. [DOI] [PubMed] [Google Scholar]
- 21.Dror Y, Freedman MH, Leaker M, et al. Low-intensity hematopoietic stem-cell transplantation across human leucocyte antigen barriers in dyskeratosis congenita. Bone Marrow Transplant. 2003;31:847–850. doi: 10.1038/sj.bmt.1703931. [DOI] [PubMed] [Google Scholar]
- 22.Brazzola P, Duval M, Fournet JC, et al. Fatal diffuse capillaritis after hematopoietic stem-cell transplantation for dyskeratosis congenita despite low-intensity conditioning regimen. Bone Marrow Transplant. 2005;36:1103–1105. doi: 10.1038/sj.bmt.1705171. author reply 1105. [DOI] [PubMed] [Google Scholar]
- 23.Ostronoff F, Ostronoff M, Calixto R, et al. Fludarabine, cyclophosphamide, and antithymocyte globulin for a patient with dyskeratosis congenita and severe bone marrow failure. Biol Blood Marrow Transplant. 2007;13:366–368. doi: 10.1016/j.bbmt.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishio N, Takahashi Y, Ohashi H, et al. Reduced-intensity conditioning for alternative donor hematopoietic stem cell transplantation in patients with dyskeratosis congenita. Pediatr Transplant. 2011;15:161–166. doi: 10.1111/j.1399-3046.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 26.Vuong LG, Hemmati PG, Neuburger S, et al. Reduced-intensity conditioning using fludarabine and antithymocyte globulin alone allows stable engraftment in a patient with dyskeratosis congenita. Acta Haematol. 2010;124:200–203. doi: 10.1159/000318721. [DOI] [PubMed] [Google Scholar]
- 27.Kharfan-Dabaja MA, Otrock ZK, Bacigalupo A, et al. A reduced intensity conditioning regimen of fludarabine, cyclophosphamide, antithymocyte globulin, plus 2 Gy TBI facilitates successful hematopoietic cell engraftment in an adult with dyskeratosis congenita. Bone Marrow Transplant. 2012;47:1254–1255. doi: 10.1038/bmt.2011.257. [DOI] [PubMed] [Google Scholar]
- 28.Gadalla SM, Savage SA. Telomere biology in hematopoiesis and stem cell transplantation. Blood Rev. 2011;25:261–269. doi: 10.1016/j.blre.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775–2783. doi: 10.1182/blood-2014-05-526285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 31.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 32.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. viii–ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 33.Ghavamzadeh A, Alimoghadam K, Nasseri P, et al. Correction of bone marrow failure in dyskeratosis congenita by bone marrow transplantation. Bone Marrow Transplant. 1999;23:299–301. doi: 10.1038/sj.bmt.1701567. [DOI] [PubMed] [Google Scholar]
- 34.Rocha V, Devergie A, Socie G, et al. Unusual complications after bone marrow transplantation for dyskeratosis congenita. Br J Haematol. 1998;103:243–248. doi: 10.1046/j.1365-2141.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 35.Svenberg P, Mattsson J, Ringdén O, Uzunel M. Allogeneic hematopoietic SCT in patients with non-malignant diseases, and importance of chimerism. Bone Marrow Transplant. 2009;44:757–763. doi: 10.1038/bmt.2009.82. [DOI] [PubMed] [Google Scholar]
- 36.Ayas M, Nassar A, Hamidieh AA, et al. Reduced intensity conditioning is effective for hematopoietic SCT in dyskeratosis congenita-related BM failure. Bone Marrow Transplant. 2013;48:1168–1172. doi: 10.1038/bmt.2013.35. [DOI] [PubMed] [Google Scholar]
- 37.Ayas M, Al-Musa A, Al-Jefri A, et al. Allogeneic stem cell transplantation in a patient with dyskeratosis congenita after conditioning with low-dose cyclophosphamide and anti-thymocyte globulin. Pediatr Blood Cancer. 2007;49:103–104. doi: 10.1002/pbc.20696. [DOI] [PubMed] [Google Scholar]
- 38.Marsh RA, Kim MO, Liu C, et al. An intermediate alemtuzumab schedule reduces the incidence of mixed chimerism following reduced-intensity conditioning hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant. 2013;19:1625–1631. doi: 10.1016/j.bbmt.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison VA. Immunosuppression associated with novel chemotherapy agents and monoclonal antibodies. Clin Infect Dis. 2014;59(Suppl 5):S360–S364. doi: 10.1093/cid/ciu592. [DOI] [PubMed] [Google Scholar]
- 40.Amarasinghe K, Dalley C, Dokal I, et al. Late death after unrelated-BMT for dyskeratosis congenita following conditioning with alemtuzumab, fludarabine and melphalan. Bone Marrow Transplant. 2007;40:913–914. doi: 10.1038/sj.bmt.1705839. [DOI] [PubMed] [Google Scholar]
- 41.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]