SUMMARY
Multiple division cycles without growth are a characteristic feature of early embryogenesis. The female germline loads proteins and RNAs into oocytes to support these divisions, which lack many quality control mechanisms operating in somatic cells undergoing growth. Here we describe a small RNA-Argonaute pathway that ensures early embryonic divisions in C. elegans by employing catalytic slicing activity to broadly tune, instead of silence, germline gene expression. Misregulation of one target, a kinesin-13 microtubule depolymerase, underlies a major phenotype associated with pathway loss. Tuning of target transcript levels is guided by density of homologous small RNAs, whose generation must ultimately be related to target sequence. Thus, the tuning action of a small RNA-catalytic Argonaute pathway generates oocytes capable of supporting embryogenesis. We speculate that the specialized nature of germline chromatin led to emergence of small RNA-catalytic Argonaute pathways in the female germline as a post-transcriptional control layer to optimize oocyte composition.
Keywords: Argonaute, small RNA, embryogenesis, germline, maternal load, chromosome, centromere, fertility, oogenesis, 22G RNA, CSR-1, C. elegans
INTRODUCTION
Maternal loading of proteins and RNAs into oocytes by the female germline limits the requirement for transcriptional and translational activity during early embryogenesis. While the benefits of such a strategy are evident, it raises the question of how the composition of the maternal is specified since homeostatic mechanisms that operate during growth may no longer be relevant. Thus, specific mechanisms are likely to exist in the female germline to ensure accurate stoichiometry of maternally loaded components. In the process of investigating a small RNA-Argonaute pathway previously implicated in chromosome segregation, we uncovered a mechanism that performs such a function in C. elegans.
Argonautes are a conserved class of proteins implicated in diverse small RNA-based processes (Carmell et al., 2002; Kuhn and Joshua-Tor, 2013; Swarts et al., 2014). Argonautes are particularly prominent in C. elegans (Billi et al., 2014; Grishok, 2013), where they have been implicated in diverse processes including RNA interference (Tabara et al., 1999), transposon silencing (Batista et al., 2008; Das et al., 2008), self/non-self discrimination (Ashe et al., 2012; Gu et al., 2009; Lee et al., 2012; Seth et al., 2013; Shirayama et al., 2012), germline immortality (Buckley et al., 2012; Yigit et al., 2006), and transgenerational epigenetic inheritance (Ashe et al., 2012; Buckley et al., 2012; Shirayama et al., 2012). Of the 27 C. elegans Argonautes (Yigit et al., 2006), only one—CSR-1—is absolutely essential for fertility and embryo viability (Claycomb et al., 2009; Yigit et al., 2006). CSR-1 is bound to a class of small 22 nucleotide RNAs with a guanosine on their 5′ ends known as 22G RNAs (Gu et al., 2009). 22G RNAs act in two different Argonaute pathways in the germline, one employing CSR-1 and a second employing WAGO class Argonautes (Claycomb et al., 2009; Gu et al., 2009). CSR-1-bound 22G RNAs are homologous to germline-expressed transcripts and CSR-1—22G RNA complexes have been suggested to act in a diverse array of processes: chromosome and centromere organization (Claycomb et al., 2009), maturation of core histone mRNAs (Avgousti et al., 2012), assembly of germline ribonucleprotein structures known as P granules (Claycomb et al., 2009; Updike and Strome, 2009), protection of germline transcription via an effect on chromatin (Wedeles et al., 2013), definition of self versus non-self in a balance with the WAGO—22G RNA pathway that acts downstream of the Piwi class Argonaute PRG-1 and its associated 21U RNAs (Seth et al., 2013), promotion of sense transcription (Cecere et al., 2014), and translational control in the mitotic zone of the germline (Friend et al., 2012). A fundamental assumption in prior studies has been that CSR-1 does not significantly control transcript levels of target genes with homology to its bound 22G RNAs. This assumption, based on microarray analysis of csr-1 mutants (Claycomb et al., 2009) and sequencing analysis following feeding RNAi-based reduction of CSR-1 (Campbell and Updike, 2015), is surprising because CSR-1 has conserved residues implicated in slicing (Yigit et al., 2006) and is required for the major slicing activity in C. elegans extracts (Aoki et al., 2007).
In prior work, we found that genes with homology to CSR-1-bound 22G RNAs are in genomic regions depleted of the centromere-specific histone H3 variant CENP-A (Gassmann et al., 2012), raising the possibility that CSR-1—22G RNA complexes limit centromeric domains on holocentric C. elegans chromosomes. This, together with the chromosome segregation defect observed in early C. elegans embryos, prompted us to investigate how CSR-1 contributes to chromosome segregation. Using high-resolution phenotyping in early embryos to compare the effects of removing CSR-1 to selective ablation of its catalytic slicing activity, we found that loss of slicing activity fully phenocopied CSR-1 removal. In addition, while chromosome and centromere organization were unaffected, CSR-1 inhibition resulted in severe defects in the microtubule cytoskeleton caused by elevated expression of a kinesin-13 microtubule depolymerase prominently represented in the ensemble of CSR-1 —22G RNA complexes. Genome-wide analysis revealed widespread 22G RNA density-dependent tuning, but not silencing, of target gene expression by CSR-1 slicing activity. Thus, CSR-1 slicing activity, guided by bound 22G RNAs, tunes the expression of a large number of germline-encoded transcripts to generate a balanced maternal load that can support embryogenesis.
RESULTS
CSR-1 Inhibition Reduces Microtubule Assembly in One-Cell Embryos
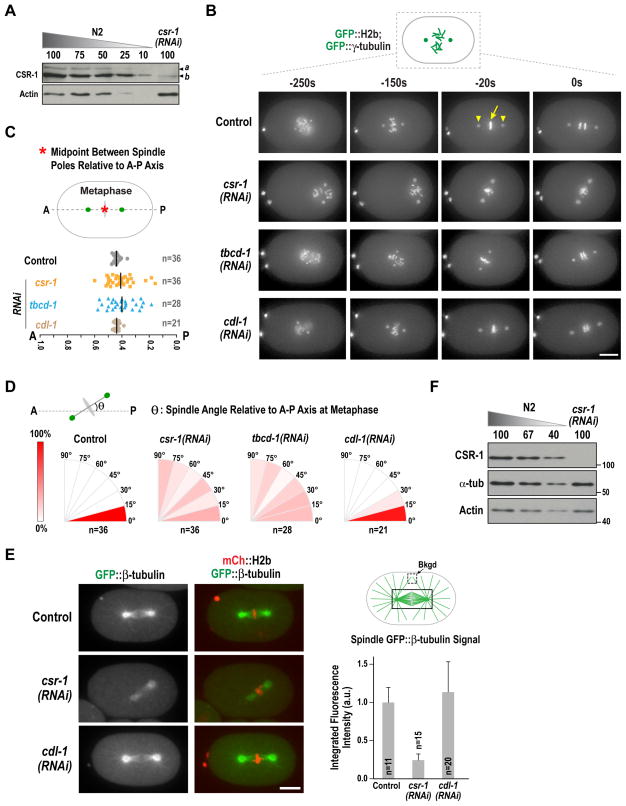
To dissect the role of CSR-1 in chromosome segregation, we analyzed the consequences of removing CSR-1 on the first embryonic cell division. As CSR-1 has been proposed to act with CDL-1, which binds the 3′ UTR of core histone mRNAs to promote their maturation and translation (Avgousti et al., 2012), we also analyzed CDL-1 depletion in parallel. CSR-1 has a short isoform b and a longer isoform a. CSR-1b is functionally critical in embryos as depleting isoform a alone did not affect embryonic viability, whereas depleting >95% of both isoforms led to penetrant embryonic lethality (Fig. 1A; S1A–C).
Figure 1. CSR-1 inhibition reduces microtubule assembly in one-cell embryos.
(A) Immunoblot comparing a standard curve of N2 wildtype worm extract to CSR-1 depleted worm extract. β-actin serves as a loading control. Bands corresponding to the two CSR-1 isoforms (a and b; Fig. S1A–C) are indicated.
(B) Images from time-lapse sequences of embryos expressing GFP::H2b (yellow arrow in Control −20s panel) and GFP::γ-tubulin (yellow arrowheads in Control −20s panel) for the indicated conditions. Time is in seconds relative to early anaphase. Scale bar, 10 μm.
(C) Plot of the position of the spindle midpoint (defined in maximal intensity projections as the midpoint between GFP::γ-tubulin foci) projected onto the embryo A-P axis at metaphase, for the indicated conditions.
(D) Plot of the distribution of the minimum angle between the spindle axis (defined in maximal intensity projections by a line connecting the GFP::γ-tubulin foci) and the embryo A-P axis at metaphase for the indicated conditions.
(E) Representative images (left) and quantification (right) of metaphase spindle GFP::β-tubulin signal for the indicated conditions. Background-subtracted integrated fluorescence intensity measurements were normalized to the control mean value. Scale bar, 10 μm.
(F) Immunoblot of α-tubulin in csr-1(RNAi) compared to a standard curve of N2 (control) worms. β-actin serves as a loading control. Molecular weight markers (in kD) are indicated on the right.
Filming one-cell embryos co-expressing GFP::histone H2b and GFP::γ-tubulin, which label the chromosomes and spindle poles, revealed a clear difference in the CSR-1 and CDL-1 depletion phenotypes (Fig. 1B). CSR-1 depletion led to phenotypes characteristic of defects in the microtubule cytoskeleton, including unstable positioning of the mitotic spindle along the A-P axis (Fig. 1B,C) and randomization of metaphase spindle angle relative to the A-P axis (Fig. 1B,D). These phenotypes were similar to those following depletion of the tubulin chaperone TBCD-1 (Fig. 1B–D; tubulin-specific cofactor D), CDL-1 depletions, which reduced global histone levels (Fig. S1D; (Avgousti et al., 2012), did not exhibit these phenotypes but instead exhibited defects in chromosome condensation and structure (Fig. 1B, −250 s panel & Fig. 2A below). Imaging in a strain expressing GFP::β-tubulin and mCherry::H2b revealed greatly reduced microtubule assembly in CSR-1 and TBCD-1 but not in CDL-1 depleted embryos (Fig. 1E, S1H). α-tubulin levels were not changed by CSR-1 depletion (Fig. 1F), suggesting that the reduction in microtubule assembly is not due to reduced availability of α/β-tubulin dimers.
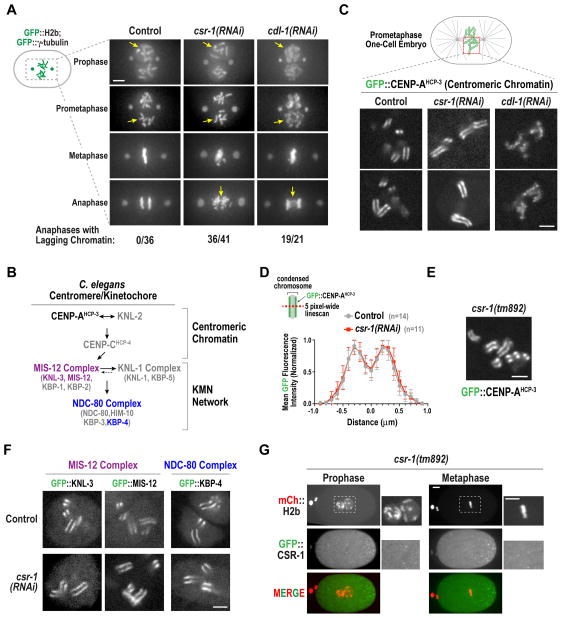
Figure 2. CSR-1 inhibition does not perturb chromosome structure or centromere/kinetochore architecture in one-cell embryos.
(A) Images from the nuclear region from time-lapse sequences of one-cell embryos expressing GFP:H2b and GFP:γ-tubulin. Arrows highlight features of chromosome structure at different stages in control, csr-1(RNAi) and cdl-1(RNAi) that are discussed in the text. Scale bar, 10 μm.
(B) Schematic summary of C. elegans centromere/kinetochore assembly.
(C) Single plane confocal images of GFP::CENP-AHCP-3 for the indicated conditions. Two examples are shown per condition. Scale bar, 5 μm.
(D) Linescan analysis of GFP::CENP-AHCP-3 fluorescence on condensed chromosomes. 5 pixel-wide individual linescans of single chromosomes were normalized based on peak intensity, centered on the valley between the peaks of GFP signal, and averaged. Error bars are the S.D. Control, n=14 chromosomes from 6 embryos. csr-1(RNAi), n=11 chromosomes from 9 embryos.
(E) Single plane confocal image of GFP::CENP-AHCP-3 in a rare one-cell embryo derived from a csr-1(tm892) homozygous worm. Scale bar, 5 μm.
(F) Single plane confocal images for the indicated GFP-fused kinetochore components. See also Fig. S2B. Scale bar, 5 μm.
(G) Maximum intensity z-stack projections of prometaphase and metaphase stage one-cell csr-1(tm892) embryos rescued by expression of GFP::CSR-1 from a single copy transgene (Fig. S2C–E). The embryos also express mCherry::H2b. Magnified images of boxed regions are shown on the right. Scale bars, 5 μm.
CSR-1 binds 22G RNAs generated by the EGO-1/DRH-3/EKL-1 complex (Gu et al., 2009). Imaging embryos depleted of EGO-1, DRH-3 or EKL-1 revealed phenotypes similar to CSR-1 depletion, indicating that the contribution of CSR-1 to microtubule assembly depends on its association with 22G RNAs (Fig. S1E–G). We conclude that whereas CDL-1 inhibition results in defects in chromatin structure consistent with its proposed role in histone production, inhibition of CSR-1 or the factors that generate its bound 22G RNAs leads to a severe defect in microtubule assembly.
CSR-1 Inhibition Does Not Affect Chromosome Structure or Centromere/Kinetochore Assembly in One-Cell Embryos
CSR-1—22G RNA complexes have been proposed to control chromosome architecture and centromere organization in the early embryo, potentially by controlling histone mRNA processing and/or by guiding deposition of the centromeric histone variant CENP-A (Avgousti et al., 2012; Claycomb et al., 2009). Our initial analysis suggested that CSR-1 depletion did not cause visible defects in mitotic chromosome structure. Consistent with this impression, closer analysis revealed that while CDL-1 depletion caused chromosome condensation defects during mitotic entry, chromosomes were normally condensed in CSR-1 depleted embryos (Fig. 2A). Both CSR-1 and CDL-1 depletion led to lagging anaphase chromatin; however, the morphology was distinct (Fig. 2A). In CSR-1 depleted embryos, condensed chromosomes were lagging, whereas in CDL-1 depleted embryos, poorly condensed chromatin was stretched between the separating chromosome masses. We conclude that the segregation defects in CSR-1 depleted embryos cannot be explained by action in the CDL-1-dependent histone mRNA maturation pathway.
To test the proposal that CSR-1 patterns centromere/kinetochore architecture (Claycomb et al., 2009; Gassmann et al., 2012), we analyzed a functional GFP fusion with the centromeric histone variant CENP-AHCP-3, which forms the structural foundation for kinetochore assembly (Fig. 2B; Oegema et al., 2001). GFP::CENP-AHCP-3 was patterned into two stripes that ran along the length of the holocentric chromosomes in early prometaphase embryos (Fig. 2C). Qualitative assessment and quantitative linescans across individual chromosomes revealed essentially identical localization in control and CSR-1-depleted embryos (Fig. 2D). In contrast, CDL-1 depleted embryos exhibited abnormal GFP::CENP-AHCP-3 localization (Fig. 2C), consistent with defective chromosome condensation (Fig. 2A). These results were confirmed by analysis of rare one-cell embryos from the csr-1(tm892) null mutant, which has severely reduced fertility (Fig. 2E; Fig. S2A), and by analysis of three outer kinetochore components (Fig. 2B; Cheeseman et al., 2004): KNL-3, MIS-12 and KBP-4 (Fig. 2F; Fig. S2B). Thus, CSR-1 depletion does not lead to detectable alterations in centromere/kinetochore architecture at the resolution of light microscopy.
In strains where the sole source of CSR-1 was GFP::CSR-1 encoded by a functional single copy transgene (Fig. S2C,D), we observed the previously described P-granule localization (Fig. S2E) but no detectable GFP signal on chromosomes in oocytes (Fig. S2E) or early mitotic embryos (Fig. 2G). Thus, there is not a substantial population of CSR-1—22G RNA complexes enriched on mitotic chromatin. We conclude that CSR-1 inhibition leads to greatly reduced microtubule assembly but not to detectable defects in chromosome or centromere/kinetochore structure.
CSR-1 Slicing Activity is Required For Its Function in Embryos and in the Germline
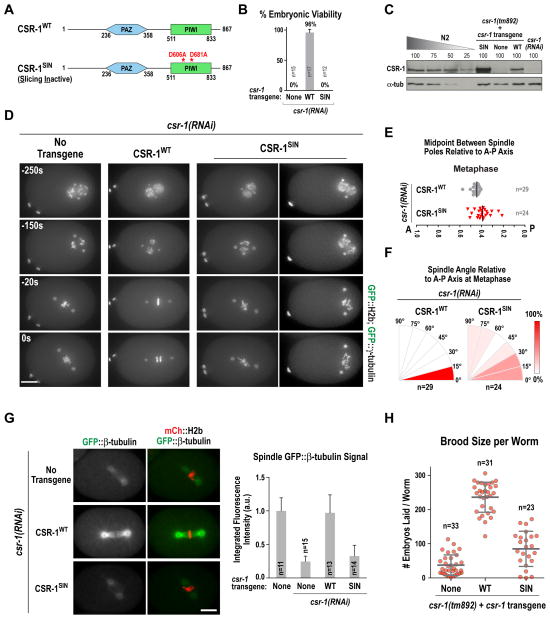
To investigate the function of CSR-1 slicing activity, we generated single-copy untagged RNAi-resistant transgenes encoding WT CSR-1 and a Slicing Inactive (SIN) mutant in which the aspartate active site residues of slicing Argonautes (D606, D681 in CSR-1b; (Aoki et al., 2007; Yigit et al., 2006) were mutated to alanine (Fig. 3A, S3A). Whereas transgene-encoded CSR-1WT rescued the lethality associated with depletion of endogenous CSR-1, CSR-1SIN did not (Fig. 3B). The same result was obtained by crossing the transgenes into the csr-1(tm892) mutant (not shown). CSR-1WT, in a homozygous csr-1(tm892) background, was expressed at a level similar to endogenous CSR-1 in control N2 worms (Fig. 3C). In contrast, despite expression from a single copy targeted transgene with an identical promoter and 5′ and 3′ UTR regions, CSR-1SIN was overexpressed (Fig. 3C; Fig. S3B), suggesting that CSR-1 slicing autoregulates its own expression (see below). The one-cell embryo phenotypes in the presence of CSR-1SIN, including a severe reduction in microtubule assembly, were essentially identical to those observed following CSR-1 depletion (Fig. 3C–G). We conclude that the early embryonic functions of CSR-1 depend on its slicing activity.
Figure 3. Selective mutation of CSR-1 slicing activity phenocopies CSR-1 depletion in one-cell embryos.
(A) Schematics of WT and Slicing-Inactive (SIN) CSR-1 expressed from targeted single copy RNAi-resistant transgene insertions (see Fig. S3A). The amino acid numbering is for the CSR-1b isoform that is essential for embryogenesis.
(B) Analysis of embryo viability for the indicated conditions. n is the number of worms analyzed; >977 progeny embryos were scored per condition. Error bars are the S.D.
(C) Immunoblotting of CSR-1 in the indicated conditions. Numbers above lanes indicated percent loading, based on number of worms. α-tubulin serves as a loading control.
(D) Images from time-lapse sequences of one-cell embryos expressing GFP:H2b and GFP::γ-tubulin, for the indicated conditions. Time is in seconds relative to anaphase. Scale bar, 10 μm
(E) Spindle positioning relative to the embryo A-P axis, measured at metaphase as in Fig. 1C, for the indicated conditions.
(F) Spindle angle relative to the embryo A-P axis at metaphase, measured as in Fig. 1D, for the indicated conditions.
(G) Representative images (left) and quantification (right) of metaphase spindle GFP::β-tubulin signal for the indicated conditions, measured as in Fig. 1E. Error bars are the S.D. Scale bar, 10 μm.
(H) Brood size per worm measured for first generation csr-1(tm892) homozygous worms, derived from balanced heterozygous mothers. When present, single copy insertion csr-1 transgenes were already homozygous in the balanced mothers.
Mutational inactivation of csr-1 leads to a significant reduction in brood size (Figs. 3H, S2D) and the germlines of both csr-1(tm892) null and csr-1SIN;csr-1(tm892) slicing activity mutant worms exhibited increased apoptotic figures and multinucleation (Fig. S3D). Interestingly, csr-1SIN;csr-1(tm892) worms laid ~2-fold more embryos than csr-1(tm892) worms suggesting a potential slicing activity-independent contribution of CSR-1 to embryo production. Like CSR-1WT, a GFP fusion with CSR-1SIN still localized to perinuclear P granules in the germline of L4 larvae (Fig S3E); in adult worms, the overexpressed CSR-1SIN slicing mutant formed aggregates in addition to perinuclear granules (not shown). We conclude that CSR-1 catalytic activity is important for embryonic divisions and for normal germline function.
CSR-1 Slicing Activity Restricts Expression of the Microtubule Depolymerase MCAKKLP-7
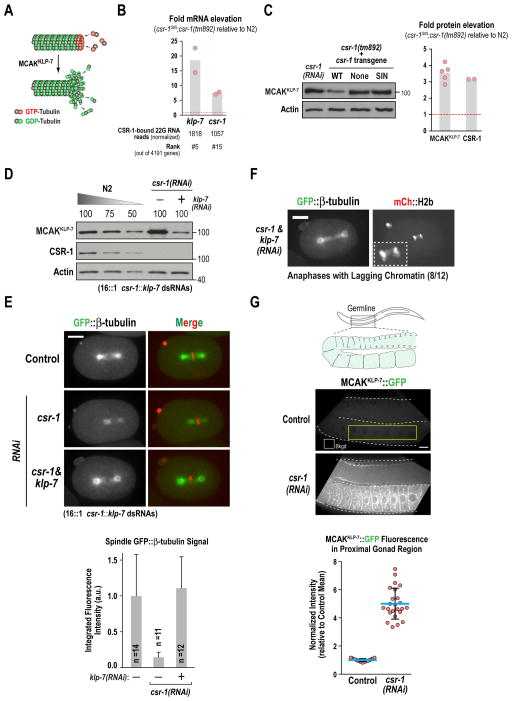
The embryonic phenotypes resulting from selective ablation of CSR-1 slicing activity suggest that CSR-1 slicing activity controls expression of a target or set of targets that regulate microtubule assembly. Examination of prior sequencing data (Claycomb et al., 2009) to identify genes encoding microtubule regulators with homology to CSR-1-bound 22G RNAs revealed that klp-7, the gene encoding the sole MCAK-related kinesin-13 in C. elegans, was ranked 5th out of 4191 genes with >25 normalized reads; csr-1 itself was ranked 15th (Fig. S4A). MCAKKLP-7 is a non-motile kinesin that acts as a potent microtubule depolymerizing enzyme, triggering growing microtubules to transition from polymerization to depolymerization (Fig. 4A; Desai et al., 1999). To determine whether increased MCAKKLP-7 expression was responsible for the reduced microtubule assembly in embryos lacking CSR-1 slicing activity, we first measured klp-7 and csr-1 mRNA levels by reverse transcription-quantitative PCR (RT-qPCR) and followed that with quantitative immunoblotting (performed as described in Fig. S4B). Since levels of MCAKKLP-7 in N2 and csr-1WT;csr-1(tm892) were equivalent (Fig. S4C), as also shown for CSR-1 (Fig. 3C), we employed a balanced heterozygous csr-1(tm892) mutant strain homozygous for the csr-1SIN transgene, picked equal number of first generation homozygous csr-1SIN;csr-1(tm892) or control N2 worms, and isolated RNA or prepared extracts for immunoblotting. Both klp-7 and csr-1 mRNAs were significantly elevated in the csr-1SIN;csr-1(tm892) mutant (Fig. 4B), indicating that CSR-1 slicing activity suppresses their levels. Immunoblotting of whole worm extracts revealed consistent ~3.5-fold and 3-fold elevation of MCAKKLP-7 and CSR-1SIN protein levels, respectively (Fig. 4C; Fig. S3B). Equivalent elevation of MCAKKLP-7 was also observed following CSR-1 depletion (Fig. 4C).
Figure 4. MCAKKLP-7 overexpression underlies the reduced microtubule assembly in CSR-1-inhibited one-cell embryos.
(A) Schematic of MCAKKLP-7 activity. MCAKKLP-7 promotes microtubule disassembly by triggering the transition from polymerization to depolymerization.
(B) Comparison of mRNA levels in csr-1SIN;csr-1(tm892) worms to N2 wildtype worms for the indicated genes; actin mRNA measured in parallel was used for normalization. Three replicates were analyzed per gene; the S.D. of replicate values was <1% and their average is plotted from two experiments (light red dots; gray bar is the mean of the two experiments). Red dashed line indicates no change in mRNA levels.
(C) Immunoblot analysis of MCAKKLP-7 and CSR-1 for the indicated conditions. Graph on the right plots measurement of protein levels by immunoblotting normalized relative to β-actin. Each dot represents an independent measurement. Red dashed lined indicates no change in protein levels.
(D–E) Co-depletion of CSR-1 and MCAKKLP-7, analyzed by immunoblotting (D) and imaging of GFP::β-tubulin (E). A mixture with a 1:16 ratio of dsRNAs targeting klp-7 and csr-1 was employed in these experiments. Metaphase spindle GFP-β-tubulin signal was measured as in Fig. 1E. Error bars are the S.D. Scale bar, 10 μm.
(F) Images of an anaphase stage one-cell embryo co-depleted for CSR-1 and MCAKKLP-7. Inset in the mCh::H2b channel shows lagging chromatin; frequency of lagging chromatin is indicated below the images. Scale bar, 10 μm.
(G) Images and quantification of endogenous locus-tagged MCAKKLP-7::GFP in the proximal region of the germline. A region outside the worm was used for background subtraction (box marked ‘bkgd’). A fixed size box (yellow outline; 360 pixels by 70 pixels) was drawn in the proximal germline region of imaged worms to measure MCAKKLP-7::GFP fluorescence. Background subtracted fluorescence intensity values, normalized to the control mean value, are plotted in the graph below. Blue lines mark the mean values; error bars are the S.D. Scale bar, 10 μm.
To determine if the 3.5-fold elevation of MCAKKLP-7 protein accounted for the reduced microtubule assembly in CSR-1 depleted embryos, we employed a 1:16 ratio of dsRNAs targeting MCAKKLP-7 and CSR-1 to reduce MCAKKLP-7 to 0.35 - 0.5-fold of wildtype levels while maintaining CSR-1 depletion (Fig. 4D). Eliminating MCAKKLP-7 elevation rescued the microtubule assembly defect in CSR-1-depleted one-cell embryos (Fig. 4E), indicating that MCAKKLP-7 elevation accounts for this prominent defect associated with loss of CSR-1 function. However, reduction of MCAKKLP-7 did not rescue the chromosome missegregation in CSR-1 depleted embryos (Fig. 4F) or restore embryonic viability (not shown), indicating it is not the sole CSR-1 target whose overexpression leads to embryonic division defects. Examination of a strain expressing a functional fusion with GFP integrated just before the stop codon at the endogenous klp-7 locus, revealed that CSR-1 depletion led to significant elevation of MCAKKLP-7::GFP fluorescence in the germline (Fig. 4G). This result suggests that excess maternally loaded MCAKKLP-7 underlies the severe microtubule assembly defect in CSR-1-depleted and CSR-1 slicing activity mutant embryos.
CSR-1 Slicing Activity Controls Protein Levels of a Number of Components Implicated in Embryonic Cell Divisions
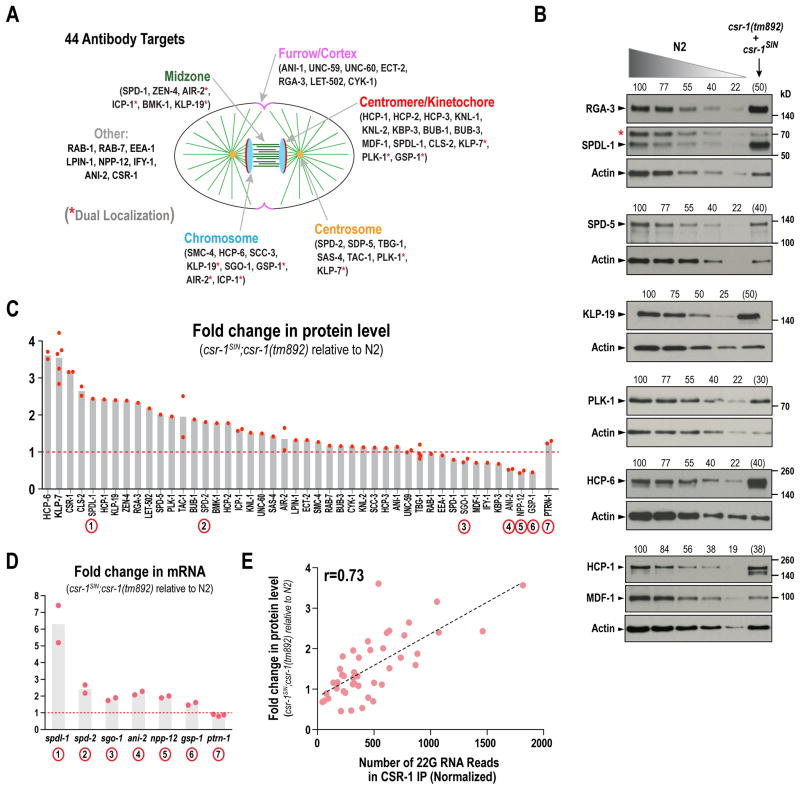
While elevated MCAKKLP-7 expression accounts for the microtubule assembly defect in CSR-1-inhibited one-cell embryos, other targets are likely also misregulated. To begin to assess the scope of CSR-1 slicing activity-mediated regulation, we employed 44 validated antibodies against C. elegans proteins important for embryonic cell division and/or germline function (Fig. 5A). The genes encoding all of these proteins have >25 normalized reads of homologous CSR-1-bound 22G RNAs (Claycomb et al., 2009). As a control, we monitored PTRN-1 (patronin), a microtubule minus end-binding protein not expressed in the germline (Wang et al., 2015) that does not have homologous CSR-1-bound 22G RNAs. Protein levels in homozygous csr-1SIN;csr-1(tm892) worm extract were compared to a standard curve for wildtype N2 worm extract on the same gel (Fig. 5B); actin was used to normalize loading. Blots were quantified when the intensity of the test protein and loading controls in the csr-1SIN;csr-1(tm892) lane fell within the N2 standard curve or required limited extrapolation (Fig. S4B). The majority of target blots were repeated 2–5 times; while the results were consistent between repeats, the criteria for quantification were only met in the subset of experiments that are plotted. This analysis revealed elevated expression of 25 of the 44 targets in the absence of CSR-1 slicing activity (Fig. 5B,C; 19 (43%) targets>1.5-fold and 25 (57%) targets>PTRN-1). For the remaining targets, protein expression either did not change significantly (12 targets=27%) or was decreased (<0.8-fold; 7 targets=16%). To assess if reduced protein levels of specific targets reflected a positive role for CSR-1 slicing activity in transcription, we measured mRNA levels for 6 target genes—2 of which exhibit elevated protein levels (spdl-1 and spd-2) and 4 of which exhibit reduced protein levels (sgo-1, ani-1, npp-12, gsp-1)—as well as for the control ptrn-1. We did not observe reduced mRNA levels for any of the tested CSR-1 target genes (Fig. 5D). Thus, the reduction in protein levels for some targets is not due to reduced transcript levels and may reflect a role for CSR-1 slicing activity in translation of specific mRNAs or be an indirect consequence of germline defects in csr-1SIN;csr-1(tm892) worms (Fig. 3H; Fig. S3D).
Figure 5. CSR-1 slicing activity modulates expression of multiple targets that participate in embryonic cell division.
(A) Schematic of 44 targets employed for analysis of CSR-1 slicing activity-dependent changes in protein levels.
(B) Representative immunoblots comparing protein levels in the absence of CSR-1 slicing activity to a N2 wildtype standard curve. Actin serves as a loading control. Numbers above lanes indicate percent loading, based on number of worms. Numbers above the test sample are shown in parenthesis, as loading was calculated based on the actin standard curve (Fig. S4B). Red asterisk indicates a background band.
(C) Plot of protein level measurements for all targets, comparing csr-1SIN;csr-1(tm892) worms to a standard curve of N2 wildtype. Light red dots represent individual measurements. Dashed red line marks no change in protein level.
(D) RT-qPCR analysis, as in Fig. 4B, comparing mRNA levels in csr-1SIN;csr-1(tm892) worms to N2 wildtype worms for the indicated genes (their corresponding gene products are marked with circled numbers on Fig. 5C).
(E) Correlation plot of the change in the level of a target protein to the normalized read count of CSR-1-bound 22G RNAs that are homologous to its encoding locus (22G RNA read data is from Claycomb et al. 2009).
The list of proteins whose expression was elevated ~2-fold in the absence of CSR-1 slicing activity included the centrosomal proteins SPD-2 and SPD-5 (Fig. 5B,C). SPD-2 is the C. elegans homolog of the human pericentriolar material (PCM) protein Cep192 and SPD-5 is the major PCM scaffold protein in C. elegans (Hamill et al., 2002; Woodruff et al., 2015). Prior work has shown that centrosome size is component-limited, with increased protein levels leading to increased centrosome size (Decker et al., 2011). Consistent with this, we observed ~2-fold increase in centrosome size in csr-1SIN;csr-1(tm892) mutant embryos (Fig. S5), supporting the conclusion that an imbalance in the maternal load caused by loss of CSR-1 slicing activity has functional consequences in the early embryo.
Plotting the fold-change in each target protein’s level against the normalized reads of CSR-1-bound 22G RNAs homologous to the gene encoding that protein (from Claycomb et al., 2009) revealed a correlation (r=0.73) between the abundance of CSR-1-bound 22G RNAs and the effect of loss of CSR-1 slicing activity on target protein expression (Fig. 5E). This correlation suggests that transcripts with greater numbers of homologous CSR-1—bound 22G RNAs are sliced to a greater extent by CSR-1 than those with fewer homologous CSR-1-bound 22G RNAs. Thus, analysis of protein levels suggests that CSR-1 slicing activity tunes down the expression of a significant proportion of its target genes and that the magnitude of the tuning correlates with the abundance of CSR-1-bound 22G RNAs.
CSR-1 Slicing Activity Tunes Target mRNAs in a Graded Fashion Dictated by 22G RNA Density
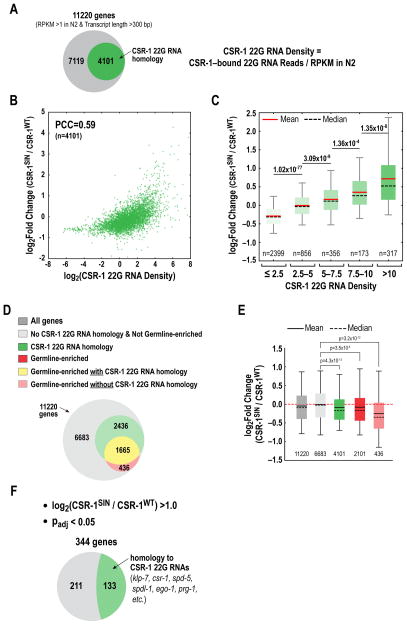
The phenotypic and immunoblotting analysis suggested that CSR-1 slicing activity is critical to generate a balanced maternal load capable of supporting early embryonic divisions. Since the immunoblotting analysis was restricted to proteins for which we had validated antibodies, we next performed mRNA-Seq analysis to obtain an unbiased genome-wide view of the role of CSR-1 slicing activity in regulating target mRNA levels. We isolated mRNA from first generation adult worms homozygous for the csr-1 deletion and for transgenes encoding CSR-1WT or CSR-1SIN. N2 wildtype worms were picked in parallel, and 2 biological duplicates were processed for the 3 genotypes (Fig. S6A,B). The CSR-1WT mapped read profile was essentially identical to N2 (Fig. S6B). With an expression threshold of RPKM>1 in N2 (RPKM=reads per kilobase of transcript per million reads) and a transcript size (defined as sum of annotated exons) >300 bp, we had 11220 genes available for comparison between the CSR-1SIN and CSR-1WT datasets.
We were particularly interested in the effect of CSR-1 catalytic activity on the expression of genes with homology to CSR-1-bound 22G RNAs. 4101 (97.9%) of the 4191 genes with homology to CSR-1-bound 22G RNAs in N2 hermaphrodites (Claycomb et al., 2009) met the RPKM and transcript size thresholds (Fig. 6A); 3866 of these 4101 genes (94%) are in the germline-expressed gene set defined by sequencing analysis of dissected gonads (Ortiz et al., 2014). Based on the correlation between the abundance of CSR-1-bound 22G RNAs and change in protein levels by immunoblotting (Fig. 5E), we hypothesized that when CSR-1’s catalytic activity is inhibited, the transcripts of genes that are more highly represented in the ensemble of CSR-1—22G RNA complexes increase to a greater degree than transcripts of genes with fewer corresponding CSR-1 22G RNAs. To test this prediction, we plotted the fold change in transcript level (CSR-1SIN/CSR-1WT) versus the ‘density’ of 22G RNAs, which we defined as the ratio of CSR-1-bound 22G RNA reads to transcript abundance (RPKM) in N2 wildtype worms, for all 4101 genes (Fig. 6A). This analysis revealed a clear correlation between 22G RNA density and elevation of transcript levels in CSR-1SIN versus CSR-1WT (Fig. 6B; Pearson Correlation Coefficient=0.59). To visualize the positive correlation between increasing CSR-1 22G RNA density and the increase in transcript levels in CSR-1SIN relative to CSR-1WT in a different manner, we split the 4101 gene set into 5 bins of increasing CSR-1 22G RNA density and plotted the change in transcript abundance between CSR-1SIN and CSR-1WT for the genes in each bin (Fig. 6C; Fig. S6C). This analysis suggested graded tuning of transcript levels by CSR-1 slicing activity from low to high 22G RNA densities, rather than activity above a specific 22G RNA density threshold. We conclude that CSR-1 catalytic activity broadly tunes the transcript levels of genes with homologous CSR-1-bound 22G RNAs, with the amplitude of its tuning being dictated by 22G RNA density.
Figure 6. Genome-wide analysis reveals tuning down of mRNA levels by CSR-1 catalytic activity that is correlated with 22G RNA density.
(A) Venn diagram showing the 11220 genes that meet the RPKM>1 in N2 and transcript length >300 bp thresholds; 4101 of these genes have homology to CSR-1—bound 22G RNAs in N2 hermaphrodites (>25 normalized reads; Claycomb et al. 2009).
(B) Plot of the change in mRNA levels between CSR-1SIN and CSR-1WT versus the density of CSR-1 22G RNAs for the 4101 genes with homologous CSR-1—bound 22G RNAs. 22G RNA density was calculated as the ratio of normalized reads to the transcript abundance (RPKM) in N2.
(C) Box-whiskers plot (5–95 percentile) of the change in mRNA levels between CSR-1SIN and CSR-1WT for gene sets of increasing 22G RNA density. Mean and median are indicated by red solid and black dashed lines, respectively. Each bin is significantly different from the prior bin; p-values indicated are from t-tests. The same plot with outliers is show in Fig. S6C.
(D) Venn diagram of gene sets; 4101 genes have homologous CSR-1-bound 22G RNAs. 2101 genes are germline-enriched, based on prior microarray analysis (Claycomb et al., 2009). 436 of the germline-enriched genes do not overlap with the 4101 CSR-1 22G RNA homology gene set.
(E) Box-whiskers plot (5–95 percentile) of the change in mRNA levels between CSR-1SIN and CSR-1WT for the indicated gene sets. Mean and median are indicated by black solid and black dashed lines, respectively. p-values shown are from t-tests. See also Fig. S6D.
(F) 344 genes exhibit >2-fold, significant (padj <0.05) increase in mRNA levels in CSR-1SIN relative to CSR-1WT. Of the 344 genes, 133 overlap with the CSR-1 22G RNA gene set (green); the genes that do not overlap (grey) are expressed at significantly lower levels than the ones that do (see Fig. S6E and Table S1).
We noticed that the baseline when comparing transcript levels of the 4101 genes with CSR-1 22G RNA homology between CSR-1SIN and CSR-1WT was lower than 1.0 (i.e. log2FoldChange < 0.0 at low 22G RNA densities; Fig. 6B,C). Consistent with this, the distribution of fold change (CSR-1SIN/CSR-1WT) in transcript levels for the 4101 genes with homology to CSR-1-bound 22G RNAs was slightly skewed to a negative value (median −0.17; mean −0.09; Fig. 6D,E; Fig. S6D). Since CSR-1-bound 22G RNAs are primarily homologous to germline-expressed genes, we wondered whether this mild negative skew reflected a direct effect or was an indirect consequence of the effect of inhibiting CSR-1 activity on germline architecture (Fig. 3H; Fig. S3D). To distinguish between these possibilities, we analyzed 2101 germline-enriched genes, defined by microarray-based comparison of a mutant that lacks a germline to control wildtype worms (Reinke et al., 2004). A majority (1665/2101) of the germline-enriched genes have homologous CSR-1 22G RNAs while a small subset (436/2101) lack CSR-1 22G RNA homology (Fig. 6D); 90% of the 436 genes in this subset were confirmed to be germline expressed by RNA sequencing of isolated gonads (Ortiz et al., 2014). The germline-enriched gene subset without CSR-1 22G RNA homology also exhibited a negatively skewed fold-change distribution (median −0.35; mean −0.25; Fig. 6E; Fig. S6D). Thus, the modest reduction in transcript levels for germline genes (both with and without homologous CSR-1-bound 22G RNAs) is potentially an indirect consequence of the effects of inhibiting CSR-1 slicing activity on germline architecture (Fig. 3H; Fig. S3D).
Finally, we identified genes whose transcript levels increased most significantly between CSR-1SIN and CSR-1WT (>2-fold with adjusted p-value <0.05; we note that the germline architecture defects in CSR-1SIN may make this an underestimate). This analysis revealed 344 genes, 133 of which were in the gene set defined by homology to CSR-1-bound 22G RNAs and included expected genes such as klp-7, csr-1, and spd-5 (Table S1). Notably, 211 of the 344 genes whose transcript levels increased >2-fold did not have reported CSR-1-bound 22G RNAs (Table S1). A comparison of transcript abundance revealed that this 211 gene set is expressed at substantially lower levels compared to the 133 genes with homology to CSR-1-bound 22G RNAs (Fig. S6E). Thus, it is possible that there are CSR-1-bound 22G RNAs homologous to these 211 genes that have not been detected/did not meet thresholds due to low abundance. 194 of these 211 genes (92%) were not germline-enriched (as defined by (Reinke et al., 2004) and 103 (49%) were absent from the most comprehensive germline-expressed gene set (Ortiz et al., 2014). Thus, if these are indeed CSR-1 regulated genes, a significant subset may be acting in non-germline contexts. Additional work will be necessary to test whether these genes are indeed CSR-1 catalytic activity-regulated and to assess the functional significance of their regulation.
DISCUSSION
CSR-1 is unique among the 27 C. elegans Argonautes in that its inhibition leads to immediate and severe embryonic division phenotypes. While prior studies have ascribed many potential roles to CSR-1, they have discounted slicing activity-dependent control of target expression. Here, by focusing on phenotypic signatures in the one-cell embryo, we show that CSR-1 function in embryonic cell division is entirely dependent on its slicing activity. This is consistent with biochemical data showing that CSR-1 provides the dominant slicing activity in C. elegans extracts (Aoki et al., 2007) and with increased mRNA levels for genes such as klp-7 and csr-1 in ego-1 mutants, where 22G RNA production is inhibited (Maniar and Fire, 2011). Notably, many CSR-1 targets have essential functions; thus, CSR-1 slicing activity, guided by the small RNA biogenesis machinery, tunes, rather than silences, target expression (Fig. 7). Below, we discuss the relationship of this finding to other proposed roles for CSR-1, the relevance of the mechanism described here to other species, and potential reasons why small RNA-catalytic Argonaute pathways may have evolved to provide a post-transcriptional layer of regulation in the female germline.
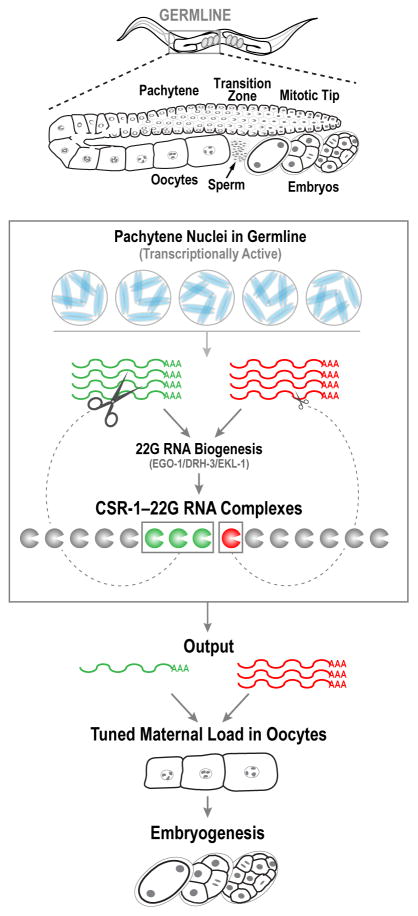
Figure 7. A single catalytic Argonaute tunes maternal load composition to support early embryogenesis.
The cartoon shows two mRNAs derived from transcriptionally active pachytene nuclei, one of which (green) is more prominently represented than the other (red) in the pool of CSR-1—22G RNA complexes. CSR-1 slicing tunes the mRNA levels guided by the proportional representation in the ensemble of CSR-1—22G RNA complexes to generate an output that supports early embryogenesis.
Relationship of Tuning Activity to Previously Proposed Roles of CSR-1
An important question emerging from our work is the relationship of CSR-1 slicing activity-dependent target tuning to its other proposed functions. Given the misregulation of a substantial number of targets, the assumption in prior studies that CSR-1 does not significantly control target expression is not valid. The reasons for this discrepancy are not clear but may involve technical differences—in the initial study of csr-1 mutants, microarrays were employed instead of sequencing (Claycomb et al., 2009); in a recent study employing sequencing (Campbell and Updike, 2015), CSR-1 was depleted by feeding-based RNAi, which has relatively low penetrance. Our analysis also focused on expression changes as a function of CSR-1 22G RNA density, instead of treating the set of genes with homology to CSR-1 22G RNAs as a single entity and imposing a fixed threshold. Regardless of the precise reasons, our findings show that a substantial number of targets are tuned by CSR-1 catalytic activity, with 133 highly tuned targets (such as MCAKKLP-7, CSR-1, SPD-5; Table S1), and lower amplitude tuning of a large proportion of germline-expressed targets. Tuning of major targets such as MCAKKLP-7 is essential, as elevated MCAKKLP-7 levels lead to severe phenotypes; the net effect of lower amplitude tuning of a large number of germline transcripts likely also contributes to embryo fitness. The fact that CSR-1 activity autoregulates its own expression is consistent with its role as a tissue-wide master regulator, by analogy with developmentally critical transcription factors that often employ autoregulation to control of their own levels (Crews and Pearson, 2009).
The fact that CSR-1—22G RNA complexes control target expression complicates interpretation of other proposed roles for the CSR-1—22G RNA pathway. We discuss a subset of these roles below but, more broadly, highlight the need to re-evaluate prior work in light of the results reported here.
Chromosome organization and segregation
Our initial motivation to study CSR-1 was based on its proposed role in chromosome/centromere organization (Claycomb et al., 2009). While our results confirm a centrally important roles for the CSR-1—22G RNA pathway in chromosome segregation, they do not support a direct role for CSR-1—22G RNA complexes in patterning holocentric chromosome structure. The phenotypic differences between depletion of the histone mRNA stem loop-binding factor CDL-1 and CSR-1 inhibition also suggest that a role in histone mRNA maturation does not account for the chromosome segregation defects associated with loss of CSR-1 activity. Instead, CSR-1 controls chromosome segregation in part, but not exclusively, via control of a key regulator of microtubule dynamics—the microtubule depolymerase MCAKKLP-7.
P-granule assembly and fertility
The CSR-1 slicing activity mutant exhibits the same phenotypic profile as loss of CSR-1 with two exceptions: the slicing mutant has a higher brood size than the null mutant and does not appear to disrupt perinuclear P granules in the germline, as reported previously for a csr-1 mutant and csr-1(RNAi) (Campbell and Updike, 2015; Claycomb et al., 2009). Recent work suggests that knockdown of csr-1 or of P-granule components leads to inappropriate expression of spermatogenesis genes (Campbell and Updike, 2015). In the CSR-1 catalytic activity mutant, there was only a mild increase in mRNA levels of spermatogenesis genes (2641 genes defined by Ortiz et al., 2014; mean log2FoldChange for this gene set in CSR-1SIN versus CSR-1WT was 0.39 (1.3-fold); median was 0.32 (1.25-fold); RPKM>1 in N2 filter was not imposed as these genes are normally repressed in gravid N2 worms). A non-catalytic role of CSR-1 in a different process, such as translational repression in the mitotic region of the germline where it acts together with the PUF domain protein FBF-1 (Friend et al., 2012), may also account for the brood size difference.
Self versus non-self
Mating-based assays analyzing transgene silencing/activation, in conjunction with genomic analysis of small RNA pools, have revealed pathways acting in the germline to define self versus non-self (Ashe et al., 2012; Lee et al., 2012; Seth et al., 2013; Shirayama et al., 2012). The Piwi family Argonaute PRG-1, together with its associated 21U RNAs, initiates recognition of non-self genes and, via the action of the EGO-1/EKL-1/DRH-3 RNA-dependent RNA polymerase complex, triggers heritable WAGO—22G RNA-mediated silencing of foreign genes (Ashe et al., 2012; Lee et al., 2012; Shirayama et al., 2012). Imperfect homology to 21U RNAs, coded in vast numbers (~30000 loci) in the genome, triggers 22G RNA generation and loading into the WAGO pathway. Self genes must be resistant to this non-self silencing pathway, and it has been proposed that CSR-1—22G RNA complexes protect self genes, potentially by acting on chromatin (Seth et al., 2013; Wedeles et al., 2013). How self gene-associated 22G RNAs are loaded into CSR-1 is not known. It is interesting to speculate whether the CSR-1—22G RNA pathway evolved to optimize maternal load by tuning levels of germline-expressed genes essential for fitness and then became co-opted to define self or vice versa.
Optimization of Maternal Load Composition by a Small RNA—Argonaute Pathway
Small RNA pathways have been long appreciated as being important for germline development and function. Here, we show that a slicing Argonaute-endogenous small RNA pathway comprised of CSR-1—22G RNAs controls target expression to generate oocytes capable of supporting early C. elegans embryogenesis. csr-1 mutant males exhibit only modestly reduced fertility (Conine et al., 2013), highlighting a greater importance of this control mechanism in the oogenic germline. CSR-1 action in the oogenic germline is guided by 22G RNA cofactors generated by the RNA-dependent RNA Polymerase (RdRP) EGO-1 (Fig. 7), which presumably employs transcripts generated by meiotic pachytene nuclei (Lerner and Goldstein, 1988) as substrates. While RdRPs are thought to have been present in the ancestor to all eukaryotes and are present in fungi, plants, nematodes as well as other metazoans, they have been lost in several animal groups including vertebrates and insects (Zong et al., 2009). Despite loss of RdRPs, vertebrates have maintained one catalytically active Argonaute, Ago2. In mouse oocytes, Ago2 acts in concert with endogenous siRNAs—some of which are derived from Dicer processing of dsRNA intermediates containing pseudogene transcripts—to tune the expression of protein-coding transcripts (Stein et al., 2015; Tam et al., 2008; Watanabe et al., 2008). Ago2 slicing activity is dispensable for oocyte formation and growth but is required for oocyte spindle assembly, meiotic divisions, and female fertility (Stein et al., 2015). As with csr-1 mutant males in C. elegans, Ago2 removal does not result in male infertility, highlighting that the essential role for Ago2 catalytic activity-based tuning is in the female germline. Intriguingly, plants harbor multiple RdRPs and also employ catalytic Argonautes to control female gamete development (Nonomura et al., 2007; Olmedo-Monfil et al., 2010). Thus, while the specific catalytic Argonautes and their small RNA cofactor generation mechanisms do not share a common ancestry, the use of catalytic Argonautes in the generation of female gametes capable of supporting early embryogenesis appears widespread.
We speculate that one reason for the existence of such a post-transcriptional layer of regulation is the special nature of germline chromatin, where tight suppression of somatic expression to maintain pluripotency may limit the precision of transcriptional control. For example, experiments in C. elegans assessing effects of ectopic expression of neuronal type-specifying master transcriptional regulators have shown that the germline is refractory to fate changes unless global chromatin states are altered (Tursun et al., 2011). Thus, the female germline appears to have adapted small RNA-catalytic Argonaute pathways to act post-transcriptionally in order to optimize maternal load composition. A post-transcriptional layer of control in the germline may also leave open promoter-based regulation of target genes in differentiated cells, where the amount of the gene product required may be different from what is needed in early embryogenesis. For example, MCAKKLP-7 controls dynamics of microtubule arrays in rapidly dividing cells of the early C. elegans embryo but also regulates microtubule dynamics in neurons (Ghosh-Roy et al., 2012) where a different amount of MCAKKLP-7 activity may be required.
As CSR-1 slicing is guided by 22G RNA density, nucleotide changes in the transcript sequence that influence 22G RNA generation can be translated via CSR-1 slicing activity into changes in expression level. Thus, we speculate that small RNA-catalytic Argonaute mediated post-transcriptional control enables evolutionary optimization of the relative representation of the array of proteins and RNAs in the maternal load by exerting selective pressure on randomly occurring sequence changes that modulate small RNA generation and, consequently, stoichiometry in the maternal load. The major question raised by our findings is precisely how 22G RNA biogenesis by the EGO-1 RNA-dependent RNA polymerase complex is controlled. Understanding the rules that govern 22G RNA synthesis may be facilitated by the development of reporters for CSR-1 targets such as MCAKKLP-7.
EXPERIMENTAL PROCEDURES
C. elegans strains (Table S2) were maintained using standard methods. A transposon-based strategy (Frokjaer-Jensen et al., 2008) was used to insert csr-1 transgenes in single copy at a defined genomic location on Chr II. All csr-1 transgenes included 2168 bp upstream of the start codon for isoform a and 1048 bp downstream of the stop codon; a segment (Fig. S3A) was modified to preserve coding information but alter nucleotide sequence, to make transgene-encoded products resistant to a dsRNA that depletes endogenous CSR-1. RNAi (conducted by injection of dsRNAs; Table S3), worm dissection, and embryo mounting for live imaging were performed as described (Cheeseman et al. 2004). For the immunoblotting, qPCR and mRNA-Seq analysis, first generation homozygotes for the untagged csr-1 transgene insertion (either WT or SIN) and the csr-1(tm892) null mutation (Fig. S2A) were picked for analysis. Transgene insertions on Chr II were first homozygosed and maintained in balanced csr-1(tm892) heterozygotes (the csr-1 locus is on Chr IV; transgene insertions are on Chr II). Homozygous csr-1(tm892) worms were isolated from balanced heterozygous csr-1(tm892) mothers based on their differential motility due to a balancer-associated unc mutation. For immunoblotting, equal numbers of worms of indicated genotypes were picked, washed in buffer and boiled with intermittent sonication in sample buffer. For analysis of mRNA levels of selected genes and mRNA-Seq, total RNA from equal number of worms was extracted using Trizol. For analysis of mRNA levels, mRNA was isolated from total RNA, reverse transcribed into cDNA, and qPCR analysis performed using primers listed in Table S4. For mRNA-Seq, libraries were generated using Illumina TruSeq Stranded mRNA Sample Prep Kit, multiplexed, and sequenced with 50 basepair (bp) single end reads to a depth of ~40 million reads per sample. Details on the imaging conditions used and on the quantitative analysis of fluorescence, immunoblotting, qPCR, and mRNA-Seq data are in the Suppl. Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Amy Pasquinelli, Andreas Rechtsteiner, James Broughton and members of the Desai and Oegema labs for helpful discussions; Kristen Jepsen and the UCSD Institute for Genomic Medicine Genomics Center for help with sequencing. This work was supported by an NIH grant (GM074215) to A.D. A.G-G. was supported by an EMBO Long Term Fellowship (ALTF 251-2012). G.W.Y. and S.S. are supported by grants from NIH (HG004659, MH107369, NS075449). A.D. and K.O. receive salary and other support from the Ludwig Institute for Cancer Research.
Footnotes
Accession Numbers
The GEO accession code for the mRNA-Seq data is GSE75128.
Author Contributions
A.G-G. and A.D. initiated the project; A.G-G. performed the majority of experiments with help from S.W., R.G. and K.O. for imaging-based phenotypic analysis and immunoblotting; S.W., S.S., and G.Y. performed analysis of sequencing data with feedback from A.D.; A.G-G., S.W., A.D. and K.O. prepared the manuscript, with input from other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki K, Moriguchi H, Yoshioka T, Okawa K, Tabara H. In vitro analyses of the production and activity of secondary small interfering RNAs in C. elegans. Embo j. 2007;26:5007–5019. doi: 10.1038/sj.emboj.7601910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgousti DC, Palani S, Sherman Y, Grishok A. CSR-1 RNAi pathway positively regulates histone expression in C. elegans. Embo j. 2012;31:3821–3832. doi: 10.1038/emboj.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi AC, Fischer SE, Kim JK. Endogenous RNAi pathways in C. elegans. WormBook : the online review of C elegans biology. 2014:1–49. doi: 10.1895/wormbook.1.170.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, Kennedy S. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AC, Updike DL. CSR-1 and P granules suppress sperm-specific transcription in the C. elegans germline. Development. 2015;142:1745–1755. doi: 10.1242/dev.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16:2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- Cecere G, Hoersch S, O’Keeffe S, Sachidanandam R, Grishok A. Global effects of the CSR-1 RNA interference pathway on the transcriptional landscape. Nat Struct Mol Biol. 2014;21:358–365. doi: 10.1038/nsmb.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139:123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Moresco JJ, Gu W, Shirayama M, Conte D, Jr, Yates JR, 3rd, Mello CC. Argonautes promote male fertility and provide a paternal memory of germline gene expression in C. elegans. Cell. 2013;155:1532–1544. doi: 10.1016/j.cell.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews ST, Pearson JC. Transcriptional autoregulation in development. Curr Biol. 2009;19:R241–246. doi: 10.1016/j.cub.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M, Jaensch S, Pozniakovsky A, Zinke A, O’Connell KF, Zachariae W, Myers E, Hyman AA. Limiting amounts of centrosome material set centrosome size in C. elegans embryos. Curr Biol. 2011;21:1259–1267. doi: 10.1016/j.cub.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Friend K, Campbell ZT, Cooke A, Kroll-Conner P, Wickens MP, Kimble J. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol. 2012;19:176–183. doi: 10.1038/nsmb.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nature genetics. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Rechtsteiner A, Yuen KW, Muroyama A, Egelhofer T, Gaydos L, Barron F, Maddox P, Essex A, Monen J, et al. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature. 2012;484:534–537. doi: 10.1038/nature10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Roy A, Goncharov A, Jin Y, Chisholm AD. Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell. 2012;23:716–728. doi: 10.1016/j.devcel.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishok A. Biology and Mechanisms of Short RNAs in Caenorhabditis elegans. Adv Genet. 2013;83:1–69. doi: 10.1016/B978-0-12-407675-4.00001-8. [DOI] [PubMed] [Google Scholar]
- Gu W, Shirayama M, Conte D, Jr, Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell. 2009;36:231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill DR, Severson AF, Carter JC, Bowerman B. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev Cell. 2002;3:673–684. doi: 10.1016/s1534-5807(02)00327-1. [DOI] [PubMed] [Google Scholar]
- Kuhn CD, Joshua-Tor L. Eukaryotic Argonautes come into focus. Trends in biochemical sciences. 2013;38:263–271. doi: 10.1016/j.tibs.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D, Jr, Mello CC. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell. 2012;150:78–87. doi: 10.1016/j.cell.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner K, Goldstein P. Electron microscopic autoradiographic analysis: evidence of RNA transcription along pachytene chromosomes of rad-4, him-4 and wild-type Caenorhabditis elegans. Cytobios. 1988;55:51–61. [PubMed] [Google Scholar]
- Maniar JM, Fire AZ. EGO-1, a C. elegans RdRP, modulates gene expression via production of mRNA-templated short antisense RNAs. Curr Biol. 2011;21:449–459. doi: 10.1016/j.cub.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K, Morohoshi A, Nakano M, Eiguchi M, Miyao A, Hirochika H, Kurata N. A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. The Plant cell. 2007;19:2583–2594. doi: 10.1105/tpc.107.053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo-Monfil V, Duran-Figueroa N, Arteaga-Vazquez M, Demesa-Arevalo E, Autran D, Grimanelli D, Slotkin RK, Martienssen RA, Vielle-Calzada JP. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature. 2010;464:628–632. doi: 10.1038/nature08828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz MA, Noble D, Sorokin EP, Kimble J. A new dataset of spermatogenic vs. oogenic transcriptomes in the nematode Caenorhabditis elegans. G3 (Bethesda, Md) 2014;4:1765–1772. doi: 10.1534/g3.114.012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- Seth M, Shirayama M, Gu W, Ishidate T, Conte D, Jr, Mello CC. The C. elegans CSR-1 argonaute pathway counteracts epigenetic silencing to promote germline gene expression. Dev Cell. 2013;27:656–663. doi: 10.1016/j.devcel.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D, Jr, Mello CC. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell. 2012;150:65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P, Rozhkov NV, Li F, Cardenas FL, Davydenko O, Vandivier LE, Gregory BD, Hannon GJ, Schultz RM. Essential Role for endogenous siRNAs during meiosis in mouse oocytes. PLoS genetics. 2015;11:e1005013. doi: 10.1371/journal.pgen.1005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts DC, Makarova K, Wang Y, Nakanishi K, Ketting RF, Koonin EV, Patel DJ, van der Oost J. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol. 2014;21:743–753. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursun B, Patel T, Kratsios P, Hobert O. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011;331:304–308. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike DL, Strome S. A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans. Genetics. 2009;183:1397–1419. doi: 10.1534/genetics.109.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wu D, Quintin S, Green RA, Cheerambathur DK, Ochoa SD, Desai A, Oegema K. NOCA-1 functions with gamma-tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in C. elegans. Elife. 2015;4 doi: 10.7554/eLife.08649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- Wedeles CJ, Wu MZ, Claycomb JM. Protection of germline gene expression by the C. elegans Argonaute CSR-1. Dev Cell. 2013;27:664–671. doi: 10.1016/j.devcel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Woodruff JB, Wueseke O, Viscardi V, Mahamid J, Ochoa SD, Bunkenborg J, Widlund PO, Pozniakovsky A, Zanin E, Bahmanyar S, et al. Centrosomes. Regulated assembly of a supramolecular centrosome scaffold in vitro. Science. 2015;348:808–812. doi: 10.1126/science.aaa3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigit E, Batista PJ, Bei Y, Pang KM, Chen CC, Tolia NH, Joshua-Tor L, Mitani S, Simard MJ, Mello CC. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127:747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Zong J, Yao X, Yin J, Zhang D, Ma H. Evolution of the RNA-dependent RNA polymerase (RdRP) genes: duplications and possible losses before and after the divergence of major eukaryotic groups. Gene. 2009;447:29–39. doi: 10.1016/j.gene.2009.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.