Introduction
About 10% of adult asthma patients suffer from aspirin exacerbated respiratory disease (AERD) characterized by more severe persistent asthma.1 Distinct differences in several eicosanoids are seen in AERD compared to aspirin tolerant asthmatics (ATA) including differences in baseline levels, after clinical reactions to aspirin, and after aspirin desensitization and treatment.2–9 AERD is characterized by higher baseline urinary leukotriene E4 (LTE4) levels compared to aspirin-tolerant asthma patients (ATA).4, 10 During a standard oral graded aspirin challenge, urinary LTE4 levels increase further.3, 8, 11 Levels of another eicosanoid, prostaglandin D2 (PGD2) increase in patients who develop bronchospasm after aspirin challenge.12–14 After an aspirin challenge, urine levels of PGE2 metabolites do not change in AERD patients but decrease in ATA.7
The diagnosis of AERD usually requires the induction of a clinically apparent hypersensitivity reaction to aspirin, which has significant morbidity because of the often severe and prolonged nature of these reactions. In this study, we investigated the effect of low-dose oral aspirin on selected clinical measurements and on eicosanoid changes of AERD patients in order to determine if it was possible to diagnose aspirin sensitivity without inducing a clinically apparent hypersensitivity reaction.
Hypersensitivity reactions in AERD patients are dose-dependent and the majority of AERD patients do not react to a low oral dose (<30 milligram) of aspirin.15, 16 We therefore challenged patients with two low aspirin doses (20 and 40 mg). Most AERD patients tolerate 20 milligram (mg) of aspirin15 but this dose still blocks platelet COX-1 and inhibits thromboxane production in healthy volunteers.17 We used the 40 mg aspirin dose because this is the initial dose used during standard oral aspirin challenges.16, 18 In addition to measuring eicosanoid levels we also analyzed other measures of reactions to aspirin including forced expiratory volume in 1 second (FEV1), nasal peak flow (NPF), and fraction of exhaled nitric oxide (FeNO). Previous reports suggested that FeNO values increase during a two-day oral aspirin desensitization procedure and during inhaled aspirin-lysine challenges in AERD patients.19, 20 On the other hand, another study found that nasal nitric oxide exhibited a dual pattern of changes during nasal challenges with aspirin-lysine.21 For all the clinical and eicosanoid parameters, we measured changes after low-dose aspirin challenges that did not induce any apparent clinical symptoms and after standard diagnostic challenges that induced clinically apparent aspirin-induced hypersensitivity reactions.
Methods
Patients
AERD and ATA patients with a history of physician-diagnosed asthma were recruited into the study. All AERD patients had history of nasal polyposis and NSAID-induced respiratory reactions. All AERD-suspected patients were confirmed as AERD with a standard oral graded aspirin challenge. All aspirin-tolerant patients occasionally used NSAIDs without any adverse reactions. They were asked to stop occasional NSAID use 2 weeks prior and for the duration of this study. All patients signed an informed consent at the time of enrollment into the study, approved by the Albert Einstein College of Medicine Institutional Review Board.
Protocol
The study consisted of three visits. Visits were scheduled at least one week apart to allow an aspirin washout period. Oral aspirin challenge with 20 mg was performed at Visit 1 and with 40 mg at Visit 2. During Visit 3 all patients underwent a standard oral graded aspirin challenge to confirm aspirin sensitivity using a previously described protocol.16 In brief, for the graded challenge, 40 mg of aspirin (dissolved Original Alka-Seltzer®) was initially administered and then the dose was doubled every 90 minutes (80, 160, and 325 mg). The diagnosis of AERD was confirmed by the presence of a positive aspirin challenge response, defined as a hypersensitivity reaction with at least one of the following symptoms: a) ≥20% decline in FEV1; b) decline in FEV1 of < 20% combined with naso-ocular reactions; c) isolated naso-ocular reactions. Patients who experienced a hypersensitivity reaction were treated based on their specific symptoms. ATA patients’ aspirin challenge followed the same dosing schedule with a cumulative dose of aspirin of 605 mg. Since the threshold for reactions to aspirin is variable in AERD patients,15 if patients developed a hypersensitivity reaction upon low dose aspirin exposure, their findings were analyzed as part of the aspirin challenge that led to a clinically-apparent hypersensitivity reaction. To confirm that including low-dose reactors data did not unduly influence the outcomes, we analyzed the data both ways: including and excluding low-dose reactors to aspirin.
All patients continued to take their prescribed inhaled corticosteroids (ICS) with or without long-acting beta-agonists (LABA) at the time of the study. On the mornings of the study visits ICS and both long-and short-acting beta-agonists were withheld. In order to enhance the safety of aspirin challenges in the AERD patients,22, 23 all study participants (AERD and ATA) took 10 mg of montelukast daily during the week prior to the standard graded oral aspirin challenge (Visit 3) or continued to take it throughout the study if it was part of their usual medical regimen. 5-lipoxygenase inhibitors were held for the duration of the study, if applicable. Spirometry, nasal inspiratory peak flow values, and respiratory symptoms were recorded at baseline, and then after aspirin administration (20 or 40 mg) at 15–30 minutes, 30–60 min, and >60 min during the first two visits. During the confirmatory standard oral graded aspirin challenge (Visit 3, 40–325 mg), recordings were done at the same time points after a hypersensitivity reaction, or when 325 mg dose of aspirin was reached. Spirometry was performed at each time point according to standard guidelines with Puritan Bennett Renaissance® II spirometer (Pleasanton, CA).24 Nasal inspiratory peak flow (NPF) was measured at each time point with an In-Check™ Nasal inspiratory flow device (Clement Clarke International, Ltd., Essex, UK) according to the manufacturer guidelines.25 The best of three efforts were recorded during spirometry and NPF measurements. The fraction of exhaled nitric oxide (FeNO) values were recorded by using NIOX® (Morrisville, NC) according to the manufacturer guidelines at the same time points prior to performing spirometry and measuring NPF. FeNO was not measured during 20 mg aspirin challenge in either group because of budgetary constraints. Blood and urine specimens were collected at the beginning of each visit. After the aspirin dose was administered during the first two visits, blood was collected at 15 min, 30 min, 60 min, and 120 min; urine was collected at 0 to 1 hour (h), 1 to 2 h, and 2 to 3 h. At the third visit, specimens were collected at the same time points after a hypersensitivity reaction, or when the 325 mg dose of aspirin was reached.
Blood and urine eicosanoid measurements
Plasma and urine specimens were immediately frozen at −80°C, then shipped overnight for analysis by Cayman Chemical, Inc., Ann Arbor, MI. Tetranor PGD2-metabolite (PGDM) and LTE4 were measured in urine and PGE2-metabolite (PGEM) was measured in plasma by ELISA.26–28 All measurements were made in triplicate. The results are expressed as pg/mg creatinine for LTE4 and tetranor PGDM and pg/ml plasma for PGEM.
Statistical Analysis
Baseline characteristics were compared between AERD patients and aspirin-tolerant asthma patients by using independent sample t-tests or by using Wilcoxon rank-sum tests when data were non-normally distributed. Logarithmic transformation was used for LTE4 data. All summary statistics were expressed as means ± SEM or as medians and interquartile range (IQR). General Linear Models (GLM) were used for multiple comparisons to reflect the fact that the outcome measurements were repeated over time within subjects. Receiver operating characteristic curves were plotted to choose the best cutoff points. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated. All statistical analyses were performed with STATA 14.0 software (StataCorp, College Station, TX). P-values of <0.05 were considered significant for all analyses.
Results
Sixteen AERD patients and 13 aspirin-tolerant asthmatics were studied. Participant characteristics are presented in Table 1. Both groups had a similar distribution of age, sex, race, and baseline FEV1, total serum immunoglobulin E (IgE), and PGEM and urinary tetranor PGDM levels. There were significant differences between the two groups in absolute counts and percentages of peripheral blood eosinophils, the number of patients with a history of nasal polyps, FeNO levels, and nasal peak flow (NPF) values. Baseline urine LTE4 levels were higher in AERD patients.
Table 1.
Baseline characteristics of participants
| AERD (n=16) | ATA (n=13) | P-value | |
|---|---|---|---|
| Age, y, mean (± SEM) | 37.8 (± 3.2) | 42.6 (± 2.4) | 0.2 |
| Sex (females/males) | 10/6 | 7/6 | 0.5 |
| Race, n (%) | 0.2 | ||
| African-American | 5 (31.2) | 2 (15.4) | |
| Hispanic | 6 (37.5) | 7 (53.8) | |
| White | 5 (31.3) | 3 (23.1) | |
| Other | 1 (7.7) | ||
| Aspirin hypersensitivity duration, years | 4 (± 1.6) | N/A | |
| Presence of nasal polyps (%) | 87.5 | 23.1 | <0.001 |
| ICS/LABA use (%) | 87 | 76 | 0.5 |
| Montelukast use, %* | 37.5 | 50 | 0.6 |
| Baseline FEV1 %predicted, mean (± SEM) | 73.0 (± 3.1) | 92.5 (± 9.4) | 0.3 |
| Nasal peak flow, L/min, mean (± SEM) | 81.7 (± 12.7) | 143 (± 28.1) | 0.04 |
| Absolute eosinophil count/mm3, peripheral blood, mean (± SEM) | 0.6 (± 0.13) | 0.15 (± 0.2) | <0.01 |
| Peripheral blood eosinophils, % leukocytes, mean (± SEM) | 10.0 (± 1.3) | 2.0 (± 1.7) | <0.001 |
| Serum IgE, IU/L, median (IQR) | 198 (102–372) | 288 (172–716) | 0.2 |
| FeNO value (ppb), median (IQR) | 29 (21–104) | 18 (13–23) | 0.01 |
| Urinary LTE4, pg/mg creatinine, median (IQR) | 660.4 (462.6–833.4) | 426.2 (356.2–455.2) | <0.01 |
| Urinary tetranor PGDM, pg/mg creatinine, mean (± SEM) | 6440.3 (± 697.5) | 6698.2 (± 828.2) | 0.8 |
| Plasma PGEM, pg/ml, mean (± SEM) | 38.8 (± 5.6) | 54.9 (± 9.1) | 0.3 |
This value represents the percentage of patients in each group taking montelukast throughout the study as part of their medical regimen. All subjects (AERD and ATA) were requested to take 10 mg of montelukast daily during the week prior to the standard graded oral aspirin challenge.
Among AERD patients, there were no clinical hypersensitivity reactions to the 20 mg aspirin challenges. There were two patients who clinically had responded to 40 mg aspirin: one had nasal congestion, rhinorrhea, and nausea/vomiting, and the other reacted with a bronchospasm and nausea/vomiting. Since these two patients had a clinical hypersensitivity reaction, their data were analyzed as part of the standard graded aspirin challenge as described in methods. Excluding them from the analysis did not alter the results (data not shown). As expected, all AERD patients experienced a hypersensitivity reaction during the standard graded aspirin challenge while none of the ATA subjects reacted to the graded aspirin challenges.
FeNO responses to low and graded aspirin challenge
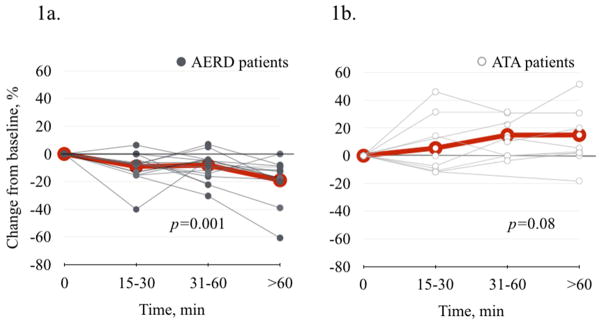
FeNO changes in response to aspirin were measured in all sixteen AERD patients and in ten ATA subjects. Sixty minutes after the 40 mg aspirin challenge FeNO levels decreased in 13/14 of the AERD patients who did not exhibit a clinical reaction to this dose (Figure 1a). In contrast, FeNO levels increased or remained the same in 9/10 of the ATA patients (Figure 1b). There was a significant decrease from baseline in mean FeNO in AERD subjects by 9.0% (±.4.0) at 15–30 min, 8.1% (±3.0) at 30–60 min, and by 19.0% (±5.1) at >60 min (p=0.001) (Figure 1a). In contrast, there was an apparent increase in FeNO in the ATA patients, but this was not statistically significant (p=0.08) (Figure 1b).
Figure 1.
FeNO change after 40 mg aspirin challenge. 1a. In AERD patients (N=14), there was a significant decrease in FeNO (p=0.001). 1b. In ATA patients (N=10), there was no significant change in FeNO (p=0.08).
Gray thin lines represent individual data points. Red thicker lines represent mean values.
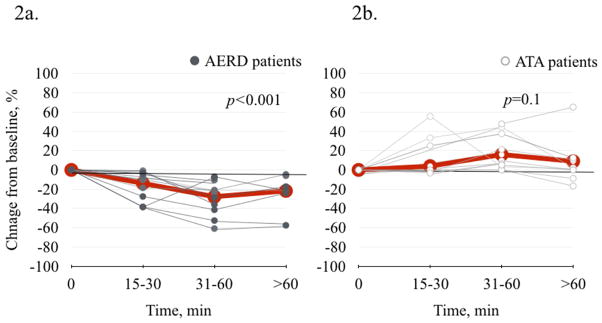
A similar pattern of FeNO changes was observed after the standard, hypersensitivity-provoking graded aspirin challenge. During the first hour after the onset of the hypersensitivity reaction, FeNO levels decreased in all 16 AERD patients (Figure 2a) while levels either increased or remained the same in all 10 of the ATA patients (Figure 2b). The decrease in FeNO in AERD patients was −13.9% (±4.0) at 15–30 min, −27.8% (±4.9) at 30–60 min, and −21.8% (±6.6) at >60 min after the onset of hypersensitivity reaction (p<0.001) (Figure 2a). For the ATA group, mean FeNO level changed by 3.9 (±5.3) at 15–30 min, by 15.9 (±6.4) at 30–60 min, and by 9.0 (±10.1) (p=0.1) (Figure 2b).
Figure 2.
FeNO change after standard oral graded aspirin challenge. 2a. In AERD patients (N=16), there was a significant decrease in FeNO (p<0.001). 2b. In ATA patients (N=10), there was no significant change in FeNO (p=0.1).
Gray thin lines represent individual data points. Red thicker lines represent mean values.
Changes in FEV1 and NPF after low and graded aspirin challenges
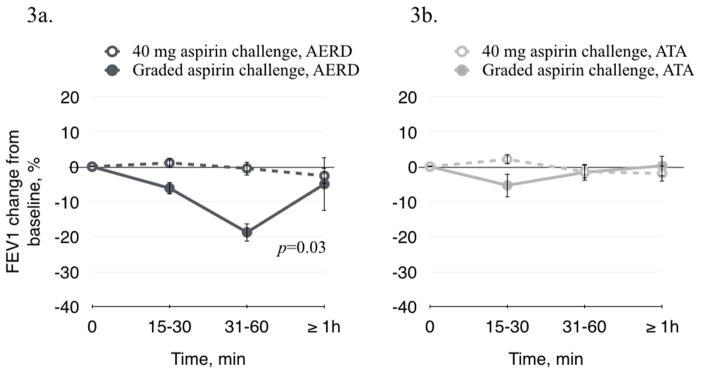
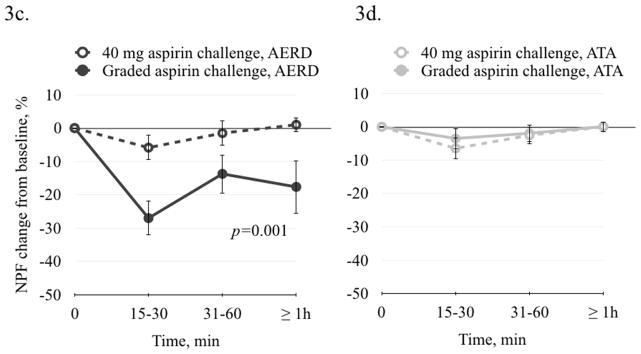
Changes in FEV1 and NPF were measured in all patients after both the low dose and standard aspirin challenges. There was no significant change in either FEV1 or NPF after 40 mg aspirin in the AERD group, confirming that this dose did not induce a clinically apparent hypersensitivity reaction (Figure 3a and 3c). During the standard graded aspirin challenge, FEV1 decreased in AERD by 6.2% (±1.8) at 15–30 min, by 18.8% (±2.6) at 30–60 min, and by 5.0 (±7.6) at >60 min (p=0.03) (Figure 3a). NPF decreased in AERD patients after the standard graded aspirin challenge by 27.0% (±5.1) at 15–30 min, by 13.7% (±5.8) at 30–60 min, and by 17.7 (±7.9) (p=0.001) (Figure 3c). These results confirm that AERD the patients were aspirin-sensitive. There was no significant change in FEV1 or NPF in the ATA subjects during the standard graded aspirin challenge. (Figures 3b and 3d).
Figure 3.
FEV1 and NPF change after 40 mg aspirin and after standard oral graded aspirin challenge. 3a and 3c. In AERD patients after 40 mg aspirin challenge, there was no significant decrease in FEV1 or in NPF. There was a significant decrease in FEV1 (p=0.03) and in NPF (p=0.001) after graded aspirin challenge. 3b and 3d. In ATA patients there was no significant change in FEV1 or in NPF after 40 mg aspirin or after graded aspirin challenge.
Changes in LTE4 after low and graded aspirin challenges
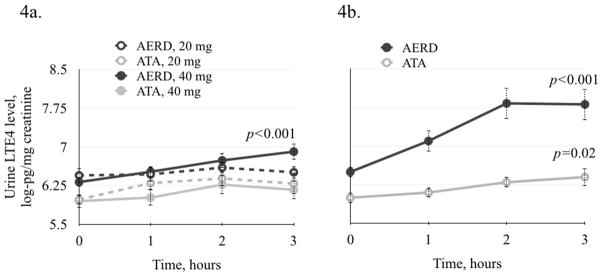
Urine levels of LTE4 were measured after low and graded aspirin challenge. There was no significant change in LTE4 level in either the AERD or ATA groups after 20 mg of aspirin (Figures 4a). In AERD patients after 40 mg of aspirin, there was a significant increase in LTE4 from 6.32 (±0.08) to 6.91 (±0.15) log-pg/mg creatinine at 3 hours (p<0.001) (Figure 4a). There was no significant increase in LTE4 in the ATA patients (Figure 4a).
Figure 4.
Change in LTE4 urine levels after low and standard oral graded aspirin challenge. 4a. After 20 mg aspirin challenge there was no significant change in LTE4 levels in either group. In AERD patients after 40 mg aspirin challenge, there was a significant increase in LTE4 levels (p=0.001). 4b. There was a significant increase in urinary LTE4 levels after standard graded aspirin challenge in AERD and in ATA patients (p<0.001 and p=0.02, respectively).
After the standard oral aspirin challenge, there was a significant increase in LTE4 in AERD patients from 6.5 (±0.1) to 7.81 (±0.3) log-pg/mg creatinine (p<0.001) (Figure 4b). There was also a significant but smaller increase in LTE4 in ATA after the graded aspirin challenge (cumulative aspirin dose of 605 mg), from 6.0 (±0.1) to 6.4 (±0.17) log-pg/mg creatinine (p=0.02) (Figure 4b).
Changes in tetranor PGDM after low and graded aspirin challenges
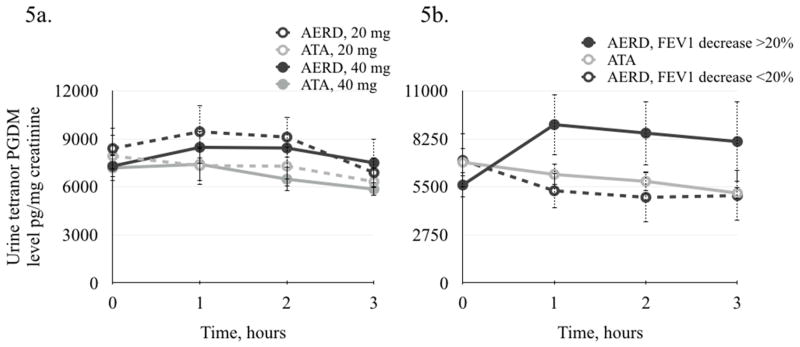
Tetranor PGDM is the major metabolite of prostaglandin D2. There was no significant change in urine tetranor PGDM concentration in either the AERD or ATA groups after the low dose, 20 mg or 40 mg aspirin challenges (Figure 5a).
Figure 5.
Change in tetranor PGDM urine levels after low and standard oral graded aspirin challenge. 5a. After low-dose aspirin challenge there was no significant change in tetranor PGDM levels in either group. 6b. After standard graded aspirin challenge, there was a significant increase in tetranor PGDM levels in AERD patients with FEV1 decrease of ≥20% (n=10, p=0.001). There was a decrease in tetranor PGDM levels in AERD patients with FEV1 decrease of <20% (n=6), and in ATA patients (n=13), (p=0.02 and p<0.01, respectively).
After the standard oral aspirin challenge, there were 10 AERD patients that exhibited a significant increase in urinary tetranor PGDM one hour after the onset of the hypersensitivity reaction (Figure 5b). In these subjects, urine tetranor PGDM levels increased from 5,607.6 (±715.6) to 9,065.8 (±1746.9) pg/mg creatinine (p=0.001). All of them developed bronchospasm with a FEV1 decrease of ≥20%. In six other AERD patients urine tetranor PGDM levels decreased, from 7,018.4 (±1538.7) to 4,900.6 (±1413.3) pg/mg creatinine (p=0.02) (Figure 5b). All six of them developed predominantly upper respiratory reactions and a FEV1 decrease of <20%. The change in urine tetranor PGDM levels in ATA patients after the graded aspirin challenge was similar to the latter AERD group. After the graded aspirin challenge, ATA patients had a significant decrease in urine tetranor PGDM levels, from 6,906.9 (±810.9) to 5,144.8 (±345.5) pg/mg creatinine (p<0.01) (Figure 5b).
The only significant differences between AERD patients who reacted with PGDM level increase vs. decrease were the magnitude of the FEV1 decrease during the challenge and the history of duration of aspirin hypersensitivity; the duration was shorter in patients who reacted with an increase in urinary tetranor PGDM than in those who had a decrease in urinary tetranor PGDM levels (3 years (±0.5) vs. 15 years (± 3.9), respectively, p=0.02).
Changes in plasma PGEM after low and graded aspirin challenges
PGEM is the major metabolite or prostaglandin E2. There was no significant change in PGEM plasma concentrations in either group after either low-dose or standard oral graded aspirin challenge (eFigure 1). PGEM levels appeared to be lower in the AERD patients at baseline and after most of the ASA challenge doses, however this difference was not statistically significant.
FeNO and LTE4
Because FeNO and LTE4 were the only parameters in this study that significantly changed in AERD patients after the 40 mg aspirin challenge dose, their diagnostic value for identification of AERD patients was evaluated.
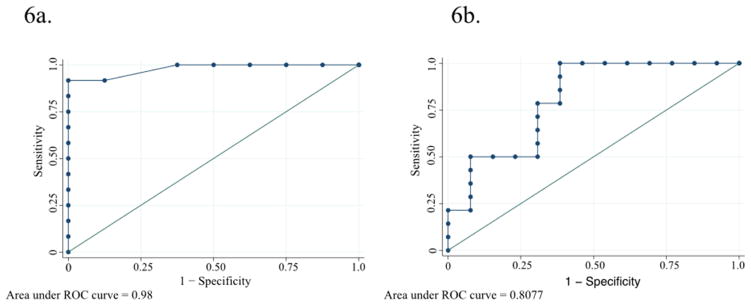
The sensitivity and specificity of FeNO changes for identifying AERD patients after 40 mg aspirin for the best-calculated cutoff point (0.8) were 90% and 100%, respectively (Figure 6a), correctly classifying 93% of participants with an area under the curve of 0.98 (95%CI 0.92–1.00) suggesting that FeNO change at >1 hour after 40 mg aspirin challenge can help discriminate between AERD and ATA patients. The positive predictive value for FeNO change at >1 hour was 100% and the negative predictive value was 83.3%.
Figure 6.
Receiver operating characteristic (ROC) curves for FeNO change (6a.) and urinary LTE4 change (6b.) after 40 mg aspirin challenge.
The sensitivity and specificity of LTE4 changes for identifying AERD patients after 40 mg aspirin for the best-calculated cutoff point (0.48) were 64.3% and 69.2%, respectively (Figure 6b), correctly classifying 66.7% with an area under the curve of 0.81 (95%CI 0.63–0.98). This suggests a much more modest and perhaps inadequate ability of LTE4 changes at >1 hour after 40 mg aspirin challenge to discirminate between AERD and ATA patients. The positive predictive value for LTE4 change at >1 hour was 69.2% and the negative predictive value was 64.3%.
Discussion
It is important to correctly identify patients as being aspirin allergic or tolerant. There is the common requirement of NSAID’s for anti-inflammatory treatment and many patients also require daily aspirin for cardioprotection. In addition, identifying AERD patients offers them the opportunity to undergo aspirin desensitization – an important treatment option for AERD that leads to the improvement in upper and lower airway symptoms.9, 29 The current standard method for diagnosing AERD is to observe clinical response (upper and/or lower respiratory symptoms) in asthma patients after a graded aspirin challenge.16 This diagnostic approach is problematic because clinical reactions to aspirin can be severe and uncomfortable to the patients. We therefore performed this study to explore the possibility of low dose aspirin challenges to diagnose AERD without inducing a clinically apparent hypersensitivity reaction.
While there were no detectable clinical signs of a hypersensitivity reaction after 40 mg aspirin in the majority of patients, FeNO changes were distinctly different between AERD and ATA subjects. FeNO levels significantly decreased from baseline in AERD patients during 40 mg aspirin challenges and during the standard graded oral aspirin challenge but did not significantly change in ATA subjects. The decrease in FeNO after administration of 40 mg aspirin was predictive of an eventually positive standard oral aspirin challenge in AERD patients. It occurred in the absence of any detectable changes in FEV1 or NPF. The decrease in FeNO during the standard graded aspirin challenge was greater than that after 40-mg-challenge, suggesting a dose-dependent response of FeNO levels to aspirin in AERD patients. FeNO has been traditionally used for monitoring asthma control over time.30 However, it is also an important biomarker for evaluating acute bronchospasm.31
In contrast to our findings, others have reported that FeNO values increase during aspirin desensitization and during inhaled aspirin-lysine challenge in AERD patients.19, 20 This discrepancy is likely due to a different timing of FeNO measurements between the studies. In the present study, FeNO values were measured shortly after aspirin administration, while peak FeNO values in one report were observed at 4 hours post inhaled aspirin-lysine challenge20 and another report documented higher FeNO values 24 hours after an allergen challenge.32 In addition, the differences in observations between the studies could be related to the different route of aspirin administration: inhaled20 vs. oral in this study. FeNO values may also vary due to the differences between devices used in studies.33
However, consistent with our observations, another report also found a decrease in nasal nitric oxide after nasal aspirin-lysine challenges at >1 hour in AERD patients who had positive challenges.21 These authors hypothesized that the decrease was due to nasal mucosa edema that blocked the release of nitric oxide from the sinuses, since it was accompanied by a significant decrease in NPF.21 The concomitant decline in FEV1 in the present study also suggests that the FeNO decrease may be due to a FeNO trapping in the airways.
We found that during the 40 mg aspirin challenge FeNO values decreased in AERD patients but there was no significant decrease in FEV1. Nitric oxide detected in exhaled air is synthesized from L-arginine by nitric oxide synthase enzymes. These enzymes are expressed in endothelial, smooth muscle cells, and inflammatory cells (i.e., neutrophils and macrophages) and are activated by inflammatory cytokines.34 In addition to inflammatory status, FeNO values can be influenced by airway caliber.31 It is possible that low-dose aspirin triggers mild bronchoconstriction that is too small to be measured by a decrease in FEV1 but that is sufficient to cause reduced airflow and a subsequent impaired nitric oxide washout from bronchi. Others observed that FeNO values decrease during methacholine and allergen challenges.31, 32, 35 In addition, it was recently reported that during LPS challenge in mice, endothelial nitric oxide synthase produces superoxide ion instead of nitric oxide, reducing nitric oxide bioavailability and perhaps leading to a lower FeNO concentration in the exhaled breath.36
Since AERD patients had no apparent clinical reaction to 40 mg aspirin challenges, the explanations of FeNO decrease due to bronchospasm or changes resulting from an acute lung injury seem to be less plausible. Although investigating the mechanism by which FeNO levels decreased in AERD patients is beyond the scope of this study, we attempted to find an explanation for the decrease in FeNO. One study investigated relationship between inducible nitric oxide synthase (iNOS) and eicosanoids in animals with glomerular immune injury.37 In that study, authors observed an induction of iNOS expression after a glomerular immune injury. iNOS expression was further enhanced by inhibition of COX by indomethacin or by inhibition of 5-lipoxygenase (5-LO) by a lypoxygenase inhibitor. Since we observed an increase in LTE4 in AERD patients after both 40 mg and a standard aspirin challenge, it is possible that high LTE4 levels may have an opposite effect on iNOS and subsequently on nitric oxide production: high LTE4 levels may suppress iNOS expression and lead to a decreased nitric oxide production. During the standard challenge some of the AERD patients had an increase in tetranor PGDM. This observation indicates that COX-inhibition was not complete or that COX could have been activated during the hypersensitivity reaction. Thus, activation of 5-LO and COX in AERD patients could lead to a suppression of iNOS expression and a subsequent decrease in nitric oxide production during both 40 mg challenge and a standard aspirin challenge. Interestingly, several aspirin-tolerant asthma patients had an apparent increase in FeNO, supporting the hypothesis of enhanced iNOS expression due to the COX inhibition by aspirin and a subsequent increase in nitric oxide production in individuals who are not allergic to aspirin. During the 40 mg aspirin challenge, some of the ATA patients also had a decrease in FeNO. Interestingly, FeNO reduction in ATA was not aspirin-dose-dependent and became less apparent during a standard graded aspirin challenge.
LTE4 was the other parameter that showed significant differences between AERD and ATA patients. We found that the baseline LTE4 level was greater in AERD subjects than in ATA study participants, although, as also previously reported, there was an overlap in LTE4 levels between both groups of asthma patients.9, 38 LTE4 was the only metabolite measured in this study that significantly increased after 40 mg of aspirin in AERD patients and not in ATA. This increase did not lead to a hypersensitivity reaction in most patients, confirming that such reactions are dose-and LTE4-level-dependent.3, 15 This finding provides further confirmation that leukotriene overproduction is a characteristic feature of AERD. It has been linked to the deficiency of PGE2 in AERD patients.39 PGE2 prevents leukotriene pathway activation and its inhibition by aspirin results in a surge of leukotrienes from eosinophils or mast cells in aspirin-sensitive and aspirin-tolerant subjects.40 The LTE4 increase after 40 mg aspirin could be considered as a predictor of clinical reactivity in AERD patients. However, the ability of LTE4 change after 40 mg aspirin to discriminate between AERD and ATA patients is probably inadequate.
We found that both COX-pathway metabolites that we measured in this study (urinary tetranor PGDM and plasma PGEM) behaved in a similar way in both AERD and ATA groups during low-dose aspirin challenges. After aspirin-induced hypersensitivity reactions, patients with a milder bronchoconstriction had urinary tetranor PGDM decrease similar to that of ATA patients, consistent with a previous report.12 In addition, we observed that a more severe bronchospasm during the challenge was associated with an increase in tetranor PGDM and a shorter duration of AERD symptoms. Notably, a duration of AERD symptoms of <10 years was previously identified as a risk factor for a more severe bronchospasm during aspirin challenges.15
Plasma PGE2 metabolite levels in this study were not significantly different between AERD and ATA patients at baseline and they also did not significantly change after aspirin challenges. Although, it is believed that AERD is due to a lack of PGE2, studies on PGEM levels and their changes have not been consistent.7, 41–45
This study has several strengths: a multi-racial and multi-ethnical population of patients with moderate to severe persistent asthma. To our knowledge, it is the first study to analyze changes in FeNO and eicosanoids during low-dose oral aspirin challenges. The study also has some limitations: a relatively small sample size and a lack of healthy controls. In addition, it is limited by a lack of placebo challenges preceding aspirin challenges and by underrepresentation of patients with nasal polyposis among aspirin-tolerant asthmatics.
In conclusion, early aspirin-induced decrease in FeNO may prove to be a useful adjunct for diagnosing AERD if confirmed in larger studies. It may also allow better monitoring and the early initiation of treatment during aspirin challenges, in order to prevent more severe procedure-induced bronchoconstriction.
Supplementary Material
Acknowledgments
Declaration of funding sources:
This publication was supported by CTSA grant number 5KL2TR001071 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The study was also supported by Investigator Initiated Research Award IISP39161 from Merck & Co., Inc.
The authors would like to acknowledge Dr. Andrzej Szczeklik for his advice and help in the design of this study.
Abbreviations
- AERD
aspirin exacerbated respiratory disease
- ATA
aspirin tolerant asthma FeNO, fraction of exhaled nitric oxide
- IQR
interquartile range
- LTE4
leukotriene E4
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- PGDM
prostaglandin D2 metabolite
- PGEM
prostaglandin E2 metabolite
- COX
cyclooxygenase
- FEV1
forced expiratory volume in 1 second
- NPF
nasal inspiratory peak flow
- NSAID
non-steroidal anti-inflammatory drugs
Footnotes
Trial registration:
ClinicalTrials.gov Identifier: NCT01320072 https://clinicaltrials.gov/ct2/show/NCT01320072?term=low+dose+aspirin+challenge&rank=1
Conflict of interest:
Dr. Elina Jerschow holds an Investigator Initiated Research Award IISP39161 from Merck & Co., Inc. Other authors have no potential conflict of interest
Contribution of each author to the manuscript:
- Elina Jerschow – conception and design of the study, data generation, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Zhen Ren – data generation, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Golda Hudes – data generation, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Marek Sanak – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Esperanza Morales – data generation, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Victor Schuster – data generation, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Simon D. Spivack – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- David Rosenstreich – conception and design of the study, data generation, analysis and interpretation of the data, preparation and critical revision of the manuscript.
All authors approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mascia K, Haselkorn T, Deniz YM, et al. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to5 treat asthma. J Allergy Clin Immunol. 2005;116:970–975. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111:743–749. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 3.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104:559–564. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 4.Dahlen B. Leukotrienes as mediators of asthma induced by aspirin and allergen. Stockholm, Sweden: Thoracic Medicine, Division of Allergology, Karolinska Institutet; 1993. [Google Scholar]
- 5.Higashi N, Mita H, Ono E, et al. Profile of eicosanoid generation in aspirin-intolerant asthma and anaphylaxis assessed by new biomarkers. J Allergy Clin Immunol. 2010;125:1084–1091. doi: 10.1016/j.jaci.2009.12.977. [DOI] [PubMed] [Google Scholar]
- 6.Klumlin M, Dahlen B, Bjorck T, Zetterstrom O, Granstrom E, Dahlen S. Urinary excretion of leukotriene E4 and 11-dehydro-thromboxane B2 in response to bronchial provocations with allergen, aspirin, leukotriene D4, and histamine in asthmatics. Am Rev Respir Dis. 1992;146:96–103. doi: 10.1164/ajrccm/146.1.96. [DOI] [PubMed] [Google Scholar]
- 7.Mastalerz L, Sanak M, Gawlewicz-Mroczka A, Gielicz A, Cmiel A, Szczeklik A. Prostaglandin E2 systemic production in patients with asthma with and without aspirin hypersensitivity. Thorax. 2008;63:27–34. doi: 10.1136/thx.2007.080903. [DOI] [PubMed] [Google Scholar]
- 8.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. Eur Respir J. 1993;6:391–399. [PubMed] [Google Scholar]
- 9.Swierczynska-Krepa M, Sanak M, Bochenek G, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: A double-blind study. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Juergens UR, Christiansen SC, Stevenson DD, Zuraw BL. Inhibition of monocyte leukotriene B4 production after aspirin desensitization. J Allergy Clin Immunol. 1995;96:148–156. doi: 10.1016/s0091-6749(95)70002-1. [DOI] [PubMed] [Google Scholar]
- 11.Juergens UR, Christiansen SC, Stevenson DD, Zuraw BL. Arachidonic acid metabolism in monocytes of aspirin-sensitive asthmatic patients before and after oral aspirin challenge. J Allergy Clin Immunol. 1992;90:636–645. doi: 10.1016/0091-6749(92)90137-q. [DOI] [PubMed] [Google Scholar]
- 12.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D: A dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashi N, Mita H, Yamaguchi H, Fukutomi Y, Akiyama K, Taniguchi M. Urinary tetranor-PGDM concentrations in aspirin-intolerant asthma and anaphylaxis. J Allergy Clin Immunol. 2012;129:557–559. 559 e551–552. doi: 10.1016/j.jaci.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan S, Dahlen B, Dahlen SE, Kumlin M. Increased urinary excretion of the prostaglandin D2 metabolite 9 alpha, 11 beta-prostaglandin F2 after aspirin challenge supports mast cell activation in aspirin-induced airway obstruction. J Allergy Clin Immunol. 1996;98:421–432. doi: 10.1016/s0091-6749(96)70167-7. [DOI] [PubMed] [Google Scholar]
- 15.Hope AP, Woessner KA, Simon RA, Stevenson DD. Rational approach to aspirin dosing during oral challenges and desensitization of patients with aspirin49 exacerbated respiratory disease. J Allergy Clin Immunol. 2009;123:406–410. doi: 10.1016/j.jaci.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Macy E, Bernstein JA, Castells MC, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–174. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen A, FitzGerald GA. Dose-related kinetics of aspirin. New Engl J Med. 1984:311. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 18.Chen JR, Buchmiller BL, Khan DA. An Hourly Dose-Escalation Desensitization Protocol for Aspirin Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015 doi: 10.1016/j.jaip.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Katial RK, Strand M, Prasertsuntarasai T, Leung R, Zheng W, Alam R. The effect of aspirin desensitization on novel biomarkers in aspirin-exacerbated respiratory diseases. J Allergy Clin Immunol. 2010;126:738–744. doi: 10.1016/j.jaci.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Rolla G, Di Emanuele A, Dutto L, et al. Effect of inhalation aspirin challenge on exhaled nitric oxide in patients with aspirin-inducible asthma. Allergy. 2004;59:827–832. doi: 10.1111/j.1398-9995.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 21.Tworek D, Kuprys-Lipinska I, Pietruszewska W, Kuna P. Two patterns of changes in nasal nitric oxide after lysine aspirin nasal challenge in patients with aspirin66 exacerbated respiratory disease. Am J Rhinol Allergy. 2012;26:428–432. doi: 10.2500/ajra.2012.26.3818. [DOI] [PubMed] [Google Scholar]
- 22.White A, Ludington E, Mehra P, Stevenson DD, Simon RA. Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Ann Allergy Asthma Immunol. 2006;97:688–693. doi: 10.1016/S1081-1206(10)61101-5. [DOI] [PubMed] [Google Scholar]
- 23.White AA, Stevenson DD, Simon RA. The blocking effect of essential controller medications during aspirin challenges in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;95:330–335. doi: 10.1016/S1081-1206(10)61150-7. [DOI] [PubMed] [Google Scholar]
- 24.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. The European respiratory journal Supplement. 1993;16:5–40. [PubMed] [Google Scholar]
- 25.Ltd. CCI; Ltd. CCI, editor. Clement Clarke International Catalogue. 2015. [Google Scholar]
- 26.Cayman Chemical. 2010. Cystenyl Leukotriene EIA Kit. [Google Scholar]
- 27.Cayman Chemical. 2010. Tetranor-PGDM EIA Kit. [Google Scholar]
- 28.Cayman Chemical. 2010. Prostaglandin E Metabolite EIA Kit. [Google Scholar]
- 29.Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003;111:180–186. doi: 10.1067/mai.2003.7. [DOI] [PubMed] [Google Scholar]
- 30.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haccuria A, Michils A, Michiels S, Van Muylem A. Exhaled nitric oxide: a biomarker integrating both lung function and airway inflammation changes. J Allergy Clin Immunol. 2014;134:554–559. doi: 10.1016/j.jaci.2013.12.1070. [DOI] [PubMed] [Google Scholar]
- 32.Roos AB, Mori M, Gronneberg R, et al. Elevated exhaled nitric oxide in allergen93 provoked asthma is associated with airway epithelial iNOS. PloS one. 2014;9:e90018. doi: 10.1371/journal.pone.0090018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014;108:830–841. doi: 10.1016/j.rmed.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. The New England journal of medicine. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 35.Giovannini M, Valli M, Ribuffo V, et al. Relationship between Methacholine Challenge Testing and exhaled nitric oxide in adult patients with suspected bronchial asthma. European annals of allergy and clinical immunology. 2014;46:109–113. [PubMed] [Google Scholar]
- 36.Wu F, Szczepaniak WS, Shiva S, et al. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L987–997. doi: 10.1152/ajplung.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lianos EA, Guglielmi K, Sharma M. Regulatory interactions between inducible nitric oxide synthase and eicosanoids in glomerular immune injury. Kidney Int. 1998;53:645–653. doi: 10.1046/j.1523-1755.1998.00791.x. [DOI] [PubMed] [Google Scholar]
- 38.Higashi N, Taniguchi M, Mita H, et al. Clinical features of asthmatic patients with increased urinary leukotriene E4 excretion (hyperleukotrienuria): Involvement of chronic hyperplastic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2004;113:277–283. doi: 10.1016/j.jaci.2003.10.068. [DOI] [PubMed] [Google Scholar]
- 39.Sestini P, Armetti L, Gambaro G, et al. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1996;153:572–575. doi: 10.1164/ajrccm.153.2.8564100. [DOI] [PubMed] [Google Scholar]
- 40.Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol. 2014;193:41–47. doi: 10.4049/jimmunol.1301753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalski ML, Pawliczak R, Wozniak J, et al. Differential metabolism of arachidonic acid in nasal polyp epithelial cells cultured from aspirin-sensitive and aspirin121 tolerant patients. Am J Respir Crit Care Med. 2000;161:391–398. doi: 10.1164/ajrccm.161.2.9902034. [DOI] [PubMed] [Google Scholar]
- 42.Laidlaw TM, Cutler AJ, Kidder MS, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014;133:1692–1701. e1693. doi: 10.1016/j.jaci.2013.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013;110:16987–16992. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol. 2011;128:66–72. e61. doi: 10.1016/j.jaci.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergology international : official journal of the Japanese Society of Allergology. 2008;57:429–436. doi: 10.2332/allergolint.o-08-545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.