Abstract
Background
Bladder dysfunction in spina bifida (SB) patients can lead to significant morbidity due to renal insufficiency. Indications for surgery vary between institutions, and the impact is unclear. Our objective was to examine trends and variations in urologic interventions and chronic renal insufficiency in SB patients.
Methods
We reviewed the Nationwide Inpatient Sample (NIS) for all SB patients treated from 1998–2011. We used ICD-9-CM codes to identify urologic surgery and chronic renal insufficiency (CRI). We calculated Spearman’s correlation coefficients between rates of SB-related bladder surgeries and rates of CRI outcomes by state. Linear regression models were fitted to investigate the associations between rates of SB-related surgery and CRI across treatment years.
Results
We identified 427,616 SB admissions (mean age 26y, 56% female); 35,249(8%) were for CRI and 11,078 (3%) were for surgery. Over the study period, CRI rates doubled (6–12%) and surgery rates declined (2.0–1.8%). There was a moderately weak, inverse association between surgery and CRI rates over time (r=−0.3, p=0.06) and by state (r=−0.3, p=0.04). On multivariate analysis, higher rates of surgery were associated with the state in which the patient was treated (p<0.001), younger age (p<0.001), and hospital teaching status (p<0.001). In contrast, CRI was not associated with SB-related surgery (p=0.67).
Conclusions
We observed a temporal and geographic trend toward decreasing urologic surgery and increasing CRI rates in SB and a wide variation in urologic surgical rates among states. Further study is needed to determine the factors behind these trends and variations in SB management.
Keywords: Urology, Spina Bifida, Neurogenic Bladder, clinical care variation, renal insufficiency
INTRODUCTION
Spina bifida (SB) is a major congenital defect in which the neural tube fails to close properly during embryonic development and is the most common permanently disabling birth defect in the US.1, 2 Due to its involvement in multiple organ systems, managing children with SB presents many complex urologic challenges. Over 90% of affected individuals will have neurogenic bladder.3 Therefore, careful and attentive bladder management is paramount for the preservation of maximal renal function and achievement of the best-possible quality of life.4, 5
The approaches to bladder management include routine monitoring with imaging and urodynamic study, anti-cholinergic medications, clean intermittent catheterization, and various surgeries including, but not limited to, enterocystoplasty (bladder augmentation), bladder neck sling, sphincterotomy, urinary diversion (vesicostomy, ileal conduit), artificial urinary sphincter, botulinum toxin injection, and creation of catheterizable stomas (appendicovesicostomy, Monti channel). The indications for surgery vary significantly between institutions and providers, and the ideal strategy remains unclear.6
As an increasingly large number of children with SB are surviving beyond infancy into childhood and adolescence as a result of modern medical and surgical advances,7 evaluation of different bladder management approaches to achieve the best long-term outcomes is critical. It is known that high bladder storage pressures related to neurogenic bladder can lead to renal insufficiency, and bladder augmentation is not infrequently performed to reduce bladder pressure in order to protect the kidneys. Institutional series have shown rather low morbidity and mortality of various urologic surgical interventions in modern series.8–10 However, the impact of those surgeries on long-term renal function is unclear. Little long-term outcome data on bladder management strategies is available to help determine the best practices in terms of indication and timing of those procedures. On the other hand, the operative morbidity of bladder augmentation is well-described, including bladder stones, malignancy, or spontaneous perforation.11, 12 Some centers therefore have endorsed a less surgically-oriented approach for the management of neurogenic bladder among children and adults with SB 13 due to the advancement of new anti-cholinergic medications with fewer side effects, close monitoring with imaging and urodynamics, and increasing awareness of potential long-term complications of SB-related surgeries.12, 14
The objective of this study is to utilize national database to expand the perspectives on the variation of CRI and SB-related urologic surgery to gain insight on their relationship. We hypothesized that urologic surgery rates, specifically bladder augmentation, would be inversely related to CRI rates.
PATIENTS & METHODS
Data Source
The Nationwide Inpatient Sample (NIS) is an all-age, all-payer database managed by the Healthcare Cost and Utilization Project and sponsored by the Agency for Healthcare Research and Quality. Data in the NIS are from a 20% stratified probability sample of US hospitals based on five hospital characteristics including ownership status, number of beds, teaching status, urban/rural location, and geographic region. NIS includes post-stratification discharge weights that may be used to calculate national estimates.15
Selection of Patients and Covariates
We identified all inpatient hospital encounters occurring between 1998 and 2011 for patients with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for SB (741.X, 756.17).
Predictor variables were a priori selected based on biologic plausibility and/or demonstrated associations in the literature. Covariates included basic patient demographics: age, gender, race, insurance payer (public vs. private), ZIP code median household income (by quartiles), year of admission, as well as hospital level factors: hospital characteristics such as hospital teaching status (teaching, non-teaching), state, and hospital size according to number of beds (small, medium, and large).
Outcome Selection
The primary outcomes were SB-related urological surgery and CRI. We defined SB-related urological surgeries to include bladder augmentation, bladder neck sling, sphincterotomy, vesicostomy, artificial urinary sphincter, botulinum toxin injection, appendicovesicostomy, and urinary diversion. Surgeries were identified by ICD-9-CM procedure codes (Appendix 1). CRI was defined based on ICD-9-CM codes (585 chronic kidney disease, 586 renal failure, unspecified) and/or Clinical Classifications Software codes (157, 158), and/or procedure codes for dialysis or renal transplant (58, 91, 105). Clinical Classifications Software codes are based on ICD-9-CM codes and were developed by AHRQ specifically for use in administrative data.16
Statistical Analysis
All analyses were weighted using NIS-specific estimated weights and covariance matrices. We calculated Spearman’s correlation coefficients to assess the relationship between rates of SB-related urological surgery in each state and rates of CRI outcomes in the same state. Linear regression models were fitted to investigate the associations between rates of SB-related urological surgery and CRI over time.
Weighted logistic regression models were used to define the associations among patient and hospital level factors with SB-related urological surgery. Model covariates were a priori determined based on our conceptual model. The importance of each covariate was examined by comparing sequential nested models. We also conducted a sensitivity analysis examining the effects of these factors using generalized estimating equations (GEE) in order to control for clustering of similar patients in each hospital and each state.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). All tests were two-sided and p-values of 0.05 or less were considered to be significant.
RESULTS
Demographics
In total, we identified 427,616 SB admissions (Table 1) from 1998–2011 NIS. Females constituted 56% of the overall cohort. Mean patient age was 26 years old; the majority were adults (56%) followed by teenagers (13%). Socio-economic analysis revealed that 53% were Caucasians, 60% were publically insured, 37% were from the southern region of the United States, and most were treated in teaching (67%) and large (63%) hospitals.
Table 1.
Cohort demographics
| Characteristics | n=427,616 | % |
|---|---|---|
| Age (Mean±SD) | 26.4±0.6 | - |
|
| ||
| Age groups | ||
| <1 y | 42,801 | 10.0 |
| 1 y | 8,790 | 2.1 |
| 2–5 y | 24,846 | 5.8 |
| 6–11 y | 37,046 | 8.7 |
| 12–18 y | 53,708 | 12.6 |
| 19–21 y | 21,560 | 5.0 |
| >21 y | 238,866 | 55.9 |
|
| ||
| Gender | ||
| male | 188,236 | 44.0 |
| female | 238,865 | 55.9 |
|
| ||
| Race | ||
| White | 228,047 | 53.3 |
| Black | 31,756 | 7.4 |
| Hispanic | 52,212 | 12.2 |
| Asian | 3,213 | 0.8 |
| Native American | 2,090 | 0.5 |
| Others | 10,112 | 2.4 |
|
| ||
| Year | ||
| 1998 | 21,141 | 4.9 |
| 1999 | 26,557 | 6.2 |
| 2000 | 23,216 | 5.4 |
| 2001 | 26,108 | 6.1 |
| 2002 | 27,768 | 6.5 |
| 2003 | 27,482 | 6.4 |
| 2004 | 30,068 | 7.0 |
| 2005 | 36,461 | 8.5 |
| 2006 | 32,355 | 7.6 |
| 2007 | 32,603 | 7.6 |
| 2008 | 34,288 | 8.0 |
| 2009 | 35,648 | 8.3 |
| 2010 | 37,585 | 8.8 |
| 2011 | 36,338 | 8.5 |
|
| ||
| Insurance | ||
| Public | 254,798 | 59.6 |
| Private | 145,482 | 34.0 |
| others | 26,391 | 6.2 |
|
| ||
| Income | ||
| quartile 1 | 87,630 | 20.5 |
| quartile 2 | 80,503 | 18.8 |
| quartile 3 | 72,882 | 17.0 |
| quartile 4 | 54,204 | 12.7 |
|
| ||
| Surgery | ||
| augmentation | 5,748 | 1.3 |
| vesicostomy | 3,881 | 0.9 |
| sphincterotomy | 76 | <0.1 |
| conduit | 1,173 | 0.3 |
| Mitrofanoff | 1,015 | 0.2 |
| botox | 1,105 | 0.3 |
|
| ||
| Renal failure | ||
| Yes | 35,249 | 8.2 |
| No | 392,368 | 91.8 |
|
| ||
| Region | ||
| Northeast | 74,504 | 17.4 |
| Midwest | 109,417 | 25.6 |
| South | 160,079 | 37.4 |
| West | 83,617 | 19.6 |
|
| ||
| Hospital teaching status | ||
| Non-teaching | 139,913 | 32.7 |
| Teaching | 284,839 | 66.6 |
|
| ||
| Hospital bedsize | ||
| Small | 62,576 | 14.6 |
| Medium | 94,012 | 22.0 |
| Large | 268,164 | 62.7 |
SB-related urological surgery and Chronic renal insufficiency
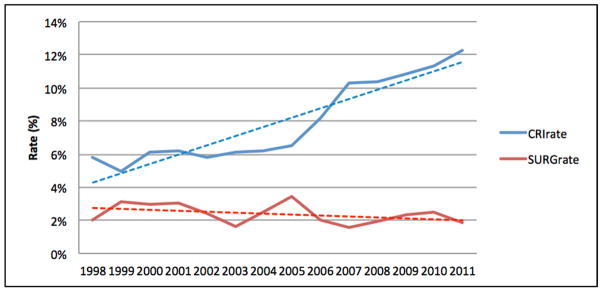
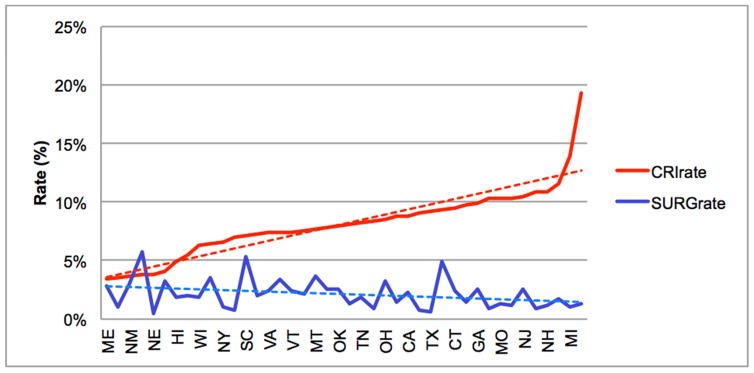
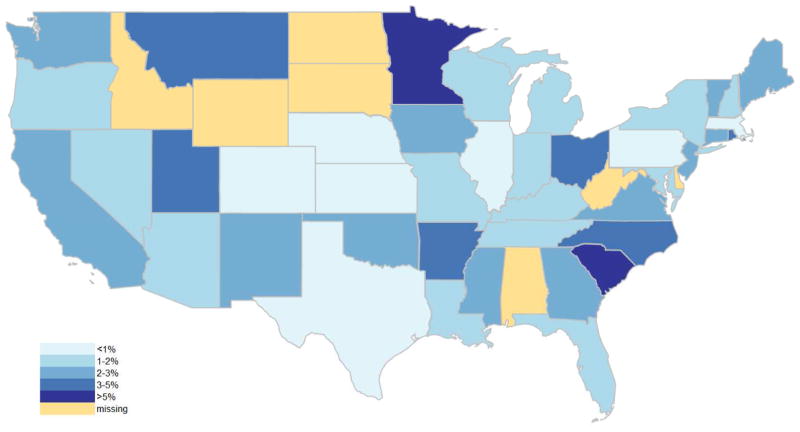
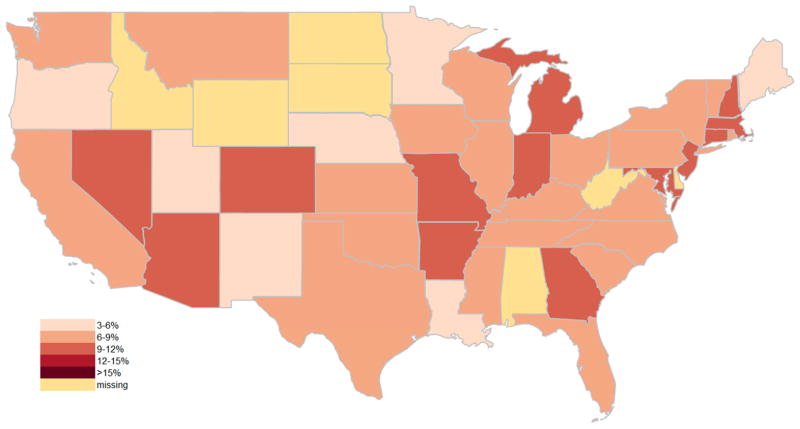
Of the included SB admissions, 35,249 (8%) were identified with CRI, while 10,106 (2%) admissions were for SB-related urological surgery. Over the study period (1998–2011), admissions with CRI doubled (6–12%) and surgery rates declined slightly (2.0 to 1.8% in Figure 1). There was a moderately weak, inverse association between SB-related urological surgery and admissions with CRI over time (r=−0.3, p=0.06). Additionally, both SB-related urological surgery rates (range 0.4–6.2%) and admissions with CRI (range 3.4–19.3%) were highly variable among states (Figures 2, 3, & 4). A significant association was observed between states in surgery and admissions with CRI (r=−0.3, p=0.03).
Figure 1.
Per-year comparison of CRI rate (red line) and SB-related urologic surgery rate (blue line); this demonstrates an inverse association over time.
Figure 2.
CRI rate versus SB-related urologic surgery rate by state
Figure 3.
heat map for SB-related urologic surgery rate
Figure 4.
heat map for CRI rate
Multivariable Analyses
The results of the bivariate and multivariate analyses are detailed in Table 2. On bivariate analysis, SB-related urologic surgery was associated with the particular state in which a patient was treated (p<0.001; each state not shown for sake of brevity), younger patient age (strongest in school age children, 6 to 11 years old; OR 8.7, p<0.001, private insurance (OR 1.4, p<0.001), treatment at a teaching hospital (OR 3.9, p<0.001), and lower likelihood of admissions with CRI (OR 0.6, p<0.001).
Table 2.
Bivariate and multivariate analysis predicting use of SB-related urologic surgery
| Covariates | unadjusted OR (95%CI) | p value | adjusted OR (95%CI) | p value |
|---|---|---|---|---|
| Age groups | ||||
| <1 y | 0.6(0.4–0.8) | <0.001 | 0.5(0.3–0.7) | <0.001 |
| 1 y | 1.9(1.2–2.9) | 0.06 | 1.4(0.9–2.2) | 0.13 |
| 2–5 y | 4.3(3.4–5.5) | <0.001 | 3.2(2.5–4.0) | <0.001 |
| 6–11 y | 8.7(6.9–10.9) | <0.001 | 6.5(5.1–8.2) | <0.001 |
| 12–18 y | 6.9(5.7–8.5) | <0.001 | 5.3(4.3–6.6) | <0.001 |
| 19–21 y | 3.1(2.4–4.0) | 0.16 | 2.7(2.1–3.5) | <0.001 |
| >21 y | ref | |||
|
| ||||
| Gender | ||||
| male | 1.0(0.9–1.1) | 0.59 | 0.9(0.8–1.0) | 0.05 |
| female | ref | |||
|
| ||||
| Year | ||||
| 1998–2004 | 1.1(0.9–1.5) | 0.33 | 1.0(0.8–1.2) | 0.93 |
| 2005–2011 | ref | |||
|
| ||||
| Insurance | ||||
| Public | ref | |||
| Private | 1.4(1.2–1.6) | <0.001 | 1.0(0.9–1.2) | 0.70 |
|
| ||||
| Renal failure | ||||
| Yes | 0.6(0.5–0.8) | <0.001 | 1.0(0.8–1.3) | 0.67 |
| No | ref | |||
|
| ||||
| Teaching status | ||||
| Yes | 3.9(2.6–5.9) | <0.001 | 2.7(1.9–3.7) | <0.001 |
| No | ref | |||
State variable was not shown in the table for brevity
On multivariate analysis, SB-related urologic surgery remained significantly associated with the particular state in which a patient was treated (p<0.001), younger patient age (again strongest among school age children, 6 to 11 years old; OR 6.5, p<0.001), and treatment at a teaching hospital (OR 2.7, p<0.001). On the other hand, admissions with CRI were not significantly associated with SB-related urologic surgery (OR 1.0, p=0.67). This was confirmed using GEE-based models to adjust for hospital- and state-level clustering (p=0.61).
DISCUSSION
To our knowledge, this study is the largest all-aged analysis investigating the association between CRI and SB-related surgery in SB patients on a population level. We found a trend towards a negative association between rates of admissions with CRI and SB-related surgery (r=−0.3, p=0.06): similarly, admissions with CRI doubled (6 to 12%) while surgery rates declined slightly (2.0 to 1.8%) from 1998 to 2011. This negative association was also observed across the states (r=−0.3, p=0.03). This result raises the concern that the pendulum of care may have swung too far towards conservative management and away from performing the surgery to protect the upper tract in terms of bladder management in SB patients.
Interestingly, in our prior analysis using national pediatric inpatient data, we did not find similar negative association between CRI and bladder augmentation rate.17 This result may suggest the protective effect of SB-related surgery does not manifest until longer-term in older patients. With more SB patients surviving well into adulthood, developing best practices in terms of appropriate timing and indications for SB-related urologic surgery is essential to maintaining renal function and a better quality of life. Further research is warranted to investigate the longer-term impact of surgery on SB patients.
In addition, we observed significant variation in SB-related surgery rates across states (range from 0.4 to 6.2%); this is consistent with other prior studies.6, 18 Such variation has been observed in other clinical situations in which consensus is lacking on the best practices.19, 20 Unwarranted variation is a reality within the medical care system and has been documented repeatedly using a wide range of data sources. Care pattern variation has also been demonstrated, in certain circumstances, to be associated with poorer clinical outcomes.21, 22 In this case, it is particularly worrisome in the setting of the observed negative association between CRI and SB-related urologic surgery.
We also found that several patient- and hospital-level characteristics contributed to higher SB-related surgery rates, including teenage/school age patients and hospital teaching status. Urologic continence procedures are typically not performed until the child both expresses a serious desire for continence and proves attainment of a level of maturity necessary to assist in his or her own post-operative management, including clean intermittent catheterization. Very young patients are unlikely to achieve these criteria. On the other hand, older adult patients are most likely stable and/or content with their current conservative management or already had the SB-related surgery performed at earlier age. Not surprisingly, more procedures were performed in teaching hospitals due to the increased comorbidity level frequently found among SB patients.
These findings must be interpreted in the context of study limitations. NIS represents a 20% stratified sample of US hospital admissions. As such, our reported results may not be generalizable to encounters not in the sample pool such as those that occur in the outpatient setting. However, NIS does provide meticulous tracking of discharge and hospital weights in order to minimize the risk of sampling bias. Although we sampled records on hospitalizations at a national level, the possibility of sampling bias still exists. The NIS sampling frame has expanded over time, so earlier years may be more subject to sampling bias than later years; however, discharge and hospital weights have been developed to minimize this risk. We believe our model adequately accounts for both patient and hospital confounders but cannot rule out the risk of bias from omitted variables, as unmeasured covariates may account for some of the variation seen in outcomes such as surgical practice patterns.
Additionally, NIS is a large administrative database that might be affected by miscoding bias. Our analysis relies on the accuracy of the diagnostic and procedure codes included in NIS; while the accuracy level of NIS is high for an administrative database, it is possible at least some portion of our cohort may be incorrectly coded. We were, therefore, deliberately broadly inclusive in our definition of chronic renal dysfunction, aiming to capture as much of this broad diagnosis as possible; however, coding alone cannot differentiate renal insufficiency due to nephrotoxic medications or sepsis versus neurogenic bladder. This type of coding could lead to either over- or under-estimation of the covariates such as CRI.
Because NIS represents admission-based rather than patient-based data, it is impossible to track a given patient across time. We were therefore unable to assess neither longer-term outcomes nor whether individual patients had multiple admissions. CRI patients may be readmitted much more than those without CRI and therefore may skew the observations. Additionally, the retrospective nature of this study limits the data that are available to us and makes it impossible to fully characterize causal relationships behind observed associations. For example, we had no data on patients’ medical or surgical history; therefore, we could not assess whether a patient with CRI had any prior SB-related surgery, nor could we evaluate the indication for the SB-related surgery at any given admission. We thus could not fully assess if the increasing CRI rate is a result from poor management or reflecting better longevity of spina bifida patients, nor could we drawn any conclusion that the variation and decreasing surgery rate were from provider’s preference or necessarily reflected the quality of SB management. However the temporal and geographic trend toward decreasing urologic surgery and increasing CRI rates are potentially concerning. A meticulously designed prospective study with long term follow up is warranted to help providers determine the best course of management for SB patient in the longer term.
CONCLUSION
We observed a significant temporal trend toward decreasing urologic surgery and increasing CRI rates, as well as a wide variation in urologic surgical rates in SB patients across states. Further study especially longitudinal data is needed to define the factors behind these trends and variations in urological SB management.
Acknowledgments
Dr. Routh is supported by grant K08-DK100534 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Wiener is supported by collaborative agreements with the Centers for Disease Control and Prevention (CDC) for the National Spina Bifida Patient Registry (U01-DD001082 and U01-DD001087). The funding sources had no role in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Key of Abbreviations
- NIS
Nationwide Inpatient Sample
- CRI
Chronic renal insufficiency
- SB
Spina bifida
Appendix 1. code dictionary
| Procedures | ICD-9 procedure codes | ICD-9 diagnosis codes |
|---|---|---|
| bladder augment | 57.87, 57.88 | |
| vesicostomy | 57.21 | |
| sphincterotomy | 57.91 | |
| conduit | 56.51, 56.71, 56.72 | |
| Mitrofanoff | V44.52 | |
| botox | 99.29 | 596.54, 596.59, 788.30, 788.31, 788.32, 788.33, 625.6 |
Footnotes
Conflict of Interest: The remaining coauthors have no financial relationships relevant to the article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 2.Mathews TJ, Honein MA, Erickson JD. Spina bifida and anencephaly prevalence--United States, 1991–2001. MMWR Recomm Rep. 2002;51:9. [PubMed] [Google Scholar]
- 3.Lloyd JC, Wiener JS, Gargollo PC, et al. Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol. 2013;190:1590. doi: 10.1016/j.juro.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Hopps CV, Kropp KA. Preservation of renal function in children with myelomeningocele managed with basic newborn evaluation and close followup. J Urol. 2003;169:305. doi: 10.1016/S0022-5347(05)64112-2. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DG. Urinary tract infection in children with myelomeningocele. Arch Dis Child. 1967;42:521. doi: 10.1136/adc.42.225.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lendvay TS, Cowan CA, Mitchell MM, et al. Augmentation cystoplasty rates at children’s hospitals in the United States: a pediatric health information system database study. J Urol. 2006;176:1716. doi: 10.1016/S0022-5347(06)00615-X. [DOI] [PubMed] [Google Scholar]
- 7.Davis BE, Daley CM, Shurtleff DB, et al. Long-term survival of individuals with myelomeningocele. Pediatr Neurosurg. 2005;41:186. doi: 10.1159/000086559. [DOI] [PubMed] [Google Scholar]
- 8.Venn SN, Mundy AR. Long-term results of augmentation cystoplasty. Eur Urol. 1998;34(Suppl 1):40. doi: 10.1159/000052275. [DOI] [PubMed] [Google Scholar]
- 9.Szymanski KM, Misseri R, Whittam B, et al. Mortality after bladder augmentation in children with spina bifida. J Urol. 2015;193:643. doi: 10.1016/j.juro.2014.07.101. [DOI] [PubMed] [Google Scholar]
- 10.Cain MP, Dussinger AM, Gitlin J, et al. Updated experience with the Monti catheterizable channel. Urology. 2008;72:782. doi: 10.1016/j.urology.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Shekarriz B, Upadhyay J, Demirbilek S, et al. Surgical complications of bladder augmentation: comparison between various enterocystoplasties in 133 patients. Urology. 2000;55:123. doi: 10.1016/s0090-4295(99)00443-4. [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe PD, Casale AJ, Kaefer MA, et al. Spontaneous bladder perforations: a report of 500 augmentations in children and analysis of risk. J Urol. 2006;175:1466. doi: 10.1016/S0022-5347(05)00672-5. [DOI] [PubMed] [Google Scholar]
- 13.Aslan AR, Kogan BA. Conservative management in neurogenic bladder dysfunction. Curr Opin Urol. 2002;12:473. doi: 10.1097/00042307-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Leslie B, Lorenzo AJ, Moore K, et al. Long-term followup and time to event outcome analysis of continent catheterizable channels. J Urol. 2011;185:2298. doi: 10.1016/j.juro.2011.02.601. [DOI] [PubMed] [Google Scholar]
- 15.Overview of the National (Nationwide) Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2014. HCUP Databases. [Google Scholar]
- 16.Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2014. HCUP CCS. [Google Scholar]
- 17.Jessica Lloyd SR, Wiener John, Routh Jonathan. Does Regional Variation in Bladder Augmentation Rates Correlate with Variation in Renal Outcomes among Children with Spina Bifida? AUA 2013. 2013:Abstract 480. [Google Scholar]
- 18.Wiener JS, Antonelli J, Shea AM, et al. Bladder augmentation versus urinary diversion in patients with spina bifida in the United States. J Urol. 2011;186:161. doi: 10.1016/j.juro.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Wang HH, Huang L, Routh JC, et al. Shock wave lithotripsy vs ureteroscopy: variation in surgical management of kidney stones at freestanding children’s hospitals. J Urol. 2012;187:1402. doi: 10.1016/j.juro.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Kleinman LC, Kosecoff J, Dubois RW, et al. The medical appropriateness of tympanostomy tubes proposed for children younger than 16 years in the United States. JAMA. 1994;271:1250. [PubMed] [Google Scholar]
- 21.Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154:789. doi: 10.1016/j.jpeds.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Arriaga AF, Lancaster RT, Berry WR, et al. The better colectomy project: association of evidence-based best-practice adherence rates to outcomes in colorectal surgery. Ann Surg. 2009;250:507. doi: 10.1097/SLA.0b013e3181b672bc. [DOI] [PubMed] [Google Scholar]