Abstract
Central neurocytomas are uncommon intraventricular neoplasms whose optimal management remains controversial due to their rarity. We assessed outcomes for a historical cohort of neurocytoma patients and evaluated effects of tumor atypia, size, resection extent, and adjuvant radiotherapy. Progression-free survival (PFS) was measured by Kaplan–Meier and Cox proportional hazards methods. A total of 28 patients (15 males, 13 females) were treated between 1995 and 2014, with a median age at diagnosis of 26 years (range 5–61). Median follow-up was 62.2 months and 3 patients were lost to follow-up postoperatively. Thirteen patients experienced recurrent/progressive disease and 2-year PFS was 75 % (95 % CI 53–88 %). Two-year PFS was 48 % for MIB-1 labeling >4 % versus 90 % for ≤4 % (HR 5.4, CI 2.2-27.8, p = 0.0026). Nine patients (32 %) had gross total resections (GTR) and 19 (68 %) had subtotal resections (STR). PFS for >80 % resection was 83 versus 67 % for ≤80 % resection (HR 0.67, CI 0.23–2.0, p = 0.47). Three STR patients (16 %) received adjuvant radiation which significantly improved overall PFS (p = 0.049). Estimated 5-year PFS was 67 % for STR with radiotherapy versus 53 % for STR without radiotherapy. Salvage therapy regimens were diverse and resulted in stable disease for 54 % of patients and additional progression for 38 %. Two patients with neuropathology-confirmed atypical neurocytomas died at 4.3 and 113.4 months after initial surgery. For central neurocytomas, MIB-1 labeling index >4 % is predictive of poorer outcome and our data suggest that adjuvant radiotherapy after STR may improve PFS. Most patients requiring salvage therapy will be stabilized and multiple modalities can be effectively utilized.
Keywords: Central neurocytoma, Intraventricular neurocytoma, Benign central nervous system tumor, Progression free survival, MIB-1 labeling index
Introduction
Central neurocytomas or intraventricular neurocytomas (IVNs) are rare central nervous system tumors derived from ectodermal/nervous system tissue [1]. These tumors are typically benign, though atypical variants have been reported and residual postsurgical tumor is believed to have potential for malignant transformation [2]. The rarity of central neurocytomas, which comprise only 0.1–0.5 % of all primary brain tumors [3, 4], has resulted in a paucity of information regarding their optimal management [5]. Typically, neurocytomas carry favorable prognoses, but can exhibit more aggressive clinical behavior [5]. Seventy percent of patients present between age 20 and 40 years. Tumor incidence shows minimal gender predominance [3].
Patients frequently present with neurological symptoms such as headaches and/or visual changes, with a duration of clinical symptoms typically less than 6 months [5]. CT scans generally demonstrate a slightly hyperdense mass within the body of the lateral ventricles [3]. MRI reveals a peri-ventricular mass that can be isointense or hypointense on T1-weighed images and isointense or hyperintense on T2-weighed images [6–8]. Post gadolinium contrast appearance can be variable, though most tumors demonstrate moderate to strong enhancement [7]. Intracranial angiography is rarely performed.
Central neurocytomas with anaplastic or atypical pathologic features behave more aggressively and might be associated with greater local relapse [9, 10]. One objective marker is MIB-1; however, the specific threshold suggesting atypia is debated [9, 11–16].
Modern management of neurocytomas has been guided by several institutional case series [4, 17–19]. Surgical resection is the primary treatment to establish pathologic diagnosis, to clear CSF pathways and to address any obstructive hydrocephalus [5]. Most studies support the idea that complete resection is an important predictor of recurrence [16, 18, 20, 21]; however, this fact remains unclear since other reports have not identified significant improvements in survival or local control [10, 17].
Adjuvant radiotherapy (RT) after gross total resection (GTR), particularly for typical neurocytoma, remains controversial since most patients have long-term tumor control [22–25]. Primary radiosurgery has been used for neurocytoma, and reduction of tumor volume was reported [26], without radiosurgical complications [27]. Adjuvant radiotherapy is frequently offered to patients with incomplete resections or evidence of atypia and options include stereotactic radiosurgery (SRS) or fractionated external beam radiotherapy (EBRT) [10]. Chemotherapy options, as part of multimodal treatment, typically include carmustine, prednisone, vincristine, and cisplatin, although responses to chemotherapy have not been well-characterized [28].
Given the numerous aspects of neurocytoma clinical management that remain unclear, we reviewed our institution's experience. In our study, we summarize 20 years of treatment approach to investigate whether extent of resection, MIB-1 labeling index, adjuvant radiotherapy and initial tumor size influence clinical outcomes.
Methods and materials
Existing pathology and neurosurgery databases were searched using keywords designed to identify all patients with neurocytomas treated at the University of California at San Francisco (UCSF) between 1994 and 2014. Prospective patients were next evaluated against a number of inclusion criteria: (1) initial surgery was performed at UCSF, (2) initial treatment record was available, and (3) confirmatory pathology was available in the medical record. Given the rarity of this tumor, we included all patients irrespective of follow-up duration. The medical records were reviewed and the following data were collected: demographic information, initial and salvage treatment approach, and outcomes. This study was approved by the UCSF Institutional Review Board.
All cases included in this series underwent a central pathologic re-review by a single neuropathologist (TT). Tumor dimensions were measured on T1 post-contrast and T2-weighted brain MRI in the transverse, antero-posterior, and vertical directions, and the product of these three diameters was used as a surrogate for the tumor volume. Initial tumor size was considered as the maximum length of the three orthogonal dimensions on the patient's pre-operative planning MRI image. Degree of resection was determined by reviewing the operative notes and post-operative imaging and/or neuroradiology reports. All complete resections and near-total resections defined radiologically were classified as GTR, with all other surgical procedures, including biopsy with cyst aspiration, considered as subtotal resection (STR).
Outcomes of interest included progression free survival (PFS) and overall survival (OS) both from the date of first surgery. If progression was not documented, patients were assumed to be progression-free and censored upon the last day of recorded contact. Patients were followed clinically and with imaging studies (MRI and/or CT) performed during follow-up period at the discretion of the treating neuro-oncologist and were not done at uniform intervals for all patients. Tumor progression was defined as ≥25 % increase of the aforementioned radiologic tumor volume estimate.
Statistical analysis
Descriptive statistics were employed to characterize the patient cohort. The Kaplan–Meier method was used to estimate median progression-free survival and overall survival. We used the log-rank test and univariate Cox proportional hazards methods to assess how prognostic factors, such as extent of resection, tumor size, MIB-1 labeling index, and adjuvant radiotherapy impacted PFS and OS. Threshold for statistical significance was p ≤ 0.05.
Results
Patient demographics
A total of 28 patients with pathologically proven central neurocytomas were treated and 26 of these patients were alive at time of analysis (Table 1). Three patients were lost to follow-up after initial surgical resection but were still included in the initial descriptive analysis. Male to female ratio was 1.15:1. Median age at diagnosis was 26 years (range 5–61 years). Most patients (86 %) had tumors centered within a lateral ventricle, 3 (11 %) had midline ventricular lesions and 1 had a parietal mass. None of the patients had MRI evidence of spinal drop metastases and 1 patient had evidence of CSF disease spread at initial diagnosis based on a lumbar puncture with positive cytology. Histopathologically, all tumors were classified as WHO Grade II. Seven of the 28 tumors (25 %) were noted as atypical by our neuropathologist. As assessed by pre-operative MRI, median maximal tumor diameter was 4.1 cm (range 0.8–8.6 cm). Figure 1 shows representative MRI findings from our series.
Table 1. Demographic and treatment characteristics.
| Entire cohort | Stable after initial treatment | Recurrent or progressive tumor | |
|---|---|---|---|
| All patients | 28 | 15 (54 %) | 13 (46 %) |
| Age at Dx (n = 28) | |||
| Mean (range) | 30 (5–61) | 31 (20–53) | 28 (5–61) |
| Gender (n = 28) | |||
| Male | 15 (54 %) | 10 | 5 |
| Female | 13 (46 %) | 5 | 8 |
| Size by MRI imaging (largest dimension in cm) (n = 27) | |||
| Mean (range) | 4.4 (0.8–8.6) | 4.1 (0.8–7.0) | 4.8 (2.5–8.6) |
| Median | 4.1 | 4.1 | 4.4 |
| Resection extent ± adjuvant radiotherapy (n = 28) | |||
| GTR (all) | 9 | 5 | 4 |
| GTR + adjuvant RT | 1 | 1 | 0 |
| GTR – adjuvant RT | 8 | 4 | 4 |
| STR (all) | 19 | 10 | 9 |
| STR + adjuvant RT | 3 | 3 | 0 |
| STR – adjuvant RT | 16 | 7 | 9 |
| Tumor MIB-1 index (percentage) (n = 24) | |||
| Mean (range) | 3.5 (0.5–7.7) | 2.7 (1.0–7.0) | 4.5 (0.5–7.7) |
| Median | 3.2 | 2.0 | 4.8 |
| Required VP shunting | 11 (38 %) | 5 | 6 |
Fig. 1.
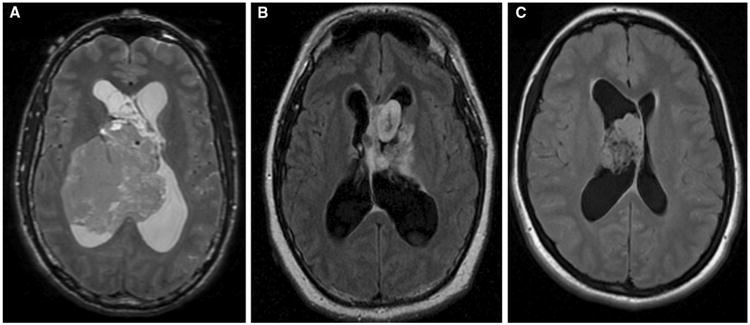
Representative neurocytoma MRI imaging findings. a T2 weighted imaging of a 44-year-old male found to have an 8.6 × 7.2 cm heterogeneous right lateral ventricle mass; predominantly isointense on T2 weighted imaging with no significant enhancement on post-contrast series. b T2 FLAIR imaging of a 23-year-old male found to have a heterogeneous lobulated mass with a mildly enhancing component and other non-enhancing components extending superiorly from the suprasellar cistern. c T2 FLAIR imaging of a 20-year-old female found to have a 3.4 × 2.5 cm highly cellular lobulated nonenhancing intraventricular mass attached to the anterior portion of the septum pellucidum
All patients underwent at least one craniotomy for definitive diagnosis and surgical resection. Initial surgical approach was available for 24 patients, with the most common approaches being transcortical transventricular (39 %) and interhemispheric transcallosal (29 %). Three patients (11 %) underwent staged resection with different approaches in separate surgeries. Median extent of initial resection was 85 % (range 30–100 %). Thirty-two percent of patients (9/28) had GTR, and 68 % (19/28) had STR. Nearly 40 % (11/28) of patients had persistent hydrocephalus that required placement of a ventriculoperitoneal shunt, placed an average of 31 days (range 5–96 days) after initial surgery. Two of these patients required at least one shunt revision.
One patient who underwent a GTR received adjuvant external beam radiotherapy (EBRT). Three STR patients (14 %) received adjuvant radiotherapy after initial resection, of which two underwent EBRT and the remaining patient had stereotactic radiosurgery. The remaining 25 patients did not receive adjuvant therapy.
Outcomes
Median duration of follow-up for the cohort was 62.2 months (range 0.03–237.7 months). Over the time period of our analysis, thirteen patients (46 %) developed recurrent or progressive disease whereas the remaining members of the cohort did not have evidence of progressive disease at last recorded interaction. We report two total cohort deaths (7 %).
Overall actuarial 2-year PFS was 75 % and estimated 5-year PFS was 54 % (Table 2). Neither age nor gender significantly impacted PFS. In terms of tumor size, actuarial 2-year PFS was 82 % for tumors >4.1 cm and 75 % for tumors ≤4.1 cm (HR 1.2, CI 0.37–3.6, p = 0.80) (Fig. 2a). In terms of extent of resection, actuarial 2-year PFS for patients with >80 % resection was 83 versus 67 % for ≤80 % resection (HR 0.67, CI 0.2–2.0, p = 0.47) (Fig. 2b). Patients who had a staged resection compared to a single operation did not have significantly different outcomes.
Table 2. Progression free survival rates by clinical parameters.
| n | Actuarial 2-year PFS (95 % CI) | Estimated 5-year PFS (95 % CI) | Hazard ratio (95 % CI) | p value (log-rank) | ||
|---|---|---|---|---|---|---|
| Entire cohort | 28 | 75 % (53–88 %) | 54 % (31–72 %) | |||
| MIB labeling index | MIB-1 ≤ 4 % | 13 | 90 % (47–99 %) | 90 % (47–99 %) | 0.18 (0.04–0.44) | 0.0026 |
| MIB-1> 4 % | 11 | 48 % (16–74 %) | 12 % (1–41 %) | |||
| Percent resection | >80 % | 14 | 83 % (46–95 %) | 48 % (16–75 %) | 0.67 (0.23–2.0) | 0.47 |
| ≤80 % | 14 | 67 % (34–86 %) | 57 % (25–80 %) | |||
| Tumor size | ≤4.1 | 14 | 75 % (41–91 %) | 54 % (21–78 %) | 0.87 (0.27–2.7) | 0.80 |
| >4.1 | 13 | 82 % (45–95 %) | 60 % (24–83 %) | |||
| Overall resection extent | GTR | 9 | 75 % (31–93 %) | 40 % (7–73 %) | 1.4 (0.41–5.2) | 0.57 |
| STR | 19 | 76 % (47–90 %) | 61 % (32–80 %) | |||
| Resection extent ± adjuvant radiotherapy | STR + RT | 3 | 100 % (NA) | 67 % (6–95 %) | NA | 0.049 |
| STR – RT | 16 | 70 % (38–88 %) | 53 % (23–76 %) |
Fig. 2.
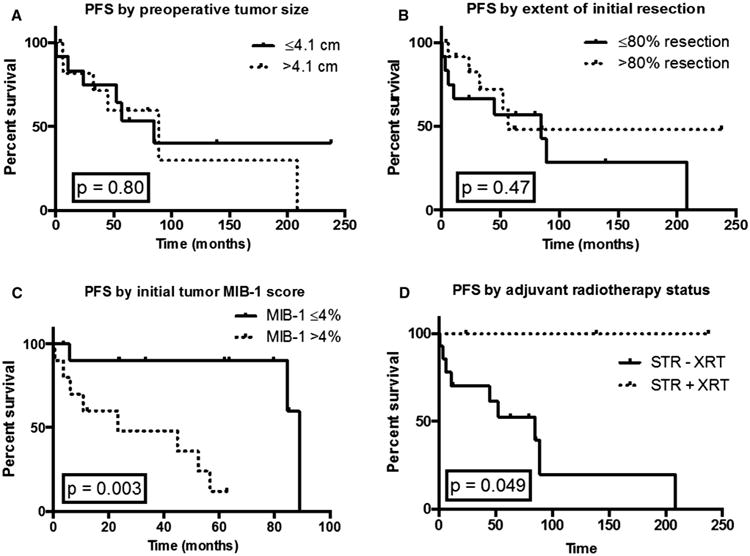
PFS based on tumor and clinical characteristics. a Two-year PFS was 82 % for tumors >4.1 cm versus 75 % for tumors ≤4.1 cm, with a hazard ratio of 1.2 (CI 0.37–3.6, p = 0.80). b Two-year PFS was 83 % for >80 % resection and 67 % for ≤80 % resection, with a hazard ratio of 0.67 (CI 0.23–2.0, p = 0.47). c Two-year PFS was 90 % for MIB-1 labeling ≤4 and 48 % for MIB-1 labeling >4 %, with a hazard ratio of 0.18, (CI 0.036–0.44, p = 0.0026). d Two-year PFS was 100 % for patients with STR plus adjuvant radiation versus 70 % for patients who underwent STR but did not undergo post-op radiation, with an undefined hazard ratio since we did not achieve events in greater than 50 % of relevant patients. However, the addition of adjuvant radiation significantly improved overall PFS (p = 0.049)
Regarding tumor atypia, 2-year PFS was 90 % for MIB labeling ≤4 versus 48 % for MIB labeling >4 % (HR 5.4, CI 2.2–27.8, p = 0.0026) (Fig. 2c). Mean initial MIB-1 index for patients with progressive/recurrent disease was 4.5 % compared to 2.7 % for patients who did not progress/recur (p = 0.061, unpaired t test). Finally, we assessed the impact of adjuvant radiotherapy and found that the overall PFS improvement for subtotally resected patients who received adjuvant radiation was significant with p = 0.049 (Fig. 2d).
Five-year overall survival (OS) for all patients was 96 %, and estimated 10-year OS was 82 %. For the two cohort deaths, one occurred 4.3 months after initial surgery and was attributed to complications of a peri-operative intracranial hemorrhage in the context of a salvage operation for progressive disease. The second death occurred 113.4 months after initial surgery and was attributed to progressive neurocytoma. These patients both had evidence of tumor atypia, with initial MIB-1 indices of 6.8 and 7.7 %, respectively.
Post-progression management
Several management strategies were utilized for the 13 patients with progressive or recurrent neurocytoma. Four patients (29 %) received salvage chemotherapy. One patient who was treated with 6 rounds of CCNU had robust radiographic response with subsequent stable disease at 3.2 years post salvage. Two patients were treated with temozolomide, one of whom had roughly three years of tumor stabilization before developing subtle progression. The other continued to have slow progressive tumor growth while on chemotherapy. At last follow-up, both patients were alive with disease.
Four patients (29 %) had repeat surgery and an additional patient had repeat surgery plus temozolomide. Two of these patients had perioperative intracranial hemorrhages and one patient died from this complication. The remaining four patients who received salvage surgery had stable disease at date of last encounter with median duration of 27 months after salvage surgery (range 4–63 months).
Two patients (21 %) had salvage radiosurgery, both of whom had radiographic evidence of treatment response. One patient received single fraction SRS to two small areas of recurrence each less than 1 cc of volume and received 16 Gy to each lesion. The second patient received a total of 30 Gy for a larger ventricular recurrence, delivered by fractionated frameless radiosurgery in five, 6 Gy fractions. Both patients had evidence of treatment effect with no new disease at date of last follow-up, 17 and 34 months after receiving salvage radiotherapy, respectively.
Discussion
In our historical cohort of benign central neurocytoma, we report a five-year PFS of 54 % with OS of 96 %. The tumor's rarity makes it challenging to define standards of care; however, most institutions, including our own, recommend resection as first line therapy [29]. Most patients will have favorable outcomes; however, clear risk factors for poorer prognosis are emerging. Specifically, a pooled analysis of over 400 patients has demonstrated that GTR is superior to STR and that atypical lesions have poorer local control and OS compared to typical variants [29]. It is now well established that a higher MIB-1 labeling index is associated with more aggressive behavior [9, 11]. In our series, all but four tumors were stained for MIB-1 (range = 0.5–7.7 %) with a higher mean index in patients who recurred. This difference trended towards significance (p = 0.061), likely the consequence of small sample size. Proposed treatment algorithms have started to incorporate MIB-1 as a consideration as to whether or not a patient should receive adjuvant therapy following STR [13, 30]. However, the pathological classification of “atypical neurocytoma” broadly, and specifically which MIB-1 levels portend poorer outcomes remain uncertain. Some of the first case series to recognize the association between MIB-1 labeling index and risk of recurrence proposed >2 % as a marker of atypical behavior [11, 13]. In our series, MIB-1 index of >2 % is suggestive of poorer outcome but it did not reach traditional levels of significance. Other studies have also found no correlation between MIB-1 and recurrence risk, suggesting that perhaps 2 % is too conservative [31]. A meta analysis performed about a decade ago proposed a higher threshold, suggesting that >3 % is associated with higher rates of local recurrence and poorer OS [15]. In our study, tumors with initial MIB-1 indices above 4 % were found to have significantly higher risk of progression. This finding aligns with other series that propose higher thresholds for atypical behavior [16]. Regardless of the threshold, consensus exists that adjuvant therapy should be strongly considered for tumors with higher MIB-1 indices.
Most cohort patients were not able to undergo GTR underscoring the technical challenge of removing intraventricular lesions. Very extensive ventricular tumors, or those that extend into the third ventricle, may require staged approaches via anterior and posterior routes. Interestingly, in our series, greater extent of initial resection and smaller initial tumor size did not appear to correlate with improved local control. While most prior case series [18, 29, 32] assert that incomplete resection is associated with increased recurrence, this finding is inconsistent. A recent retrospective study of 45 central neurocytoma patients reported no significant difference in local control or survival between GTR and STR, but found local control and survival advantages in patients with lower mitotic indices [17]. This study was confounded by the fact that one-third of the population received adjuvant radiation. In a recent systematic review, resection extent was not predictive of improved local control [10]. Our cohort may be too small to assess true outcome differences between STR and GTR.
Three patients underwent adjuvant radiotherapy after STR ranging from 70 to 95 %; however, MIB-1 did not guide that decision. In fact, two tumors did not have staining available since they were resected prior to the early published correlations between MIB-1 and recurrence risk [11]. Acknowledging the inherent limitation of low patient numbers, our data suggest there may be improved local control for the small subcohort that underwent adjuvant radiotherapy compared to those that did not. Numerous reports are emerging which suggest that adjuvant radiotherapy can significantly improve local control, and some also note OS improvements [33].
The appropriate treatment of recurrent disease remains ambiguous, with options including re-operation, EBRT, SRS and/or chemotherapy [34]. Even within our series, the diversity of salvage treatment modalities underscores the heterogeneity of management. The use of image guided craniotomy has allowed for directed approaches for small tumors typically high in the ventricle along the wall opposite the original surgical approach. Anecdotally, we report two patients whose recurrent disease regressed following SRS. Genc et al. have published the largest series examining utilization of SRS for residual or recurrent neurocytomas and reported after a mean of almost 37 months of follow-up, durable reduction in tumor size for 15/22 patients, stable disease in 6/22 and one patient with progressive disease [14]. They reported a trend towards better response in tumors with lower MIB-1; however, these results were not significant and were attributed to short follow-up time. A recent systematic review concluded that both fractionated EBRT and SRS represent reasonable options for recurrent or residual tumors, and found a statistically non-significant trend towards better local tumor control and survival rates with fewer complications amongst residual neurocytomas treated with SRS [10]. The authors cautioned that the available evidence is mostly low-level, and suggest that the risks and benefits of both approaches continue to be weighed patient-by-patient. We agree that more prospective data would be helpful.
Based on 20 years of experience, we offer several treatment recommendations. We agree that maximal safe resection should remain first line therapy. Postoperatively, MIB-1 label and evidence of tumor atypia should influence which patients are considered for adjuvant therapy. Our findings support the use of a MIB-1 label of 4 % as a guideline for atypical tumors and we recommend multi-disciplinary discussions for patients with MIB-1 staining in this range. Based on our small cohort we also found that the addition of adjuvant RT may reduce the odds of disease progression for incompletely resected patients. Finally, SRS may be an effective salvage modality, particularly for patients with small recurrences.
Acknowledgments
This research was supported in part by the NIH Brain Tumor SPORE grant P50 CA097257 (DHK), Nancy and Stephen Grand Philanthropic Fund (DHK), and The V Foundation (DHK) as well as by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR000144 (BSI).
Footnotes
Compliance with ethical standards: Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, Toga M. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol (Berl) 1982;56:151–156. doi: 10.1007/BF00690587. [DOI] [PubMed] [Google Scholar]
- 2.Mozes P, Szanto E, Tiszlavicz L, Barzo P, Cserhati A, Fodor E, Hideghety K. Clinical Course of Central Neurocytoma with Malignant Transformation-An Indication for Craniospinal Irradiation. Pathol Oncol Res POR. 2013 doi: 10.1007/s12253-013-9697-y. [DOI] [PubMed] [Google Scholar]
- 3.Hassoun J, Söylemezoglu F, Gambarelli D, Figarella-Branger D, von Ammon K, Kleihues P. Central neurocytoma: a synopsis of clinical and histological features. Brain Pathol Zurich Switz. 1993;3:297–306. doi: 10.1111/j.1750-3639.1993.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharma MC, Deb P, Sharma S, Sarkar C. Neurocytoma: a comprehensive review. Neurosurg Rev. 2006;29:270–285. doi: 10.1007/s10143-006-0030-z. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt MH, Gottfried ON, von Koch CS, Chang SM, McDermott MW. Central neurocytoma: a review. J Neurooncol. 2004;66:377–384. doi: 10.1023/b:neon.0000014541.87329.3b. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Wen L, Henning TD, Feng XY, Zhang YL, Zou LG, Zhang ZG. Central neurocytoma: clinical, pathological and neuroradiological findings. Clin Radiol. 2006;61:348–357. doi: 10.1016/j.crad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I. From the Radiologic Pathology Archives: Intraventricular Neoplasms: Radiologic-Pathologic Correlation. RadioGraphics. 2013;33:21–43. doi: 10.1148/rg.331125192. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Jia D, Shen J, Zhang J, Li G. Clinical and imaging features of central neurocytomas. J Clin Neurosci Off J Neurosurg Soc Australas. 2013;20:679–685. doi: 10.1016/j.jocn.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Christov C, Adle-Biassette H, Le Guerinel C. Recurrent central neurocytoma with marked increase in MIB-1 labelling index. Br J Neurosurg. 1999;13:496–499. [PubMed] [Google Scholar]
- 10.Garcia RM, Ivan ME, Oh T, Barani I, Parsa AT. Intraventricular neurocytomas: a systematic review of stereotactic radiosurgery and fractionated conventional radiotherapy for residual or recurrent tumors. Clin Neurol Neurosurg. 2014;117:55–64. doi: 10.1016/j.clineuro.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Söylemezoglu F, Scheithauer BW, Esteve J, Kleihues P. Atypical central neurocytoma. J Neuropathol Exp Neurol. 1997;56:551–556. doi: 10.1097/00005072-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Sharma MC, Rathore A, Karak AK, Sarkar C. A study of proliferative markers in central neurocytoma. Pathology (Phila) 1998;30:355–359. doi: 10.1080/00313029800169626. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie IR. Central neurocytoma: histologic atypia, proliferation potential, and clinical outcome. Cancer. 1999;85:1606–1610. doi: 10.1002/(sici)1097-0142(19990401)85:7<1606::aid-cncr24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Genc A, Bozkurt SU, Karabagli P, Seker A, Bayri Y, Konya D, Kilic T. Gamma knife radiosurgery for cranial neurocytomas. J Neurooncol. 2011;105:647–657. doi: 10.1007/s11060-011-0635-0. [DOI] [PubMed] [Google Scholar]
- 15.Rades D, Schild SE, Fehlauer F. Prognostic value of the MIB-1 labeling index for central neurocytomas. Neurology. 2004;62:987–989. doi: 10.1212/01.wnl.0000115392.21898.e3. [DOI] [PubMed] [Google Scholar]
- 16.Kaur G, Kane AJ, Sughrue ME, Oh M, Safaee M, Sun M, Tihan T, McDermott MW, Berger MS, Parsa AT. MIB-1 labeling index predicts recurrence in intraventricular central neurocytomas. J Clin Neurosci Off J Neurosurg Soc Australas. 2013;20:89–93. doi: 10.1016/j.jocn.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leenstra JL, Rodriguez FJ, Frechette CM, et al. Central neurocytoma: management recommendations based on a 35-year experience. Int J Radiat Oncol Biol Phys. 2007;67:1145–1154. doi: 10.1016/j.ijrobp.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Hallock A, Hamilton B, Ang LC, Tay KY, Meygesi JF, Fisher BJ, Watling CJ, Macdonald DR, Bauman GS. Neurocytomas: long-term experience of a single institution. Neuro-Oncol. 2011;13:943–949. doi: 10.1093/neuonc/nor074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Zhou R, Liu J, Tang J. Central neurocytoma. J Clin Neurosci Off J Neurosurg Soc Australas. 2012;19:849–853. doi: 10.1016/j.jocn.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Qian H, Lin S, Zhang M, Cao Y. Surgical management of intraventricular central neurocytoma: 92 cases. Acta Neurochir (Wien) 2012;154:1951–1960. doi: 10.1007/s00701-012-1446-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim JW, Kim DG, Kim IK, et al. Central neurocytoma: long-term outcomes of multimodal treatments and management strategies based on 30 years' experience in a single institute. Neurosurgery. 2013;72:407–413. doi: 10.1227/NEU.0b013e3182804662. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa K, Aoki Y, Sakata K, Sasaki Y, Matsutani M, Akanuma A. Radiation therapy of well-differentiated neuroblastoma and central neurocytoma. Cancer. 1993;72:1350–1355. doi: 10.1002/1097-0142(19930815)72:4<1350::aid-cncr2820720433>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Schild SE, Scheithauer BW, Haddock MG, Schiff D, Burger PC, Wong WW, Lyons MK. Central neurocytomas. Cancer. 1997;79:790–795. doi: 10.1002/(sici)1097-0142(19970215)79:4<790::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Kim DG, Paek SH, Kim IH, Chi JG, Jung HW, Han DH, Choi KS, Cho BK. Central neurocytoma: the role of radiation therapy and long term outcome. Cancer. 1997;79:1995–2002. doi: 10.1002/(sici)1097-0142(19970515)79:10<1995::aid-cncr22>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni V, Rajshekhar V, Haran RP, Chandi SM. Longterm outcome in patients with central neurocytoma following stereotactic biopsy and radiation therapy. Br J Neurosurg. 2002;16:126–132. doi: 10.1080/02688690220131714. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RC, Elder JB, Parsa AT, Issacson SR, Sisti MB. Radiosurgery for the treatment of recurrent central neurocytomas. Neurosurgery. 2001;48:1231–1237. doi: 10.1097/00006123-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hara M, Aoyagi M, Yamamoto M, Maehara T, Takada Y, Nojiri T, Ohno K. Rapid shrinkage of remnant central neurocytoma after gamma knife radiosurgery: a case report. J Neurooncol. 2003;62:269–273. doi: 10.1023/a:1023310829796. [DOI] [PubMed] [Google Scholar]
- 28.Brandes AA, Amistá P, Gardiman M, Volpin L, Danieli D, Guglielmi B, Carollo C, Pinna G, Turazzi S, Monfardini S. Chemotherapy in patients with recurrent and progressive central neurocytoma. Cancer. 2000;88:169–174. doi: 10.1002/(sici)1097-0142(20000101)88:1<169::aid-cncr23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Rades D, Schild SE. Treatment recommendations for the various subgroups of neurocytomas. J Neurooncol. 2006;77:305–309. doi: 10.1007/s11060-005-9047-3. [DOI] [PubMed] [Google Scholar]
- 30.Rades D, Fehlauer F, Schild SE. Treatment of atypical neurocytomas. Cancer. 2004;100:814–817. doi: 10.1002/cncr.20032. [DOI] [PubMed] [Google Scholar]
- 31.Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Knosp E. Recurrent central neurocytomas. Cancer. 2005;104:135–142. doi: 10.1002/cncr.21109. [DOI] [PubMed] [Google Scholar]
- 32.Vasiljevic A, François P, Loundou A, Févre-Montange M, Jouvet A, Roche PH, Figarella-Branger D. Prognostic factors in central neurocytomas: a multicenter study of 71 cases. Am J Surg Pathol. 2012;36:220–227. doi: 10.1097/PAS.0b013e31823b8232. [DOI] [PubMed] [Google Scholar]
- 33.Chen YD, Li WB, Feng J, Qiu XG. Long-term outcomes of adjuvant radiotherapy after surgical resection of central neurocytoma. Radiat Oncol Lond Engl. 2014;9:242. doi: 10.1186/s13014-014-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudhari KA, Kaliaperumal C, Jain A, Sarkar C, Soo MYS, Rades D, Singh J. Central neurocytoma: a multi-disciplinary review. Br J Neurosurg. 2009;23:585–595. doi: 10.3109/02688690903254350. [DOI] [PubMed] [Google Scholar]


