Abstract
Aims
Haptoglobin (Hp) genotype 2-2 increases cardiovascular diabetes complications. In type 2 diabetes, α-tocopherol was shown to lower cardiovascular risk in Hp 2-2, potentially through HDL function improvements. Similar type 1 diabetes data are lacking. We conducted a randomized, cross-over pilot of α-tocopherol supplementation on HDL function (i.e. cholesterol efflux (CE) and HDL-associated lipid peroxides (LP)) and lipoprotein subfractions in type 1 diabetes.
Methods
Hp genotype was assessed in members of two Allegheny County, PA type 1 diabetes registries and the CACTI cohort; 30 were randomly selected within Hp genotype, and 28 Hp 1-1, 31 Hp 2-1 and 30 Hp 2-2 were allocated to daily α-tocopherol or placebo for 8 weeks with a 4-week wash-out.
Results
Baseline CE decreased with the number of Hp 2 alleles (p-trend=0.003). There were no differences in LP or lipoprotein subfractions. In intention-to-treat analysis stratified by Hp, α-tocopherol increased CE in Hp 2-2 (beta=0.79, p=0.03) and LP in Hp 1 allele carriers (betaHp 1-1=0.18, p=0.05 and betaHp 2-1 =0.21, p=0.07); reduced HDL particle size (beta=−0.07, p=0.03) in Hp 1-1 carriers; increased LDL particle concentration in Hp 1-1 and decreased it in Hp 2-2 carriers. However, no significant interactions were observed by Hp.
Conclusions
In this type 1 diabetes study, HDL function worsened with the number of Hp 2 alleles. α-tocopherol improved HDL function in Hp 2-2 carriers and appeared to adversely affect lipid peroxides and lipoprotein subfractions among Hp 1 allele carriers. As no significant interactions were observed, findings require replication in larger studies.
Keywords: Type 1 Diabetes, Haptoglobin genotype, HDL function, NMR lipoprotein subfractions, vitamin E, double-blinded placebo-controlled randomized trial
Introduction
Reactive oxygen species have been implicated in the etiology and progression of diabetes complications [1–3] leading to the hypothesis that antioxidants could protect against vascular disease. As clinical trials have generally failed to demonstrate reduced cardiovascular risk with α-tocopherol supplementation [4], it has been recently proposed that the success of antioxidant therapy may be limited to highly susceptible subgroups, such as individuals with diabetes and the Haptoglobin (Hp) 2-2 genotype [5].
One of the major physiological roles of Hp is to bind to free hemoglobin, inhibiting oxidative tissue damage that would ensue following release of heme-iron in circulation [6]. In humans, the Hp insertion polymorphism is defined by the absence (Hp 1 allele) or presence (Hp 2 allele) of a 1.7kb in-frame duplication of exons 3 and 4 of the Hp gene. The Hp 1 and Hp 2 allelic protein products differ in their ability to protect against hemoglobin-driven oxidative stress, with Hp 2 being an inferior antioxidant [7]. Moreover, the Hp protein is HDL-associated, with increased Hp binding in Hp 2-2 genotype carriers [8]. Once bound to HDL, Hp has been proposed to tether hemoglobin to HDL, resulting in its oxidative modification and loss of function.
Prospective type 2 diabetes investigations have reported increased cardiovascular risk with Hp 2-2 [10–13] and improvements in HDL function with α-tocopherol in Hp 2-2 (but not Hp 1-1) carriers [8–9], suggesting that vitamin E may modify the Hp 2-2 associated cardiovascular risk. Retrospective analyses of the HOPE trial demonstrated that α-tocopherol supplementation in type 2 diabetes with Hp 2-2 reduces myocardial infarction and cardiovascular mortality [14]. Similarly, in the prospective ICARE trial, α-tocopherol significantly reduced cardiovascular events by 53% [15]. In type 1 diabetes, we demonstrated a twofold increased CAD incidence among Hp 2-2 versus Hp 1-1 carriers [16]. Coronary artery calcification incidence was also reported to increase with the number of Hp 2 alleles in type 1 diabetes, but not in controls [17], providing further support for a role of Hp in CAD in this population.
However, the Hp genotype - HDL function association in type 1 diabetes and the ability of vitamin E to modulate this relationship have not been investigated. We therefore conducted a pilot/feasibility study for a future antioxidant trial. The hypotheses tested were that: a) HDL function, as measured by HDL-mediated cholesterol efflux, would worsen and HDL-associated lipid peroxides would increase with the number of Hp 2 alleles; and b) α-tocopherol supplementation would improve HDL function and decrease lipid peroxides in, the most susceptible, Hp 2-2 genotype group. A secondary hypothesis tested was that large HDL and LDL particle concentrations would be lower among Hp 2-2 carriers and that vitamin E would favorably affect their distribution.
Methods
We conducted a randomized, double-blinded, placebo-controlled crossover study to evaluate the effects of α-tocopherol supplementation on HDL function in type 1 diabetes, stratifying by Hp genotype (ClinicalTrials.gov Identifier: NCT01098994). Participants for this endeavor were recruited from two type 1 diabetes registries in Allegheny County, Pennsylvania: the Pittsburgh Allegheny County Insulin Dependent Diabetes Mellitus Registry (ACR) and the Children’s Hospital of Pittsburgh/Epidemiology of Diabetes Complications study (EDC). ACR, a detailed description of which has been previously published [18], was designed to ascertain all new type 1 diabetes cases (<20 years) within 1/1/1965-12/31/1979. The EDC is an ongoing, retrospectively-defined, incident cohort of childhood-onset type 1 diabetes [19]. Given the low prevalence of Hp 1-1 and to assure timely sample analysis for all Hp groups, the CACTI study was also used to recruit individuals with type 1 diabetes and the Hp 1-1 genotype [20]. The Universities of Pittsburgh and Colorado IRB approved the study protocol and all participants provided a written informed consent.
Recruitment took place within 2010–2012. Other than being local residents, eligibility criteria comprised either an age ≥30 years and diabetes duration ≥10 years or a cardiovascular disease history, matching eligibility criteria for a future trial of α-tocopherol supplementation on CAD by Hp genotype in type 1 diabetes. Exclusion criteria included acute coronary syndrome in the prior six months, pancreatic transplant, unwillingness to limit antioxidant vitamin use to trial medications, or a known allergy to vitamin E. Willing and eligible participants were scheduled for a first clinical visit, where study aims were explained in detail, eligibility was re-assessed, and written informed consent and a blood sample for Hp genotype assessment were obtained, if needed (the majority of EDC participants had already had Hp genotype determined). Samples collected were stored in −70 C freezers and batched until Hp genotype was assessed by an ELISA test [21]. All follow-up procedures concluded by December 2013.
Subsequent to Hp assessment and following a four-week washout period for those reporting prior antioxidant supplementation to eliminate carry-over effects, block randomization (i.e. within Hp genotype) was performed to assign treatment order until approximately 30 within Hp type were enrolled, allowing for a 13% dropout rate. Thus, participants within each Hp genotype were randomly allocated to eight weeks of daily α-tocopherol or placebo followed by a second wash-out period before being assigned to the second intervention. α-Tocopherol was supplied as capsules containing 400 IU d-α-tocopherol acetate, 79 mg rice flour, and 10 mg magnesium stearate (Nutricap Labs, Farmingdale, NY). The placebo was identically supplied and formulated except that it contained no d-α-tocopherol acetate. Protocol adherence was evaluated by clinic attendance, pill count and by comparing plasma α-tocopherol after vitamin E or placebo.
Total lipid peroxides (nanomoles) associated with HDL were assessed (blinded to Hp and treatment sequence) in 1 μg immunopurified HDL as previously reported [22]. Participant serum was also assessed (similarly blinded) for its ability to promote the efflux of 3H-cholesterol from macrophages as previously described [23–24]. Plasma α-tocopherol was measured under subdued lighting [25–26]. Frozen EDTA plasma samples were shipped on dry ice to Liposcience, Inc. (Raleigh, NC) for NMR lipoprotein subfraction analysis. Particle concentrations of lipoproteins of different sizes were calculated from the measured amplitudes of their spectroscopically distinct lipid-methyl group [27]. The sum of the diameter of each subclass multiplied by its relative mass percentage based on the amplitude of its methyl NMR signal was used to derive the weighted-average lipoprotein particle size [27]. NMR lipoprotein variables evaluated were those relating to HDL and LDL cholesterol.
Power
Twenty-five active participants in each Hp group were required to achieve 94% power for a non-zero contrast of the means versus the alternative that the contrast is zero, assuming cholesterol efflux in Hp 1-1, 2-1 and 2-2 of 13%, 12%, and 11% respectively, using an F-test with α=0.05, a linear trend across means and a within-group SD=2%. Additionally, for the cross-over trial, 25 active participants within Hp genotype were required to achieve 92% power to detect a two unit difference between vitamin E and placebo, assuming a square root of the within mean square error of 2.00 and α=0.05 (NCSS, PASS, and GEISS 2006. NCSS Kaysville, UT).
Prior to trial initiation, identification numbers were randomly assigned (by R.G.M.), using simple random sampling without replacement, to each vitamin and placebo bottle to mask the contents. Each vitamin bottle was paired to a bottle from the placebo set and treatment order was randomly determined for each pair. Upon study entry, participants were assigned to a bottle pair using probability-proportional-to-size sampling weighted for gender. Participants and investigators were masked to the treatment assignment for the duration of the study.
Statistical analysis
Participant characteristics were compared across Hp genotypes using descriptive statistics. The presence of carryover effects (i.e. the significance of the coefficient for treatment sequence) was evaluated in mixed models, with the outcome being plasma α-tocopherol, and with random effects for participant ID nested within treatment sequence. Intent-to-treat analysis was subsequently conducted stratified by Hp genotype and involving participants randomly assigned to a treatment group. Separate mixed models were constructed for each Hp genotype, taking into account the time period (intervention A/wash-out/intervention B) and using random effects for participant ID nested within treatment sequence. Though the study by design was not powered to detect modification of the treatment effect by Hp genotype (we only had approximately 58% and 12% power to detect a significant interaction for cholesterol efflux and lipid peroxides associated with HDL, respectively), we tested the presence of effect modification by including an interaction term in a separate mixed model and plotted the treatment effect estimates.
Results
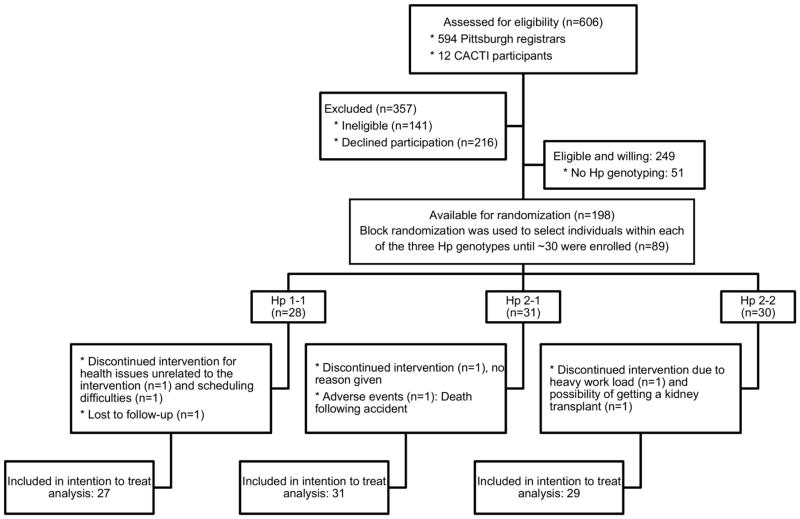
Of 594 Pittsburgh registrars, 141 were ineligible and 216 declined participation. Of the 237 eligible/willing participants, Hp was unavailable for 51 (due to inability to reestablish contact for, or attend, a clinic visit within a reasonable timeframe). The distribution of the Hp genotype among the remaining 186 individuals was 14.5% Hp 1-1, 46.8% Hp 2-1 and 38.7% Hp 2-2 and was in Hardy-Weinberg equilibrium (p=0.93). Additionally, 12 Hp 1-1 carriers were recruited from the CACTI study. Block randomization was subsequently used and 128 were successfully enrolled: 28 with Hp 1-1, 31 with Hp 2-1 and 30 with Hp 2-2 (Figure 1).
Figure 1.
Flow diagram of the HapE cross-over trial
Five participants discontinued the intervention: two with Hp 1-1 (for health reasons unrelated to the intervention and scheduling difficulties); one with Hp 2-1 (no reason provided); and two with Hp 2-2 (due to heavy work load and the possibility of a kidney transplant). In addition, one individual (Hp 1-1) was lost to follow-up after having partially completed the study protocol and another participant (Hp 2-1) died following an accident unrelated to the intervention. Two of these individuals who did not provide any follow-up data (the one who discontinued participation for health issues unrelated to the intervention and the one withdrawn because of pending transplant), and were not included in the analysis, leaving 87 randomly assigned to a treatment group and included in the intention-to-treat analysis (27 Hp 1-1; 31 Hp 2-1 and 29 Hp 2-2, Figure 1). No side-effects were reported with the exception of two participants reporting heartburn, one while on vitamin E and the other while on placebo.
No baseline differences in demographic characteristics were observed by Hp (Table 1). However, there was a significant trend toward lower plasma α-tocopherol and HDL-mediated cholesterol efflux with the number of Hp 2 alleles, while HDL-associated lipid peroxides did not differ by Hp. Generally, lipoproteins measured by NMR also did not differ by Hp, with the exception of an increase in large LDL particle concentration with the number of Hp 2 alleles. As previous research studies in type 1 diabetes have not identified differences in HbA1c by Hp genotype, and given that HbA1c was not anticipated to change in such a short time frame, glycemic control was not assessed in this study.
Table 1.
Participant characteristics at study entry by Hp genotype
| Hp 1-1 (n=27) | Hp 2-1 (n=31) | Hp 2-2 (n=29) | Overall p-value | |
|---|---|---|---|---|
| Age (years) | 49.1 (8.2) | 49.3 (8.7) | 50.7 (6.2) | 0.71 |
| Females (%, n) | 51.8 (14) | 61.3 (19) | 51.7 (15) | 0.69 |
| Age at onset (years) | 12.4 (8.4) | 9.3 (4.9) | 10.5 (4.9) | 0.17 |
| Diabetes duration (years) | 36.7 (8.5) | 40.1 (7.1) | 40.2 (4.3) | 0.11 |
| α-tocopherol (mg/L) | 10.3 (2.1) | 9.5 (2.3) | 8.9 (2.1) | 0.07 (p-trend=0.02) |
| HbA1c (%)* | 7.6 (7.0, 8.7) | 7.9 (6.7, 9.4) | 7.2 (6.1, 10.0) | 0.69 |
| HDL function measures | ||||
| Cholesterol efflux (%) | 11.9 (3.7) | 11.2 (3.8) | 9.0 (3.6) | 0.009 (p-trend=0.003) |
| Lipid peroxides (nmol) | 1.1 (0.73, 2.4) | 1.3 (0.73, 2.9) | 1.1 (0.89, 2.4) | 0.65 (p-trend=0.22) |
| NMR lipoprotein measures | ||||
| HDL cholesterol (mg/dL) | 56.5 (49.0, 76.0) | 57.0 (51.0, 73.0) | 54.0 (49.0, 59.0) | 0.19 |
| HDL particles (μmol/L) | 35.5 (31.5, 38.9) | 36.8 (30.8, 41.2) | 34.2 (30.0, 37.3) | 0.23 |
| Large HDL particles (μmol/L) | 7.7 (3.9, 11.2) | 7.9 (5.5, 11.6) | 7.1 (5.3, 8.2) | 0.47 |
| Medium HDL particles (μmol/L) | 11.9 (9.5, 15.4) | 13.0 (9.6, 18.5) | 10.8 (8.6, 13.7) | 0.15 |
| Small HDL particles (μmol/L) | 16.1 (9.3, 18.5) | 13.5 (8.2, 18.5) | 13.6 (10.7, 18.1) | 0.83 |
| HDL size (nm) | 9.6 (9.1, 10.0) | 9.7 (9.2, 10.0) | 9.5 (9.2, 9.7) | 0.52 |
| LDL particles (nmol/L) | 754.5 (657.0, 939.0) | 801.0 (659.0, 1052.0) | 873.0 (816.0, 993.0) | 0.28 |
| IDL particles (nmol/L) | 62.0 (28.0, 107.0) | 60.0 (19.0, 87.0) | 50.0 (30.0, 98.0) | 0.78 |
| Large LDL particles (nmol/L) | 438.0 (255.0, 567.0) | 471.0 (372, 601.0) | 596.0 (404.0, 756.0) | 0.07 |
| Small LDL particles (nmol/L) | 394.5 (67.0, 464.0) | 93.0 (55.0, 563.0) | 94.0 (76.0, 496.0) | 0.77 |
| LDL size (nm) | 21.0 (20.6, 21.3) | 21.2 (20.5, 21.3) | 21.2 (20.8, 21.3) | 0.73 |
Data are means (SD), median (25th, 75th percentile) or percent (n)
HbA1c values were obtained during the 2006–07 follow-ups of the EDC and CACTI studies and were available for 16, 14 and 19 study participants with the Hp 1-1, Hp 2-1, and Hp 2-2 genotypes, respectively
Adherence, as assessed by pill count (93% and 91% during the first and second periods, respectively) and plasma α-tocopherol concentrations, which increased by 69.2% with supplementation (p-value for treatment <0.0001), was high during the crossover study. No carryover effects were detected for α-tocopherol in the overall sample (p=0.31), Hp 1-1 (p=0.82), Hp 2-1 (p=0.20) or Hp 2-2 (p=0.64).
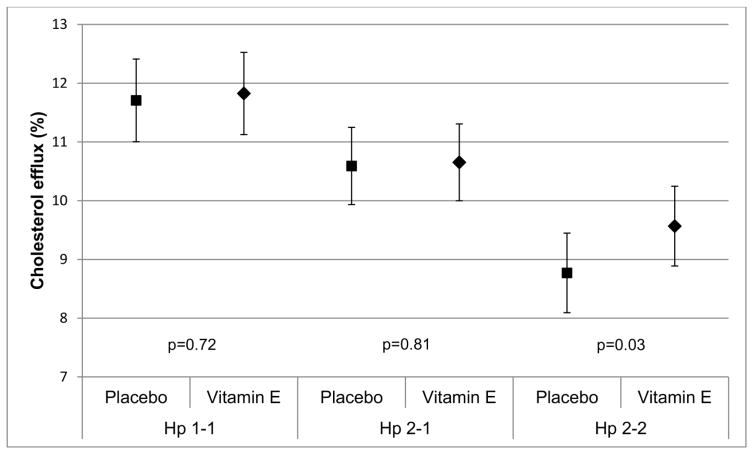
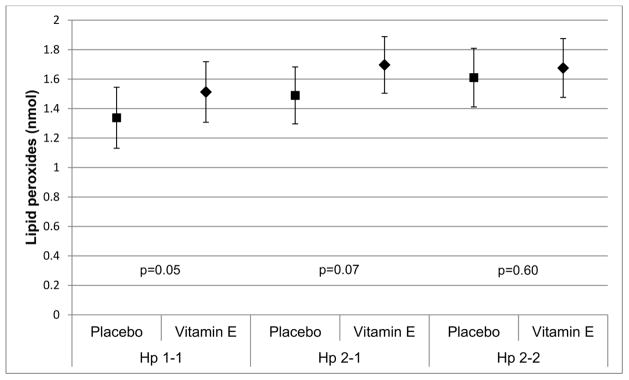
Vitamin E treatment increased cholesterol efflux by 3.3% in the Hp 2-2 group, but decreased efflux by 2.3% and 2.9% in the Hp 1-1 and Hp 2-1 groups. Intention-to-treat analyses (Table 2) accounting for the nesting of participants within treatment sequence and adjusting for the time period suggested a small increase in HDL-mediated cholesterol efflux that reached significance in Hp 2-2 carriers (β=0.79, p=0.03). Treatment appeared to somewhat increase HDL-associated lipid peroxides in Hp 1 carriers (βHp 1-1=0.18, p=0.05 and βHp 2-1=0.21, p=0.07) but had no effect in those homozygous for the Hp 2 allele (p=0.60). Though the study was not powered to detect interactions, we tested for effect modification by Hp genotype. As expected, the interaction term for a difference in the effect of treatment by Hp did not reach statistical significance (p=0.25 and 0.63 for cholesterol efflux and HDL-associated lipid peroxides, respectively, Figure 2).
Table 2.
Effect of vitamin E vs. placebo on HDL function measures in intention to treat analyses stratified by Hp*
| Effect of vitamin E vs. Placebo | ||
|---|---|---|
| β estimate (std. error) | p-value | |
| Cholesterol efflux (%) | ||
| Hp 1-1 (n=27, 105 obs) | 0.13 (0.36) | 0.72 |
| Hp 2-1 (n=31, 120 obs) | 0.08 (0.33) | 0.81 |
| Hp 2-2 (n=29, 112 obs) | 0.79 (0.36) | 0.03 |
| Lipid peroxides (nmol) | ||
| Hp 1-1 (n=27, 105 obs) | 0.18 (0.09) | 0.05 |
| Hp 2-1 (n=31, 120 obs) | 0.21 (0.11) | 0.07 |
| Hp 2-2 (n=29, 112 obs) | 0.07 (0.12) | 0.60 |
Models took into account the time period; random effects for participant ID nested within treatment sequence were used.
Figure 2. Effect of vitamin E treatment (mean, standard error), adjusting for period and using random effects for participant ID nested within treatment sequence.
Figure 2A. Treatment effect on cholesterol efflux (p-valuetreatment = 0.72 for Hp 1-1, p-valuetreatment = 0.81 for Hp 2-1 and p-valuetreatment = 0.03 for Hp 2-2)
Figure 2B. Treatment effect on HDL-associated lipid peroxides (p-valuetreatment = 0.05 for Hp 1-1, p-valuetreatment = 0.07 for Hp 2-1 and p-valuetreatment = 0.60 for Hp 2-2)
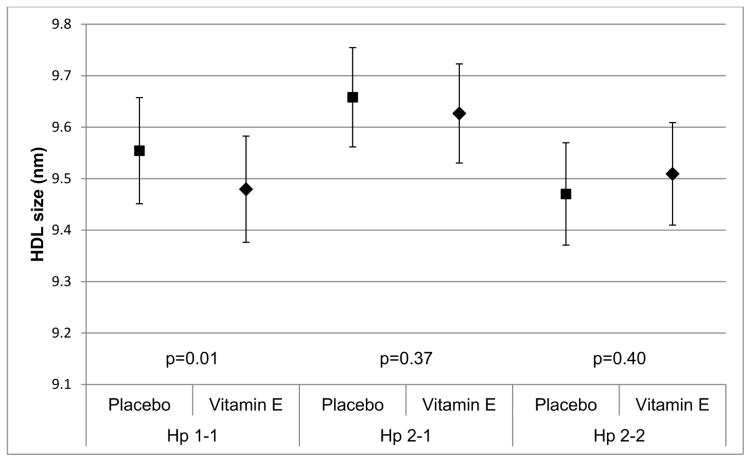
Figure 2C. Treatment effect on HDL size (p-value treatment = 0.03 for Hp 1-1, p-value treatment = 0.37 for Hp 2-1 and p-value treatment = 0.40 for Hp 2-2)
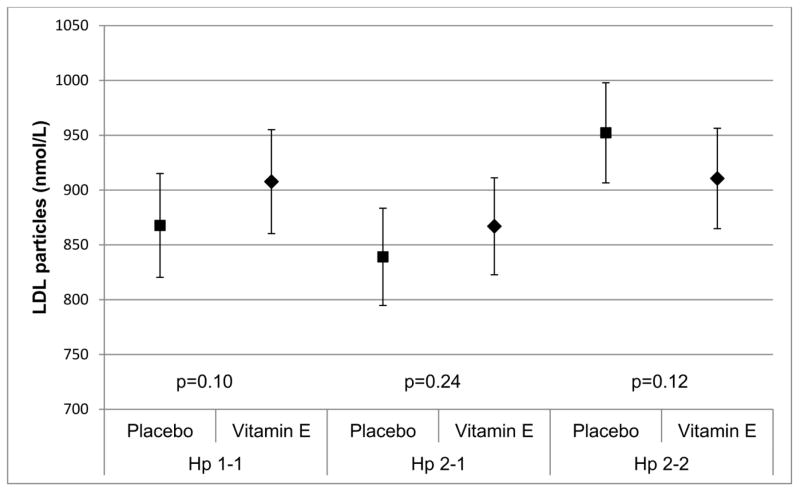
Figure 2D. Treatment effect on total LDL particle concentration (p-value treatment = 0.10 for Hp 1-1, p-value treatment = 0.24 for Hp 2-1 and p-value treatment = 0.12 for Hp 2-2)
HDL particle concentration increased (β=1.13, p=0.08), mostly attributable small HDL particles increases (β=1.76, p=0.05), with vitamin E among Hp 1-1 participants, resulting in an overall reduction in HDL particle size in this group (β=−0.07, p=0.03) (Table 3). However, treatment generally did not affect HDL particles in Hp 2 allele carriers. Though not statistically significant, treatment also appeared to increase LDL particle concentration in Hp 1 allele carriers (particularly Hp 1-1), although reductions were noted in the Hp 2-2 group, especially in intermediate density lipoprotein (IDL) particles. Nevertheless, the interaction terms for a difference in the treatment effect by Hp did not reach significance.
Table 3.
Effect of vitamin E vs. placebo on NMR lipoprotein measures in intention to treat analyses stratified by Hp*
| Hp 1-1 | Hp 2-1 | Hp 2-2 | ||||
|---|---|---|---|---|---|---|
| β (std. error) | p-value | β (std. error) | p-value | β (std. error) | p-value | |
|
| ||||||
| n=27, 103 obs | n=31, 116 obs | n=29, 110 obs | ||||
| HDL cholesterol (mg/dL) | 0.20 (1.03) | 0.85 | 0.32 (1.02) | 0.75 | −0.20 (1.25) | 0.88 |
| HDL particles (μmol/L) | 1.13 (0.64) | 0.08 | 0.75 (0.63) | 0.24 | 0.06 (0.58) | 0.92 |
| Large HDL particles (μmol/L) | 0.03 (0.21) | 0.87 | −0.23 (0.21) | 0.30 | 0.04 (0.27) | 0.88 |
| Medium HDL particles (μmol/L) | −0.54 (0.81) | 0.51 | 1.27 (0.70) | 0.07 | 0.04 (0.71) | 0.96 |
| Small HDL particles (μmol/L) | 1.76 (0.90) | 0.05 | −0.32 (0.68) | 0.63 | −0.07 (0.72) | 0.92 |
| HDL size (nm) | −0.07 (0.03) | 0.03 | −0.03 (0.04) | 0.37 | 0.04 (0.05) | 0.40 |
| LDL particles (nmol/L) | 45.93 (27.91) | 0.10 | 30.53 (25.85) | 0.24 | −41.59 (26.25) | 0.12 |
| IDL particles (nmol/L) | 4.81 (9.12) | 0.60 | 2.86 (8.85) | 0.75 | −14.28 (7.56) | 0.06 |
| Large LDL particles (nmol/L) | 28.82 (19.75) | 0.15 | 6.57 (23.99) | 0.78 | −6.89 (23.84) | 0.77 |
| Small LDL particles (nmol/L) | 11.74 (23.51) | 0.62 | 21.05 (34.46) | 0.54 | −19.79 (30.03) | 0.51 |
| LDL size (nm) | 0.005 (0.05) | 0.92 | −0.05 (0.06) | 0.36 | −0.04 (0.05) | 0.52 |
Models took into account the time period; random effects for participant ID nested within treatment sequence were used.
Discussion
The notion of individualized medicine and pharmacogenomics has evolved over the past 50 years [31], although such optimization of drug therapy has yet to be successfully applied in medicine. Recent type 2 diabetes trials [14–15; 32] suggest that a polymorphism in the Hp gene may provide the opportunity to reduce cardiovascular events with α-tocopherol supplementation among those with the susceptible Hp 2-2 genotype (approximately 43% and 36% of type 1 and type 2 diabetes, respectively). Though the scope of our short-duration mechanistic trial was not to assess event reduction with such therapy, our findings suggest that α-tocopherol may improve HDL-mediated cholesterol efflux, a dysfunction in which may be at least partly responsible for the observed cardiovascular susceptibility in Hp 2-2 carriers in type 1 diabetes. Our results further suggest that vitamin E may reduce LDL particle concentration in this Hp subgroup, with no benefit, indeed somewhat adverse changes in lipoprotein subfractions and lipid peroxides, observed in Hp 1 allele carriers. To our knowledge, similar data on the effect of α-tocopherol supplementation on NMR lipoprotein subfractions by Hp genotype in type 1 diabetes are unavailable.
Being a pilot, our study was not powered to detect a pharmacogenetic effect. However, our findings must be viewed in light of the strong type 2 diabetes evidence, which motivated this study. Our results on HDL function are indeed consistent with those previously obtained in type 2 diabetes [8–9]. The HDL function differences we observed by Hp genotype and with supplementation were not large. However, it is critical to emphasize that they were of the same magnitude as those recently reported to be independently associated with cardiovascular events [28]. This improvement in HDL function with α-tocopherol in the large subset of the type 1 diabetes population associated with HDL dysfunction (Hp 2-2) contrasts the lack of a similar benefit from drugs used in trials to raise HDL cholesterol [29]. Nevertheless, as discrepancies exist regarding the direction of the cholesterol efflux-cardiovascular disease association [30], further research is needed to understand the complex interplay between HDL and atherosclerosis development.
In type 2 diabetes, low HDL is an important, independent determinant of cardiovascular risk. Its role in type 1 diabetes, however, is unclear, as HDL mass (or cholesterol) is generally not reduced, leading, early on [33], to the concept of HDL dysfunction. As previously published [8], the noted HDL dysfunction in diabetes with Hp 2-2 and α-tocopherol’s potential to reverse this impairment relate to the ability of the Hp 2-2 protein to bind to Apo A1 on HDL and thereby tether hemoglobin to HDL. We have proposed that the specificity of the interaction of Hp genotype and diabetes on HDL function is due to the impaired clearance of hemoglobin in diabetes with Hp 2-2 and the impaired ability of Hp 2-2 to prevent heme release from hemoglobin when the latter is glycated, which occurs to a greater degree in the diabetic state [8]. We have previously shown that this heme-iron associated with HDL in diabetes with Hp 2-2 leads to the oxidative modification of HDL, resulting not only in impaired HDL-mediated reverse cholesterol transport, but also rendering the HDL proatherogenic [8].
Whether measures of HDL function, and consequently the effects of α-tocopherol supplementation, relate to cardiovascular event development is not currently known in type 1 diabetes, where clinical trial data are lacking. These findings, however, provide some support for the hypothesis that α-tocopherol supplementation, while not beneficial for the general or diabetes population overall, may benefit those most susceptible to cardiovascular disease, i.e. individuals with diabetes who carry the Hp 2-2 genotype. However, definitive conclusions on this matter, which could affect up to 43% of individuals with type 1 diabetes, will have to await results from a randomized clinical trial. Of importance in this regard is our failure to demonstrate a reduction in lipid peroxides among Hp 2-2 carriers and, importantly, the small increase noted in lipid peroxide and LDL particle concentration along with a decrease in HDL particle size among Hp 1 allele carriers with vitamin E therapy. As previous findings in individuals with type 2 diabetes have suggested a marked increase in the amount of lipid peroxides in the HDL of Hp 2-2 compared to Hp 1-1 carriers while experiments in diabetic mice have shown a reduction in HDL-associated lipid peroxides with α-tocopherol supplementation [8], our results are puzzling. However, it is perhaps possible that a greater abnormality in lipid peroxide concentration than observed here is required to derive benefits from vitamin E supplementation. Indeed, the concentration of lipid peroxides was similar across Hp genotype groups at our study baseline. Moreover, similar to our findings, a worsening of HDL function with α-tocopherol supplementation has also been reported in individuals with type 2 diabetes and the Hp 2-1 genotype, despite improved HDL function among Hp 2-2 carriers with supplementation in the same study [9]. In an effort to explain the disparate findings according to Hp genotype, researchers further investigated the effect of vitamin E on the mass or activity of the antioxidant, HDL-associated [34–35], proteins GPx-3 and paraoxonase, in addition to the amount of redox-active nontransferrin-bound iron. Interestingly, vitamin E was found to be associated with a non-significant 50% increase in HDL associated glutathione peroxidase and a 25% reduction in redox active iron in Hp 2-2 carriers but a 3- to 4-fold decrease in HDL associated glutathione peroxidase (p=0.06) and no change in redox active iron among Hp 2-1 carriers [9]. Paradoxical as they may appear, these findings must be viewed in light of emerging evidence that the generation of reactive oxygen species is an essential feature of normal physiology and that dysregulation of oxidant signaling can promote pathological conditions [36–37]. It is therefore conceivable that “excessive” supplementation, and thus “excessive” suppression of oxidant signaling, may have occurred in the present study among carriers of the Hp 1 allele. Given evidence in type 1 diabetes of greater coronary artery disease risk among those with the Hp 2-1 compared to the Hp 1-1 genotype, however, whether lower vitamin E supplementation doses could ameliorate or eliminate the observed excess risk in this subgroup remains unanswered. Nevertheless, these findings further underscore the need for a full pharmacogenetic intervention trial before considering vitamin E supplementation in those with type 1 diabetes and the Hp 1-1/2-1 genotype. Moreover, if confirmed in other cohorts, these results may also partially explain the null or harmful effects observed with antioxidant supplementation in previously conducted trials.
Limitations of this pilot include the lack of power to detect effect modification by Hp, as such ability requires much larger samples. Another was the small number of covariates measured. However, previous type 1 diabetes studies have not reported differences in clinical characteristics by Hp genotype, with the exception of non-HDL cholesterol [16, 34]. It is thus unlikely that any further risk factor differences would have been observed here.
In conclusion, our results suggest that HDL function worsens with the number of Hp 2 alleles in type 1 diabetes. Our findings further support that α-tocopherol supplementation may improve HDL function in those with the Hp 2-2 genotype. However, as this pilot was not adequately powered to detect a significant modification of the effect of treatment with vitamin E by Hp genotype, our findings require replication in larger trials, specifically designed to evaluate the presence of a pharmacogenetic effect.
Acknowledgments
We are indebted to study participants and staff for their invaluable contributions to the HapE study. The authors thank Dr. Yuefang Chang, Department of Neurological Surgery, University of Pittsburgh, for providing statistical expertise and Dr. Jim Otvos, LipoScience, NC for assessment of NMR lipoprotein subfractions. This study was funded by the American Diabetes Association, grant number 1-10-CT-12.
List of abbreviations
- ACR
Pittsburgh Allegheny County Insulin Dependent Diabetes Mellitus Registry
- CACTI
Coronary Artery Calcification in Type 1 Diabetes
- CAD
Coronary artery disease
- CHR
Children’s Hospital of Pittsburgh
- EDC
Epidemiology of Diabetes Complications study
- HDL
High density lipoprotein cholesterol
- HOPE
Heart Outcomes Prevention Evaluation
- Hp
Haptoglobin
- ICARE
Israel CArdiovascular events Reduction with vitamin E
Footnotes
ClinicalTrials.gov Identifier
Author’s contributions
T.C. conceived of the study and participated in the design and coordination, conducted the statistical analysis and wrote the manuscript. A.P.L. researched data and edited the manuscript; R.G.M. researched/analyzed the data and reviewed the manuscript; R.A., J.S.B., D.F., C.E.F., G.P., R.V., R.W.E. and T.J.O. researched data and reviewed/edited the manuscript. T.C. is the grantor of this work.
Conflict of interests
A.P.L. is the author of patents owned by his institution which claim that the Hp genotype is predictive of diabetic CVD and that antioxidant therapy may be able to reduce this risk. No other author has any competing interests in the manuscript.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed Consent
Informed consent was obtained from all individuals for being included in the study.
References
- 1.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 2.Baynes JW, Thorpe SR. The role of oxidative stress in diabetic complications. Current Opinion in Endocrinology. 1996;3:277–284. [Google Scholar]
- 3.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361(9374):2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 5.Vardi M, Levy NS, Levy AP. Vitamin E in the prevention of cardiovascular disease-the importance of proper patient selection. J Lipid Research. 2013;54:2307–2314. doi: 10.1194/jlr.R026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12:189–261. doi: 10.1007/978-1-4615-8315-8_3. [DOI] [PubMed] [Google Scholar]
- 7.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004;43:3899–3906. doi: 10.1021/bi0362626. [DOI] [PubMed] [Google Scholar]
- 8.Asleh R, Blum S, Kalet-Litman S, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes. 2008;57:2794–2800. doi: 10.2337/db08-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farbstein D, Blum S, Pollak M, et al. Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. Atherosclerosis. 2011;219(1):240–244. doi: 10.1016/j.atherosclerosis.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy AP. Haptoglobin genotype and vascular complications in patients with diabetes. New Engl J Med. 2000;343:969–970. doi: 10.1056/NEJM200009283431313. [DOI] [PubMed] [Google Scholar]
- 11.Levy AP, Hochberg I, Jablonski K, et al. Haptoglobin genotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol. 2002;40:1984–1990. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 12.Suleiman M, Aronson D, Asleh R, et al. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;54:2802–2806. doi: 10.2337/diabetes.54.9.2802. [DOI] [PubMed] [Google Scholar]
- 13.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care. 2003;26:2628–2631. doi: 10.2337/diacare.26.9.2628. [DOI] [PubMed] [Google Scholar]
- 14.Levy AP, Gerstein HC, Miller-Lotan R, et al. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care. 2004;27:2767. doi: 10.2337/diacare.27.11.2767. [DOI] [PubMed] [Google Scholar]
- 15.Milman U, Blum S, Shapira C, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 16.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes. 2008;57:1702–1706. doi: 10.2337/db08-0095. [DOI] [PubMed] [Google Scholar]
- 17.Simpson M, Snell-Bergeon JK, Kinney GL, et al. Haptoglobin genotype predicts development of coronary artery calcification in a prospective cohort of patients with type 1 diabetes. Cardiovasc Diabetol. 2011;10:99. doi: 10.1186/1475-2840-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaPorte RE, Fishbein HA, Drash AL, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) registry: The incidence of insulin dependent diabetes in Allegheny County, Pennsylvania (1965–1976) Diabetes. 1981;30:279–284. doi: 10.2337/diab.30.4.279. [DOI] [PubMed] [Google Scholar]
- 19.Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications of IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 20.Dabelea D, Kinney G, Snell-Bergeon JK, et al. Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52(11):2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 21.Levy NS, Vardi M, Blum S, et al. An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype. Clin Chem Lab Med. 2013;51:1615–1622. doi: 10.1515/cclm-2013-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype and diabetes-dependent differences in iron mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435–441. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 23.Asleh R, Miller-Lotan R, Aviram M, et al. Haptoglobin genotype is a regulator of reverse cholesterol transport in diabetes in vitro and in vivo. Circ Res. 2006;99:1419–1425. doi: 10.1161/01.RES.0000251741.65179.56. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblat M, Vaya J, Shih D, Aviram M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: a possible role for lysophosphatidylcholine. Atherosclerosis. 2005;179:69–77. doi: 10.1016/j.atherosclerosis.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Driskell WJ, Neese JW, Bryant CC, Bashor MM. Measure of Vitamin A and Vitamin E in human serum by high performance liquid chromatography. J Chromatography. 1982;231:439–444. doi: 10.1016/s0378-4347(00)81869-1. [DOI] [PubMed] [Google Scholar]
- 26.Miller KW, Lorr NA, Yang CS. Simultaneous determination of plasma retinol, alpha-tocopherol, lycopene, alpha-carotene and beta-carotene by high performance liquid chromatography. Analytical Biochemistry. 1984;138:340–345. doi: 10.1016/0003-2697(84)90819-4. [DOI] [PubMed] [Google Scholar]
- 27.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khera AV, Patel PJ, Reilly MP, Rader DJ. The addition of niacin to statin therapy improves high-density lipoprotein cholesterol levels but not metrics of functionality. J Am Coll Cardiol. 2013;62(20):1909–1910. doi: 10.1016/j.jacc.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans WE, Relling MV. Moving toward individualized medicine with pharmacogenomics. Nature. 2004;429:464–468. doi: 10.1038/nature02626. [DOI] [PubMed] [Google Scholar]
- 32.Blum S, Vardi M, Levy NS, Miller-Lotan R, Levy AP. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis. 2010;211:25–27. doi: 10.1016/j.atherosclerosis.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orchard TJ. Dyslipoproteinemia and diabetes. Endocrinology and Metabolism Clinics of North America. 1991;19:361–380. [PubMed] [Google Scholar]
- 34.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen N, Liu Y, Greiner CD, Holtzman JL. Physiologic concentrations of homocysteine inhibit the human plasma GSH peroxidase that reduces organic hydroperoxides. J Lab Clin Med. 2000;136:58–65. doi: 10.1067/mlc.2000.107692. [DOI] [PubMed] [Google Scholar]
- 36.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orchard TJ, Sun W, Cleary PA, Genuth SM, Lachin JM, McGee P, Paterson AD, Raskin P, Anbinder Y, Levy AP DCCT/EDIC Research Group. Haptoglobin genotype and the rate of renal function decline in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes. 2013;62:3218–3223. doi: 10.2337/db13-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]