Abstract
AIMS
Cardiac outcomes after acute coronary syndrome (ACS) are worse in patients with depression, but identifying which depressed patients are at increased risk, and by what means, remains difficult.
METHODS AND RESULTS
We analyzed inpatient ECGs from 955 patients admitted with non-ST elevation ACS (NSTE-ACS) in the Prescription Use, Lifestyle, and Stress Evaluation (PULSE) study. Patients with QRS duration ≥120 milliseconds or whose rhythm was not normal sinus were excluded (sample size=769). Depressive symptoms were measured by Beck Depression Inventory score ≥10. ECG markers of ventricular hypertrophy included Cornell voltage-duration product (CP-LVH) and strain pattern in the lateral leads. In multivariable logistic regression models, depressive symptoms were associated with increased odds of CP-LVH, ECG-strain, and the combination of the two (odds ratios 1.74 to 2.33, p values <0.01). The combination of both CP-LVH and ECG-strain was predictive of one-year risk of myocardial infarction (MI) or death among patients with depressive symptoms (hazard ratio 4.91, 95% CI 1.55–15.61, p=0.007), but not among those without depressive symptoms (p value for interaction 0.043).
CONCLUSION
In our NSTE-ACS cohort, ECG markers of hypertrophy were both more common, and more predictive of MI/mortality, among those with depressive symptoms. Cardiac hypertrophy is a potential target for therapy to improve outcomes among depressed NSTE-ACS patients.
Keywords: electrocardiography, hypertrophy, depression, acute coronary syndrome
INTRODUCTION
Among patients with acute coronary syndrome (ACS), individuals with depressive symptoms are at higher risk for ACS recurrence and cardiac mortality compared with those without depressive symptoms.1, 2 Several mechanisms have been postulated to explain this excess risk, including reduced adherence to medical treatments and behavioral recommendations among depressed patients,3, 4 increased inflammation,5 endothelial dysfunction,6 abnormal platelet reactivity,7 and reduced heart rate variability.8
Abnormal cardiac sympathetic activation has previously been observed in patients with major depression.9, 10 A recent animal model has suggested that abnormal cardiac sympathetic activation may play an important role in the poor prognosis associated with depression after myocardial infarction.11 To test this in humans, electrocardiographic markers may reflect the consequences for cardiac performance of the autonomic dysregulation commonly found in depressed patients,12, 13 and so may also help to identify those patients with depression who are at particularly high risk for recurrent cardiac events. Electrocardiographic left ventricular hypertrophy (LVH) and strain pattern consisting of repolarization abnormalities in the lateral leads predict cardiac mortality in patients with hypertension,14 aortic stenosis,15 and ACS.16 We assessed the relation between ECG markers of LVH and depression in patients with non ST elevation ACS (NSTE-ACS), expecting that depressed patients may more commonly have repolarization abnormalities. Also, we hypothesized that that depressed patients with these ECG abnormalities may be at particular risk for adverse outcomes after ACS.
METHODS
Patient selection and depression/anxiety symptom evaluation
Institutional Review Board approval for this study was obtained at Columbia University. The cohort was drawn from the Prescription Use, Lifestyle, and Stress Evaluation (PULSE). a prospective cohort study of depression after ACS,17 Participants were recruited from patients admitted to Columbia University Medical Center between February 2009 and June 2010. ACS events were defined according to American Heart Association/American College of Cardiology criteria18 as either acute MI or unstable angina. All patients had symptoms consistent with acute myocardial ischemia and at least one of the following: ischemic electrocardiographic changes (i.e. ST depression and/or T-wave abnormalities), an angiogram indicative of coronary artery disease on current admission, and/or documented history of coronary artery disease according to a stress test during the index admission or previous coronary angiogram. Patients who presented with an acute rise in serum troponin I levels >0.4 ng/ml were categorized as MI. A study cardiologist confirmed ACS eligibility for all patients.
A total of 1087 patients were enrolled in PULSE, and for this analysis we drew our sample from the 955 patients who were admitted with NSTE-ACS (87.9% of the total ACS sample). Participants were followed as long as one-year after their initial NSTE-ACS event. Cardiovascular events including myocardial infarction and/or death were identified by participant report with medical record adjudication, electronic medical record search, and National Death Index search during follow-up.
The Beck Depression Inventory (BDI),19 a 21-item self-report measure of depressive symptom severity, was administered within one week after the index ACS event, while patients were still hospitalized. The BDI is a well-validated questionnaire with the most extensive evidence of validity for predicting MI recurrence and death compared to all other self-report measures. Based on prior studies in cardiac patients, 20–23 the BDI score was dichotomized, and a score ≥10 was considered to indicate significant depressive symptoms.
Twelve-lead ECGs were acquired during hospitalization for ACS, analyzed by physician/medical student researchers and then over-read by a cardiologist (WW). All ECG interpretations were performed blinded to depression status. For this study we excluded patients with QRS duration ≥120 milliseconds or those whose rhythm was not normal sinus. Left ventricular hypertrophy (CP-LVH) was defined according to Cornell voltage-duration product {[RaVL+SV3+(6 mV in women)]*QRS duration} ≥2440 mV-ms.24 ECG-strain was defined according to the presence of ≥1 mm ST depression and/or T-wave inversion in any lateral lead (I, aVL, V5, or V6).16 Measurements were taken using CardioCalipers software (Iconico, Inc., New York, New York).
Statistical Analyses
All analyses were performed with SPSS Version 22 software (IBM Corp., Armonk, NY). Participants with and without depressive symptoms were compared using t-tests for continuous measures and chi-square tests for categorical measures. We estimated logistic regression models for odds ratios of the association of different ECG markers of hypertrophy with depressive symptoms. Three separate regression models were estimated, for CP-LVH, ECG-strain, and the presence of both CP-LVH and ECG-strain together. Full multivariable models included age, sex, black race, body mass index, ACS type, hypertension, diabetes, reduced estimated glomerular filtration rate <60 ml/min/1.73 m2, left ventricular ejection fraction <0.40, heart rate by ECG, systolic blood pressure on admission, smoking, reported history of antidepressant use prior to admission, and history of beta blocker use.
We used proportional hazards models to estimate the risk of MI/death within one year of the index ACS event associated with different ECG markers. Then, we estimated models separately in patients with and without depressive symptoms. Finally, we estimated model in all patients together, with multiplicative interaction terms between depressive symptoms and each ECG marker to assess for effect modifiers.
RESULTS
Among 955 participants with NSTE-ACS in PULSE, 109 were excluded for QRS duration ≥120 msec, 67 for rhythm other than normal sinus, and 10 for poor quality/missing ECGs, resulting in a sample size of 769 for analysis. Participants with depression were more likely to be female and/or black, to have diabetes mellitus, and to report smoking. Average heart rate was higher among those who reported depressive symptoms. The proportion of participants who presented with MI or left ventricular ejection fraction <0.40 were not significantly different between those with and without depression.
ECG markers of hypertrophy, including CP-LVH, ECG-strain, and the combination of CP-LVH and ECG-strain were each more frequent in the depressed group (Table 1). In separate age/sex/race- adjusted logistic regression models (Table 2), depressive symptoms were associated with increased odds of CP-LVH (odds ratio = 1.76, 95% CI 1.13–2.76), ECG-strain (OR 1.59, 95% CI 1.13–2.24), and the combination of CP-LVH and ECG-strain (OR 1.85, 95% CI 1.09–3.13). In full multivariable models, the association of depressive symptoms with these ECG markers was stronger (odds ratios 1.74 to 2.33, p values 0.005 to 0.008). The relation between depression and ECG markers did not differ between men and women (p value for interaction 0.898 to 0.943).
Table 1.
Characteristics of categories of participants based on cutoff values for Beck Depression Inventory (BDI) score
| BDI <10 (n=516) | BDI ≥10 (n=253) | P value | |
|---|---|---|---|
| Age, years | 63.3 (11.1) | 61.7 (11.2) | 0.07 |
| Female, % | 28.1 | 48.6 | <0.01 |
| Black % | 17.0 | 27.6 | <0.01 |
| BMI >25 | 78.1 | 79.8 | 0.64 |
| Hypertension, % | 78.9 | 83.8 | 0.12 |
| LVEF<0.40, % | 7.2 | 9.1 | 0.39 |
| ECG Heart rate, BPM | 66.7 (11.4) | 68.8 (12.2) | 0.02 |
| Diabetes mellitus, % | 31.6 | 41.9 | 0.02 |
| eGFR <60 ml/min/1.73 m2 | 24.7 | 25.4 | 0.86 |
| Smoking, % | 48.4 | 56.5 | 0.04 |
| Beta blocker, % | 52.3 | 58.1 | 0.14 |
| Antidepressant, % | 11.0 | 31.2 | <0.01 |
| Non-STEMI, % | 35.3 | 34.0 | 0.75 |
| BDI score | 4.2 (2.7) | 15.8 (6.0) | <0.01 |
| CP-LVH, % | 9.9 | 19.0 | <0.01 |
| ECG-strain, % | 23.8 | 36.0 | <0.01 |
| CP-LVH & ECG-strain, % | 6.4 | 12.6 | <0.01 |
Values are mean (standard deviation) or percentages. P values correspond to chi-square tests for categorical variables, analysis of variance for continuous variables.
BMI = body mass index; LVEF = left ventricular ejection fraction; BPM = beats per minute; BDI= Beck Depression Inventory; ECG=electrocardiogram; CP-LVH = left ventricular hypertrophy by Cornell product
Table 2.
Odds ratios for different ECG indicators of hypertrophy associated with depressive symptoms (BDI ≥10).
| Age/sex/race adjusted | P value | Multivariable | P value | |
|---|---|---|---|---|
| Model 1: CP-LVH | ||||
| BDI ≥10 | 1.76 (1.13, 2.76) | 0.013 | 1.95 (1.19, 3.18) | 0.008 |
| Model 2: ECG-strain | ||||
| BDI ≥10 | 1.59 (1.13, 2.24) | 0.008 | 1.74 (1.18, 2.56) | 0.005 |
| Model 3: CP-LVH & ECG-strain | ||||
| BDI ≥10 | 1.85 (1.09, 3.13) | 0.023 | 2.33 (1.28, 4.25) | 0.006 |
All multivariable models also adjust for body mass index, ACS type, hypertension, diabetes mellitus, left ventricular ejection fraction <0.40, estimated glomerular filtration rate<60 ml/min/1.73 m2, admission systolic blood pressure, ECG heart rate, smoking, antidepressant use, and beta blocker use.
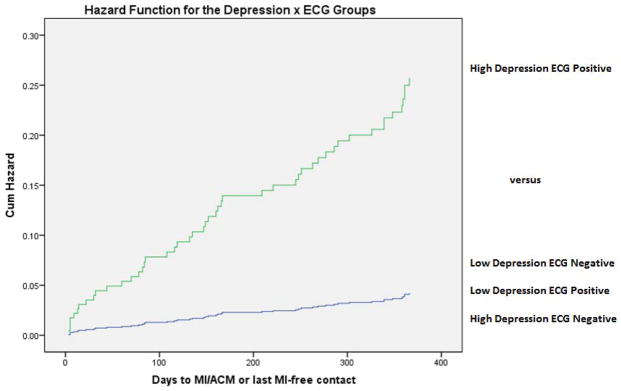
Nominal 1-year proportions for MI/death according to depressive symptoms and each ECG marker of hypertrophy are shown in Table 3. The presence of either depressive symptoms or an ECG marker alone was not associated with higher risk. However, when both depression and an ECG marker were present, the risk of MI/death was particularly high. Among patients with depressive symptoms and the combination of CP-LVH and ECG-strain (N=33, 4.3% of the sample), the nominal 1-year risk of MI/death was 24.2% (p for interaction 0.054). Adjusted hazard functions for MI/death also demonstrated evidence for an interaction between depressive symptoms and the combined presence of CP-LVH and ECG-strain (Figure). A proportional hazards model with full multivariable adjustment demonstrated a statistically significant interaction between depressive symptoms and the combination of CP-LVH and ECG-strain (p=0.043). In the subgroup of participants with depressive symptoms, the presence of CP-LVH and ECG-strain together was associated with elevated risk of MI/death in full multivariable models (hazard ratio 4.91, 95% CI 1.55–15.61, p value 0.007), whereas the risk was not elevated in the subgroup without depressive symptoms (hazard ratio 0.591, 95% CI 0.13–2.74, p value 0.502).
Table 3.
One-year nominal risk for MI/ACM according to depressive symptom category and absence or presence of ECG indicator of hypertrophy.
| BDI <10 | BDI ≥10 | P value for interaction | |
|---|---|---|---|
| CP-LVH | |||
| absent | 27/465 (5.8%) | 11/205 (5.4%) | |
| present | 3/51 (5.9%) | 8/48 (16.7%) | 0.119 |
| ECG-strain | |||
| absent | 20/393 (5.1%) | 7/162 (4.3%) | |
| present | 10/123 (8.1%) | 12/91 (13.2%) | 0.264 |
| CP-LVH & ECG-strain | |||
| absent | 28/483 (5.8%) | 11/220 (5.0%) | |
| present | 2/33 (6.1%) | 8/33 (24.2%) | 0.054 |
Figure.
Hazard functions for time to myocardial infarction or all-cause mortality, comparing subjects both with Beck Depression Inventory score ≥10 and left ventricular hypertrophy with strain (high depression, ECG positive) versus the average of the other 3 groups. Hazard functions are adjusted for age, sex, body mass index, ACS type, hypertension, diabetes mellitus, left ventricular ejection fraction <0.40, estimated glomerular filtration rate<60 ml/min/1.73 m2, admission systolic blood pressure, ECG heart rate, smoking, antidepressant use, and beta blocker use.
DISCUSSION
In this cohort of patients who presented with NSTE-ACS, ECG markers of cardiac hypertrophy including CP-LVH, ECG-strain, and the combination of the two were significantly more frequent among those with depressive symptoms. This relation held even with adjustment for multiple cardiovascular risk factors such as reduced left ventricular ejection fraction, reduced eGFR, and hypertension. The strength of association with depressive symptoms was greatest for the combination of CP-LVH and ECG-strain. Our findings are consistent with a prior cross-sectional study of 2420 subjects without known cardiovascular disease, among whom depressive symptoms measured by BDI score were associated with increased left ventricular mass index and with measures of diastolic dysfunction by echocardiography.25 Our study extends this work to an ACS sample and with application of validated ECG criteria for detection of hypertrophy.
The ECG markers identified in our analysis suggest that depressive symptoms are associated with changes in cardiac structure that occur over the course of months or years. We cannot rule out the possibility that individuals with more severe cardiac disease develop depressive symptoms as a consequence. However, it is also possible that depressive symptoms themselves contribute to hypertrophy, and a potential mechanism for the association involves elevated sympathetic activity. An investigation of cardiac norepinephrine spillover using coronary sinus sampling in 39 subjects with major depressive disorder and 76 healthy subjects demonstrated reduced norepinephrine reuptake among those with major depression.26 Similarly, a recent rodent model of myocardial infarction demonstrated cardiac sympathetic hyperinnervation and left ventricular hypertrophy in rats with induced depression.11 Our study is consistent with the idea that depression leads to subsequent hypertrophy, potentially due to increased cardiac sympathetic activation.
In this study, 33% of participants were depressed, and 12% showed the CP-LVH with ECG-strain pattern. Neither depression nor CP-LVH with ECG-strain pattern were independently associated with increased risk, but patients with the confluence of depressive symptoms and CP-LVH with ECG-strain pattern were at particularly high risk for death/MI by one year, compared with patients with depressive symptoms alone or ECG markers alone. In the Global Utilization of STrategies to Open occluded arteries (GUSTO IV) study of 7443 NSTE-ACS patients, there was a two-fold difference in mortality at one year among those with ECG-LVH with strain pattern (14.3%) compared with those without (7.1%), and this difference was significantly more pronounced in women than men.16 In our study, the association of CP-LVH and ECG-strain with depressive symptoms was present regardless of sex. Women are known to report depressive symptoms more frequently than men after ACS,27 and in our NSTE-ACS cohort, women comprised a greater proportion of patients with depressive symptoms. Thus, it is possible that differences in the burden of depressive symptoms may explain part of the sex interaction noted in GUSTO IV.
The risk of cardiac mortality associated with depressive symptoms in ACS is widely recognized, but trials of therapies to modify this risk have generally reported null findings 28 or have not been powered for cardiac mortality outcomes.29–31 Our findings suggest that 12-lead ECGs may offer important markers of cardiac risk among ACS patients with depressive symptoms to guide future trials of therapy.
The ECG markers in our study may also serve as a potential target of therapy in depressed patients with cardiac disease. In the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study, reduction in LVH according to CP-LVH and ECG-strain was associated with lower risk of cardiovascular mortality and sudden cardiac death.32 Treatment that is focused on interruption of cardiac disease progression may offer more benefits, certainly in terms of cardiac events, than treatment of depressive symptoms per se.
Limitations of our study include the small number of endpoints, such that our findings may be the result of chance. However, the interaction between depressive symptoms and CP-LVH and ECG-strain was preserved with adjustment for numerous other predictive variables including hypertension, diabetes, and reduced left ventricular ejection fraction. Another limitation of our analysis is the single time point for ECG capture and analysis, such that we cannot assess for the impact of changes in depressive symptoms on ECG markers. Also, we did not collect magnetic resonance imaging or echocardiographic information regarding ventricular hypertrophy, such that we cannot correlate our ECG findings with other imaging modalities. ECG measures of hypertrophy have generally been found to be specific, but not sensitive for ventricular hypertrophy based on these other methods.33 In addition, we used a marker for depressive symptoms based on a self-report inventory, rather than an interview-based diagnosis for depression.
In conclusion, in our NSTE-ACS cohort, ECG markers of hypertrophy were both more common, and more predictive of MI/mortality, among those with depressive symptoms. Cardiac hypertrophy is a potential target for therapy to improve outcomes among depressed NSTE-ACS patients.
Acknowledgments
This research was supported by grants HL128310, HL088117 and HL117832 from NHLBI.
References
- 1.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 3.Rieckmann N, Kronish IM, Haas D, Gerin W, Chaplin WF, Burg MM, Vorchheimer D, Davidson KW. Persistent depressive symptoms lower aspirin adherence after acute coronary syndromes. Am Heart J. 2006;152(5):922–7. doi: 10.1016/j.ahj.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165(21):2508–13. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG, Dimsdale JE. Depressed Mood and Flow-Mediated Dilation: A Systematic Review and Meta-Analysis. Psychosomatic Medicine. 2011;73(5):360–369. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serebruany VL, Glassman AH, Malinin AI, Sane DC, Finkel MS, Krishnan RR, Atar D, Lekht V, O’Connor CM. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagul Fibrinolysis. 2003;14(6):563–7. doi: 10.1097/00001721-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Carney RM, Freedland KE. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med. 2009;76(Suppl 2):S13–7. doi: 10.3949/ccjm.76.s2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Lavekar GS, Raju TR, Gangadhar BN. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J Affect Disord. 2007;100(1–3):137–41. doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Koschke M, Boettger MK, Schulz S, Berger S, Terhaar J, Voss A, Yeragani VK, Bar KJ. Autonomy of autonomic dysfunction in major depression. Psychosom Med. 2009;71(8):852–60. doi: 10.1097/PSY.0b013e3181b8bb7a. [DOI] [PubMed] [Google Scholar]
- 11.Shi S, Liang J, Liu T, Yuan X, Ruan B, Sun L, Tang Y, Yang B, Hu D, Huang C. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: evidence from an animal experiment. PLoS One. 2014;9(7):e101734. doi: 10.1371/journal.pone.0101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whang W, Julien HM, Higginbotham L, Soto AV, Broodie N, Bigger JT, Garan H, Burg MM, Davidson KW. Women, but not men, have prolonged QT interval if depressed after an acute coronary syndrome. Europace. 2012;14(2):267–71. doi: 10.1093/europace/eur246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whang W, Peacock J, Soto AV, Borda T, Bhatt AB, Richardson SI, Burg M, Davidson KW. Relationship between premature ventricular complexes and depressive symptoms in non-ST-elevation acute coronary syndrome. European Heart Journal: Acute Cardiovascular Care. 2013;2(1):61–67. doi: 10.1177/2048872613476101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Julius S, Snapinn S, Dahlof B. Electrocardiographic strain pattern and prediction of cardiovascular morbidity and mortality in hypertensive patients. Hypertension. 2004;44(1):48–54. doi: 10.1161/01.HYP.0000132556.91792.6a. [DOI] [PubMed] [Google Scholar]
- 15.Greve AM, Boman K, Gohlke-Baerwolf C, Kesaniemi YA, Nienaber C, Ray S, Egstrup K, Rossebo AB, Devereux RB, Kober L, Willenheimer R, Wachtell K. Clinical implications of electrocardiographic left ventricular strain and hypertrophy in asymptomatic patients with aortic stenosis: the Simvastatin and Ezetimibe in Aortic Stenosis study. Circulation. 2012;125(2):346–53. doi: 10.1161/CIRCULATIONAHA.111.049759. [DOI] [PubMed] [Google Scholar]
- 16.Westerhout CM, Lauer MS, James S, Fu Y, Wallentin L, Armstrong PW. Electrocardiographic left ventricular hypertrophy in GUSTO IV ACS: an important risk marker of mortality in women. Eur Heart J. 2007;28(17):2064–9. doi: 10.1093/eurheartj/ehm223. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer JA, Davidson KW, Schwartz JE, Shimbo D, Newman JD, Gurland BJ, Maurer MS. Prevalence and characteristics of anergia (lack of energy) in patients with acute coronary syndrome. Am J Cardiol. 2012;110(9):1213–8. doi: 10.1016/j.amjcard.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38(7):2114–30. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 19.Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961;4(6):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–60. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 21.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91(4):999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 22.Whang W, Shimbo D, Kronish IM, Duvall WL, Julien H, Iyer P, Burg MM, Davidson KW. Depressive symptoms and all-cause mortality in unstable angina pectoris (from the Coronary Psychosocial Evaluation Studies [COPES]) Am J Cardiol. 2010;106(8):1104–7. doi: 10.1016/j.amjcard.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, Mankad S, Rogers WJ, Sopko G, Cornell CE, Sharaf B, Merz CN. Depression is associated with cardiac symptoms, mortality risk, and hospitalization among women with suspected coronary disease: the NHLBI-sponsored WISE study. Psychosom Med. 2006;68(2):217–23. doi: 10.1097/01.psy.0000195751.94998.e3. [DOI] [PubMed] [Google Scholar]
- 24.Okin PM, Roman MJ, Devereux RB, Kligfield P. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995;25(2):417–23. doi: 10.1016/0735-1097(94)00371-v. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Kim SH, Lim SY, Cho GY, Baik IK, Lim HE, Na JO, Han SW, Ko YH, Shin C. Relationship between depression and subclinical left ventricular changes in the general population. Heart. 2012;98(18):1378–83. doi: 10.1136/heartjnl-2012-302180. [DOI] [PubMed] [Google Scholar]
- 26.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25(10):2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 27.Frazier L, Yu E, Sanner J, Liu F, Udtha M, Cron S, Coulter S, Bogaev RC. Gender Differences in Self-Reported Symptoms of Depression among Patients with Acute Coronary Syndrome. Nurs Res Pract. 2012;2012:109251. doi: 10.1155/2012/109251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. Jama. 2003;289(23):3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 29.van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–6. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 30.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McLvor M. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 31.Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, Albanese G, Kronish I, Hegel M, Burg MM. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;170(7):600–8. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction - A LIFE review. J Electrocardiol. 2014;47(5):630–5. doi: 10.1016/j.jelectrocard.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Rautaharju PM, Soliman EZ. Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: A critical appraisal. Journal of Electrocardiology. 2014;47(5):649–654. doi: 10.1016/j.jelectrocard.2014.06.002. [DOI] [PubMed] [Google Scholar]