Abstract
Background
Studies in vitro demonstrate that neuronal membrane/lipid rafts (MLRs) establish cell polarity by clustering pro-growth receptors and tethering cytoskeletal machinery necessary for neuronal sprouting. However, the effect of MLR and MLR-associated proteins on neuronal aging is unknown.
Methods
Here we assessed the impact of neuron-targeted overexpression of a MLR scaffold protein, caveolin-1 (via a synapsin promoter; SynCav1), in the hippocampus in vivo in adult (6-months-old) and aged (20-month-old) mice on biochemical, morphologic and behavioral changes.
Results
SynCav1 resulted in increased expression of Cav-1, MLRs, and MLR-localization of Cav-1 and tropomyosin-related kinase B (TrkB) receptor independent of age and time post gene transfer. Cav-1 overexpression in adult mice enhanced dendritic arborization within the apical dendrites of hippocampal CA1 and granule cell neurons, effects that were also observed in aged mice, albeit to a lesser extent, indicating preserved impact of Cav-1 on structural plasticity of hippocampal neurons with age. Cav-1 overexpression enhanced contextual fear memory in adult and aged mice demonstrating improved hippocampal function.
Conclusions
Neuron-targeted overexpression of Cav-1 in the adult and aged hippocampus enhances functional MLRs with corresponding roles in cell signaling and protein trafficking. The resultant structural alterations in hippocampal neurons in vivo are associated with improvements in hippocampal dependent learning and memory. Our findings suggest Cav-1 as a novel therapeutic strategy in disorders involving impaired hippocampal function.
Keywords: membrane/lipid rafts, caveolin, neuroplasticity, TrkB, CA1, dentate gyrus
Introduction
Synaptic plasticity occurs when endogenous neurotrophins and neurotransmitters bind to membrane receptors in neuronal microdomains to initiate neuritogenesis. Key plasmalemmal organizers and regulators of these pro-growth signaling events are membrane/lipid rafts (MLRs), which are discrete plasmalemmal regions enriched in cholesterol, sphingolipids, and scaffolding proteins such as caveolins (Cavs) and flotillins (1-3). MLRs found at the leading edge of polarized neurites (1, 4) are essential for neurite growth and guidance (5, 6), synapse formation/stabilization (7). Cav-1 organizes a multitude of neuronal receptors (3, 8, 9) and modulators of cytoskeletal dynamics (10-13). Loss or disruption of MLRs from the leading edge of neuronal growth cones results in growth cone collapse and inhibition of neuritogenesis (14, 15), indicating the functional significance of MLRs in neuronal survival and differentiation as well as numerous synaptic functions.
Neurotrophins, such as nerve growth factor, brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, and NT-4, are capable of binding to specific tropomyosin-related kinase (Trk) receptors and to the p75 neurotrophin receptor (16, 17). Binding of NTs to cognate Trk receptors results in the activation and recruitment of a number of downstream signaling proteins that are involved in neurotransmission, neuronal survival and differentiation, and retrograde signaling of developing and mature neurons (18, 19). Notably, the localization of Trk receptors to specific neuronal microdomains has been suggested to be crucial for determining the biological functions of neurotrophins. For example, recent in vitro and ex vivo studies have indicated that TrkB at synaptic sites are localized in MLRs (20-25), suggesting a role for lipid rafts in TrkB signaling. Furthermore, the localization of TrkB to MLRs is functionally important for BDNF-induced downstream signaling and neuronal plasticity in isolated corticohippocampal neurons (25). Supporting this study, previous work from our group demonstrated that neuron-targeted Cav-1 overexpression in corticohippocampal neurons enhanced MLR formation, increased pro-growth signaling (TrkB signaling, receptor-mediated cAMP formation), and promoted dendritic growth and arborization of primary neurons (9). However, whether Cav-1 overexpression-induced enhanced neuronal signaling and functional plasticity occurs in vivo is unknown, and the importance of Cav-1 overexpression in cognitive improvement has not been investigated.
With age there exists a greater vulnerability to cognitive deficits (26-28). Structural and functional changes in the aged brain that may underlie these deficits include a reduction in the number of synapses in the hippocampus (a brain region important for cognitive function), impairment in long-term potentiation (LTP) of hippocampal neurons, and a reduced capacity of the hippocampal neurons to evoke neuroplasticity (27, 29-31). Restoration of molecular signals that assist with structural and functional plasticity in the hippocampus might protect against age related cognitive decline. To that end, identification and targeting of the molecular events that promote neuronal survival and differentiation as well as associated synaptic functions in the hippocampus may yield new therapies to enhance functional neuroplasticity, thereby attenuating aged related cognitive dysfunction dependent on the hippocampus. In the context of this hypothesis, plasmalemmal organization and subcellular distribution of receptors and downstream signaling components essential for neuronal signaling and plasticity are altered with age (32-34), thus limiting the functional efficacy of neurotrophins. Because MLRs organize synaptic receptors necessary for plasticity, genetic interventions that enhance neuronal MLR formation may promote neuroplasticity dependent on MLR-neurotrophin signaling and reverse age-related cognitive decline. Therefore, we tested whether in vivo delivery of neuron-targeted Cav-1 in the aged hippocampus would enhance neurotrophin (TrkB)-induced signaling, structural plasticity of hippocampal neurons and improve hippocampal cognition in aged mice. The findings from the present study provide in vivo evidence to show that a single genetic intervention that increases MLRs in adult and aged subjects promotes molecular signals governing neuroplasticity, enhances structural plasticity of hippocampal neurons and improves hippocampal-dependent cognitive function.
Materials and Methods
Animals
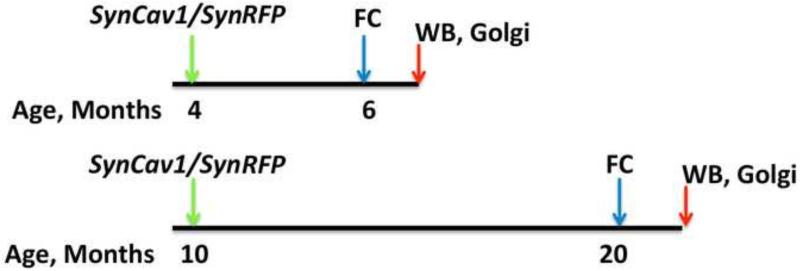
All mice (C57BL/6; Jackson Laboratories) were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science, Washington, D.C.). All animal use protocols were approved by the Veterans Administration San Diego Healthcare System Institutional Animal Care and Use Committee (IACUC, San Diego, California) prior to procedures performed. Male adult (4 month) or aged (10 month) mice were housed under normal conditions with ad libitum access to food and water and received stereotaxic injections of adeno-associated virus serotype 9 (AAV9) (at 4-month-old or 10-month-old, discussed below) and were tested for cognitive behaviors at 6 or 20 months of age (Figure 1). Two weeks after behavior testing all mice were euthanized by rapid decapitation and brain tissue was processed for western blotting or Golgi-Cox staining. Additional details on methods are provided in the supporting information.
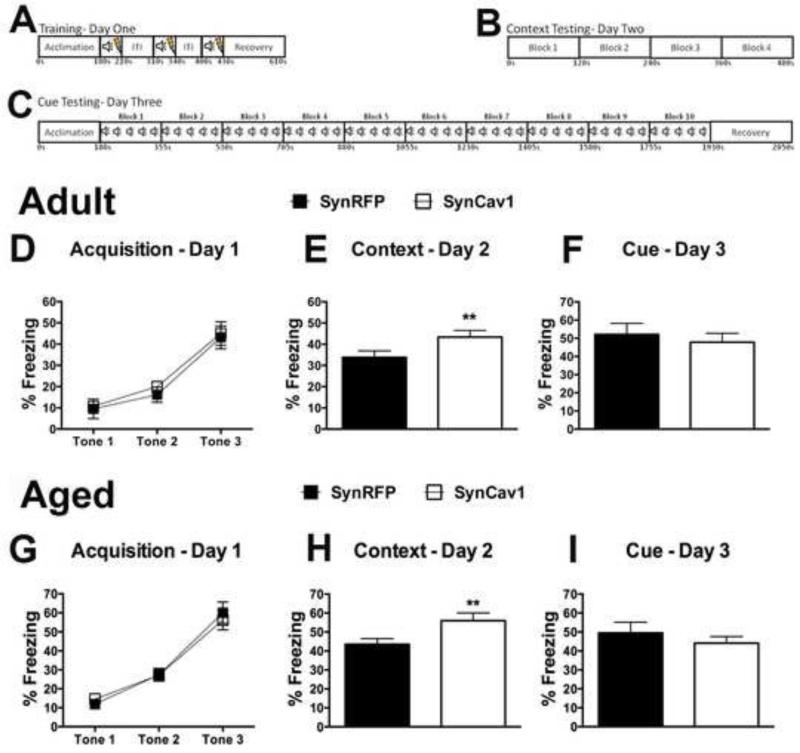
Figure 1.
Schematic illustration of experimental design in adult and aged animals. Green arrows indicate injection of virus into the hippocampus and blue arrow indicate fear conditioning (FC) behavioral timeline. Red arrows indicated euthanasia for brain tissue collection. Age of the animal is indicated in months.
Stereotactic Injection
Mice were anesthetized and prepared for sugery with a protocol modified from a previously described study (35). Hippocampal-targeted injections were controlled using Injectomate software (Neurostar – Berlin, Germany). Injections were made using a 33 gauge 10 l Hamilton Gas Tight syringe. At each coordinate, the needle was lowered at a rate of 0.32 mm/sec. After 60 sec, 0.5 l of AAV9 containing synapsin-red fluorescent protein (SynRFP) or synapsin-caveolin-1 (SynCav1) was injected over 60 sec (0.5 μl/min injection rate at a viral titer of 109 g.c./μl) at 3 locations (rostral to caudal) in each hippocampal hemisphere with an indwelling time of 1 min. Sagital brain sections were stained to confirm location and spread of RFP protein in adult (data not shown) and aged (supplementary figure 1) animals. Sections were also stained for hematoxylin and eosin, and histopathological analysis did not reveal any gross morphology or cell death in hippocampal sections from adult and aged mice (supplementary figure 1).
Biochemical characterization of membrane/lipid rafts
Mice were sacrificed by rapid decapitation under isoflurane (5%) anesthesia. The whole brain was quickly removed and hippocampal tissue (bilateral, CA regions and dentate gyrus combined, 50-100 mg) was dissected and homogenized using a carbonate lysis buffer (500 mM sodium carbonate, pH 11.0) containing protease and phosphatase inhibitors (33). Protein was quantified by Bradford assay and normalized to 0.5 mg/ml. Sucrose density gradients were prepared as previously reported (9). Membranes samples (f4-5 and f10-12) were incubated overnight with primary antibodies for Cav-1 (Cell Signaling #3238, 1:1000), TrkB (BD Biosciences 610102, 1:1000), or CT-B (Invitrogen C-22841, 1:1000) and probed with species-specific secondary antibodies conjugated to HRP. Densitometric analysis (arbitrary units) was conducted as previously described (33).
Golgi-Cox impregnation
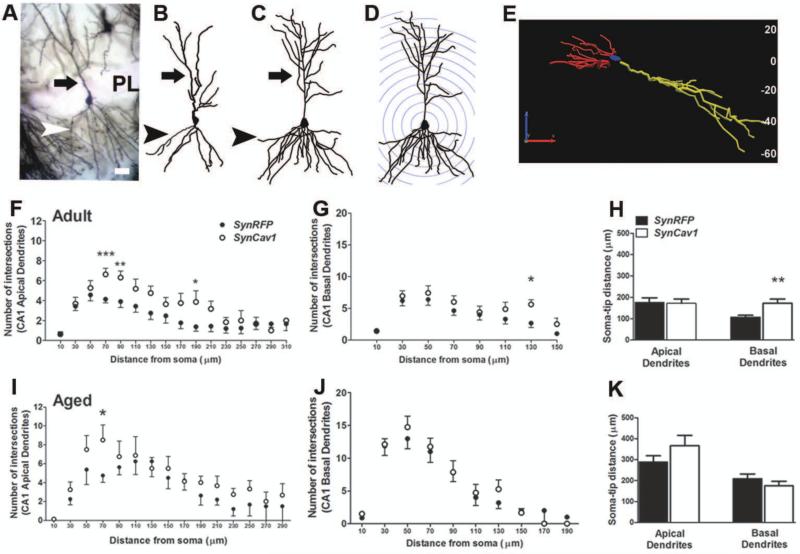
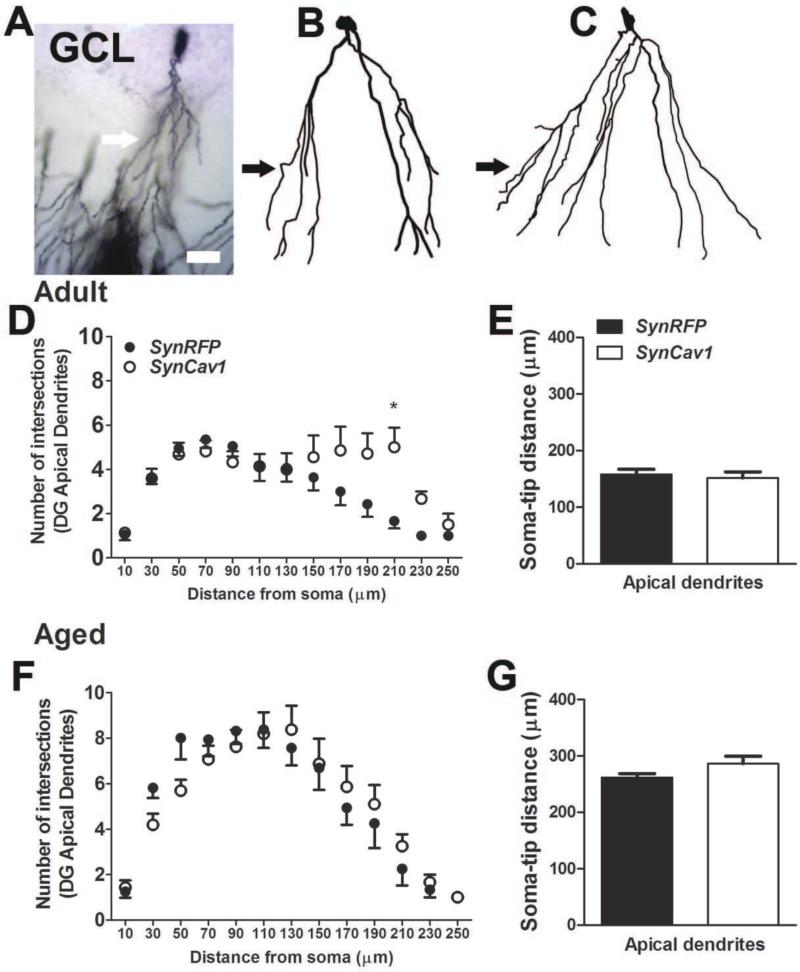
Mice were sacrificed by rapid decapitation (5% isoflurane) and whole brain was quickly removed and submerged in Golgi-Cox solution A + B (FD Neurotechnologies Inc.) for 8 days at room temperature, followed by solution C for 4 days at room temperature and stored at −80°C until processed for staining. Frozen brain tissue was coronally cut on a cryostat at 80-μm-thick sections and stained with solution D + E and dehydrated according to manufacturer's instructions. To evaluate hippocampal neuron morphology, a Zeiss AxioImager microscope and a computer-based system (Neurolucida; Micro-BrightField) were used to generate three dimensional neuron tracings that were subsequently visualized and analyzed using NeuroExplorer (MicroBrightField). For each animal, two to five CA1 pyramidal neurons and three to four dentate gyrus (DG) granule cell neurons were traced at 40x magnification with an oil immersion lens (equipped with a 10x eye piece) (Fig. 3a-e and 4a-c). For CA1 neurons, both the apical and basal dendrites were traced, whereas for DG neurons only apical dendrites were traced and morphological measurements were analyzed separately as described by Kim et al 2014 (36).
Figure 3.
SynCav1 enhances CA1 pyramidal neuron dendritic arborization and soma-tip length in adult mice. A-E, 3D reconstruction and Sholl ring analysis at 20 μm ring distance with a starting ring at 10 μm. A representative example of Golgi-Cox stained CA1 pyramidal neuron in the hippocampus (A) along with neuron tracings in Neurolucida (B, SynRFP; C, SynCav1) and corresponding 3D reconstruction and Sholl ring of the neuron in C (D). xyz orientation of the cell in C and the corresponding depth of the cell shown in micrometers are indicated in E. xyz analysis indicated that the entire cell in its xyz axis is within 80 mm thickness. Arrow in A-D points to the apical dendrites; arrowhead in A-D points to basal dendrites; scale bar in A represents 20 μm (applies AE). F-H, Total number of intersections of apical dendrites (F; filled circles, SynRFP in black and SynCav1 in white) and basal dendrites of CA1 pyramidal neurons (G), and length of soma to tip distance of the dendrites (H) in adult mice. I-K, Total number of intersections of apical dendrites (I; filled circles, SynRFP in black and SynCav1 in white) and basal dendrites of CA1 pyramidal neurons (J), and length of soma to tip distance of the dendrites (K) in aged mice. n = 4-6 animals in each group and neuron data are from 10-22 neurons in each group. ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 compared with SynRFP.
Figure 4.
SynCav1 enhances granule cell neuron dendritic arborization in adult mice. A-E, 3D reconstruction and Sholl ring analysis at 20 μm ring distance with a starting ring at 10 μm. A representative example of Golgi-Cox stained DG neuron in the hippocampus (A) along with neuron tracings in Neurolucida (B, SynRFP; C, SynCav1). Arrow in A-C points to the apical dendrites; scale bar in A represents 20 μm (applies A-C). D, Total number of intersections of apical dendrites (filled circles, SynRFP in black and SynCav1 in white) of DG granule cell neurons in adult mice. E, Length of soma to tip distance of the dendrites in adult mice. F, Total number of intersections of apical dendrites (filled circles, SynRFP in black and SynCav1 in white) of DG granule cell neurons in aged mice. G, Length of soma to tip distance of the dendrites in aged mice. n = 4-6 animals in each group and neuron data are from 16-22 neurons in each group. ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 compared with SynRFP.
Fear conditioning behavior
Fear conditioning was performed as previously described (37, 38). Foot shocks were delivered through the floor consisting of 36 stainless steel rods wired to a shock generator. Presentation of unconditioned stimuli (US: scrambled foot shock) and conditioned stimuli (CS: auditory tone) were controlled by computer (Med Associates Inc.). Freezing was determined using analysis software (Video Freeze®, Med Associates Inc., ANY-MAZE, San Diego Instruments). Training: After an acclimation period (2 min), mice were presented with a tone (CS: 90 dB, 5 kHz) for 30s that co-terminated with a foot shock (US: 2.0s, 1.0 mA) in dark chambers. A total of three tone-shock pairings were presented with a varying intertrial interval of 30-90s. Freezing was measured during each CS to measure fear acquisition level across groups. Context fear was tested twenty-four hours later. Cued fear was tested twenty-four hours after context fear. To remove contextual cues, the chambers were altered across several dimensions (odor - scent; visual – light chambers and walls are altered via plastic inserts; tactile - new floor covering) to minimize generalization from the conditioning context. The session started with a 3 min acclimation period, during which time no tones were presented (“pre-tone” period), then 10 blocks of 5 CSs were presented for 30s each with an inter-trial interval of 5s. Freezing was recorded during each CS presentation. For analysis, total freezing was averaged as total freezing during all CS presentations.
Statistical analyses
Data were checked for normal distribution and then analyzed with 2-way ANOVA, Mann-Whitney or student's t-test. Following significant effects with 2-way ANOVA, unpaired t-tests were performed as appropriate. For the analysis we used GraphPad Prism 6 (La Jolla, CA). Data are compared as SynRFP versus SynCav1 and presented as mean ± SEM. Significance was assumed when p ≤ 0.05. Experimental groups were blinded to the experimenters and code was broken only for analysis.
Results
SynCav1 significantly enhances TrkB, CT-B and Cav-1 in adult and aged hippocampal MLR fractions
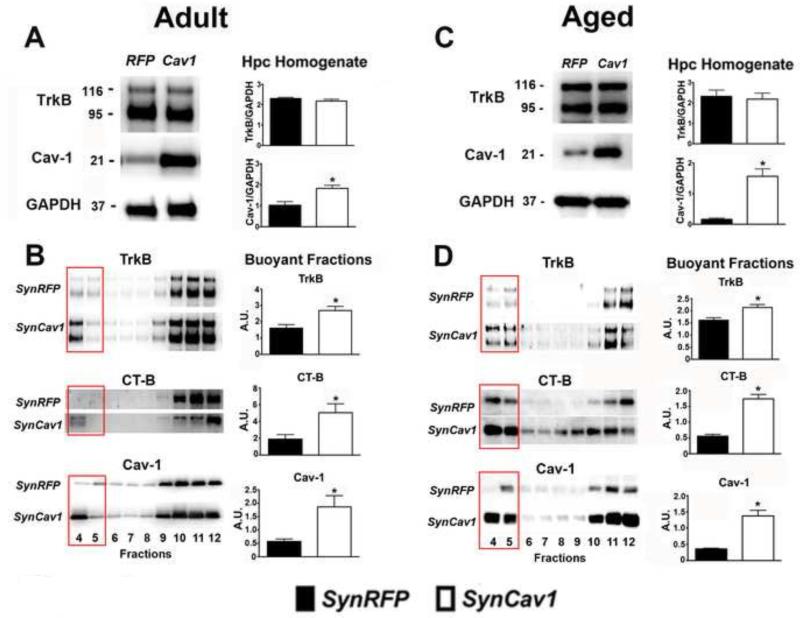
To test whether SynCav1 gene delivery in vivo increased MLR formation in adult and aged mice (Figure 1), hippocampal tissue was homogenized and subjected to sucrose density fractionation followed by Western blot analysis. Whole hippocampal tissue homogenates demonstrated a significant increase in Cav-1 protein expression in both adult (~ 1.8 fold over SynRFP; t8 = 3.55; p = 0.008; n = 5/group; Figure 2A) and aged (~ 9.7 fold over SynRFP; t7 = 6.45; p < 0.001; n = 4-5/group; Figure 2C) brains after SynCav1 gene delivery. No significant change in TrkB whole hippocampal homogenate was found in either adult (Figure 2A) or aged (Figure 2C) brains. Sucrose density fractionation of these hippocampal homogenates revealed a significant increase in TrkB expression, CT-B binding (indicative of increased gangliosides and MLR formation), and Cav-1 expression in the MLR fraction of adult (Figure 2B; TrkB: t8 = 3.17; p = 0.01; CT-B: t8 = 2.64; p = 0.03; Cav-1: t8 = 3.00; p = 0.02; n = 5/group) and aged mice (Figure 2D; TrkB: t7 = 3.37; p = 0.01; CT-B: t8 = 7.83; p < 0.001; Cav-1: t6 = 4.38; p = 0.005; n = 3-5/group), indicating that SynCav1 overexpression produced significant biochemical and subcellular changes in the hippocampus of both adult and aged mice compared with SynRFP overexpression.
Figure 2.
SynCav1 significantly enhances TrkB, CT-B and Cav-1 in adult and aged hippocampal membrane/lipid raft fractions. Hippocampal homogenates from adult (A) and aged (C) mice were western blotted for TrkB, Cav-1, and GAPDH. Hippocampal tissue was subjected to sucrose density fractionation followed by western blot analysis for TrkB, CT-B and Cav-1. Quantitation of protein expression in MLR (buoyant fractions 4 & 5 are denoted by an open rectangle box) isolated from adult hippocampi (B). Quantitation of protein expression in MLR (buoyant fractions 4 & 5) isolated from aged hippocampi (D). Six month and 20 month old mice per gene injection from the behavioral cohorts were used for quantitation. All fractions were generated from equal protein loading of 0.5 mg/ml. Data represent arbitrary units (A.U.) mean ± SEM. Significance was assumed when *p ≤ 0.05.
SynCav1 enhances dendritic arborization of CA1 and DG neurons in the hippocampus in adult and aged mice
In adult mice, three-dimensional Sholl analysis of CA1 (Figure 3A-E) and DG (Figure 4A-C) neurons from each group (n = 4-6 mice/group, 2-5 neurons per animal) demonstrated that SynCav1 significantly increased dendrite arborization in CA1 pyramidal neurons (Figure 3F-G; effect of Cav-1, apical: F1,388 = 13.82, p < 0.001; basal: F1,200 = 4.2, p = 0.04 by 2-way ANOVA), and increased total basal dendrite length (Figure 3H; t37 = 2.90; p = 0.006 by unpaired t test) with no significant difference in total apical dendrite length (p = 0.89). Enhanced arborization was evident in apical dendritic segments 70 μm (p = 0.001), 90 μm (p = 0.009), and 190 μm (p = 0.02) from soma and basal dendritic segments 130 μm (p = 0.02) from soma by unpaired t test. SynCav1 did not alter the number of dendritic spines on CA1 apical dendrites (number of spines per 10 μm: 16.9 ± 0.74 vs 18.9 ± .81; t30 = 1.89; p = 0.07) nor basal dendrites (number of spines per 10 μm: 18.1 ± .67 vs 18.4 ± .75; t30 = 0.25; p = 0.80). In aged mice, SynCav1 significantly increased CA1 apical dendrite arborization (Figure 3I; effect of Cav-1, F1,166 = 4.8, p = 0.03; by 2-way ANOVA), without altering arborization within basal dendrites (Figure 3J; effect of Cav-1, p = 0.29) and total apical (p = 0.20) or basal (p = 0.32) dendrite length of CA1 neurons (Figure 3K). Enhanced arborization was evident in apical dendritic segment 70 μm from soma (p = 0.05 by unpaired t test).
Within the dentate gyrus, SynCav1 significantly increased apical dendrite arborization in the DG neurons (effect of Cav-1, F1,318 = 3.85, p = 0.05 by 2-way ANOVA, Figure 4D), with no difference in soma-tip distance (Figure 4E; p = 0.67) in adult mice. Enhanced arborization was evident in apical dendritic segments at 210 μm from soma (p = 0.03). SynCav1 did not alter the apical dendrite arborization in the DG neurons of aged mice (Figure 4F; effect of Cav-1, p = 0.75) nor soma to tip distance (Figure 4G; p = 0.11).
SynCav1 enhances contextual fear memory dependent on the hippocampus in adult and aged mice
Mice were weighed and baseline tested for gross motor function and anxiety in the open field paradigm to exclude any obvious dysfunction before testing learning and memory behaviors (supplementary figure 3; all p > 0.05). To assess the behavioral consequence of SynCav1, we performed hippocampal injections with either SynCav1 or SynRFP in young adult mice (4 month) and then monitored the mice at 6 months (2 month post gene delivery) in the fear conditioning paradigm (Figure 5A-C). We performed an unpaired t-test on baseline freezing, context freezing, and cued freezing measures after testing for normality and exclusion of outliers using ROUT's method (Q = 1%). A 2-way ANOVA with gene as the between subject factor and tone as a within subject factor was performed for percent freezing during the learning phase on day 1. Fear conditioning revealed no baseline difference in freezing behavior between groups (SynRFP: 3.88 ± 0.61 vs SynCav1: 5.47 ± 0.62; t25 = 1.83; p = 0.08; n = 13-14/group) and during the acquisition period, indicated by no significant effect of ‘gene’ (F1,25 = 1.58, p = 0.22) or ‘time * gene’ - interaction (F2,50 = 0.12, p = 0.89) (Figure 5D; n = 13-14/group) by repeated measure 2-way ANOVA. In both groups, similar increases in freezing over time with repeated CS-US pairing trials were apparent (F2,50 = 56.64, p < 0.001). On day 2, SynCav1-injected mice showed significantly increased freezing response during the contextual recall test, an effect specific to hippocampal-dependent learning and memory (39) (Figure 5E; t26 = 2.19; p = 0.037; n = 14/group). No significant difference between groups was observed during exposure to the cue in an altered context on day 3 (Figure 5F; p = 0.12; n = 14/group).
Figure 5.
SynCav1 gene delivery improves contextual fear learning and memory in adult and aged mice. Schematic detailing the fear conditioning protocol for (A) training day one, (B) context testing day two, and (c) cue testing day three. SynCav1 gene delivery improves contextual fear learning and memory in young adult mice. D, SynRFP and SynCav1 (n = 13-14/group) injected adult mice (4 months) showed similar acquisition in response to the conditioned stimulus at 6 months of age; this was reflected in A significant effect of time during the repetitive exposure of the tone/shock pairings, with no significance for ‘gene’ or ‘time * gene’ – interaction. E, SynCav1-injected mice exhibited increased freezing to context re-exposure (p = 0.037). F, No significant difference between groups was observed after re-exposure to the conditioned stimulus in the altered context. G, SynRFP and SynCav1 (n = 17-20/group) injected mice (10 months) showed similar acquisition in response to the conditioned stimulus at 20 months of age; this was reflected in a significant effect of time during the repetitive exposure of the tone/shock pairings. H, SynCav1-injected mice exhibited increased freezing to context re-exposure (p = 0.017). I, No significant difference between groups was observed after re-exposure to the conditioned stimulus in the altered context. Data are presented as mean ± SEM. Significance was assumed when p ≤ 0.05.
To assess the behavioral consequence of SynCav1 in aged mice (Figure 5G-I), we performed hippocampal injections with either SynCav1 or SynRFP in middle aged mice (10 month) and then monitored the mice at 20 months (10 month post gene deliver) in the fear conditioning paradigm. We performed unpaired t-tests on the baseline freezing, context freezing, and cued freezing measures after testing for normality and exclusion of outliers using ROUT's method (Q=1%). A 2-way ANOVA with group as the between subject factor and trial as a within subject factor was performed for percent freezing during the learning phase on day 1. Fear conditioning revealed no baseline difference between groups (SynRFP: 7.80 ± 1.18 vs SynCav1: 8.55 ± 1.27; t33 = 0.44; p = 0.66; n = 17-18/group) and responses during the acquisition period, represented by no significant effect of ‘gene’ (F1,35 = 0.01, p = 0.92) or ‘time * gene’ - interaction (F2,70 = 0.47, p = 0.62; Figure 5G; n = 17-20/group) by repeated measures 2-way ANOVA. In both groups, similar increases in freezing over time with repeated CS-US pairing trials were apparent (F2,70 = 98.36, p < 0.001). On day 2, SynCav1-injected mice showed significantly increased freezing response during the contextual recall test, an effect specific to hippocampal-dependent learning and memory (39) (Figure 5H; t33 = 2.52; p = 0.017; n = 17-18/group). No significant difference between groups was observed during exposure to the cue in an altered context on day 3 (Figure 5I; t34 = 1.23; p = 0.23; n = 17-19/group).
Discussion
Although previous work has shown that markers of MLRs, such as CT-B (40, 41) and/or flotillins (42, 43), are elevated during neuronal sprouting and repair after CNS trauma, the functional significance of overexpression of a MLR-associated scaffold protein, Cav-1, in the adult and aged brain is unknown. In this study, we have demonstrated that hippocampus-targeted overexpression of Cav-1 in adult and aged mice recruits TrkB receptors to MLRs in the hippocampus. These molecular events were accompanied by morphological alterations in hippocampal neurons, visualized as enhanced dendritic arborization within apical dendrites in adult and aged mice, indicating enhanced structural plasticity of these neurons. Moreover, the molecular and structural changes in the hippocampus in animals overexpressing Cav-1 specifically in neurons were associated with improved hippocampal-dependent behavior, suggesting direct functional effects from Cav-1 on neuroplasticity and memory. Furthermore, our biochemical findings demonstrate that Cav-1 regulates the subcellular distribution of TrkB towards membrane microdomains (i.e., MLRs) in vivo, a cellular event necessary for TrkB activation, which in turn promotes structural plasticity and improves cognitive function. Although the mechanism underlying Cav-1-induced TrkB relocation to MLRs in vivo is not known, Cav-1 may regulate TrkB endocytosis and recycling as suggested by recent in vitro evidence for Cav-1 mediated increases in TrkB activation (i.e., phosphorylation) and MLR localization via enhanced sphingolipids and Rac-mediated signaling (44).
In the adult mammalian brain, inhibitory/repulsive molecules present a major obstacle for nerve regeneration after injury and diseases (45). Thus, understanding neurotrophin-induced signal transduction mechanisms in the context of neuronal growth and guidance will provide potential strategies to combat growth inhibition of regenerating nerve fibers. For many guidance cues, the formation of ligand-receptor complexes on the plasma membrane in neuronal subdomains represents the first step in signal transduction, followed by additional cellular events that result in synaptic plasticity (46). The fact that these important events occur at or within the plasma membrane suggests that the membrane lipid environment is crucial for signal transduction of these extracellular cues. Notably, recent in vitro data indicates that the lipid environment of the plasma membrane plays a role in guidance signaling, and membrane components including MLRs are important for functional receptor-signaling complexes to generate specific signaling cascades for distinct dendritic and axonal responses (1, 3, 47-49). Furthermore, BDNF-TrkB signaling within MLRs plays a role in chemotrophic guidance of nerve growth cones in adult brain and neurons isolated from adult cortex, indicating a functional role for MLRs in mediating localized signal transduction during growth cone guidance (24, 50, 51). In the adult rodent brain, BDNF is heavily expressed in the hippocampus, which is the anatomical component of learning and memory, and hippocampal neurons undergo several forms of plasticity during adulthood (27, 29, 52).
Importantly, the present findings in adult mice demonstrate that neuron-specific overexpression of Cav-1 in the hippocampus induces recruitment of TrkB receptors to MLRs and produces enhancements in dendritic structure of hippocampal neurons and hippocampal dependent behaviors. The observation that overexpression of Cav-1 in the hippocampus of adult mice significantly enhanced CA1 apical and basal dendrite arborization and total spine density in the stratum radiatum and stratum oriens and enhanced granule cell apical dendritic arborization approximately 150-200 μm distal from soma indicates that functional MLRs promote structural and potentially functional plasticity (53). These results suggest a novel strategy of enhancing neuroplasticity in the aged hippocampus that specifically rely on manipulation of MLRs and their associated components (including neurotrophins, current findings, and other MLR-associated glutamatergic receptor systems (9).
It is possible that genetic interventions that increase MLRs and concurrently neuronal structural plasticity have the potential to improve behavior in the aged brain. For example, a key brain region that is impaired during aging is the hippocampus (27). The aging brain demonstrates region-specific alterations in neuronal morphology, reduced structural plasticity (30, 31) and dendritic branching (54), and a decreased capacity to regenerate (27, 55). With age the brain undergoes changes in plasma membrane biophysical properties (i.e., decreased cholesterol, gangliosides, and phosphoinositides) (56), impaired cholesterol synthesis and lipoprotein transport (55), changes in the molecular composition of the postsynaptic membrane (54) accompanied with reduced MLR-associated synaptic proteins (57), and diminished pre-synaptic vesicle exocytosis and subsequent neurotransmitter release; the latter are due to cholesterol depletion or cholesterol redistribution from cyto to exofacial leaflet of the plasma membrane ultimately leading to altered recruitment of SNARE complexes (56). Akin to these findings, previous work from our group demonstrated that Cav-1 and synaptic components (e.g., PSD-95, NMDAR, AMPAR, TrkB) are decreased in the aged brain, specifically in synaptosomes and MLRs isolated from the hippocampus (33, 54). Age-related changes in membrane lipid composition and concomitant reduction in functional synaptic components (both pre- and post-synaptic) may contribute to the maladaptive plasticity of the aged brain. Although some therapeutic approaches attempt to initiate structural and functional plasticity through delivery of exogenous pro-growth stimuli (such as neurotrophins (58)), the lack of efficacy may be due to the absence of receptors and their downstream effector molecules from the appropriate plasma membrane microdomains. Therefore, interventions that promote MLR formation in neurons may enhance the signaling efficacy of endogenous neurotransmitters and neurotrophins that converge upon pro-growth signaling. The present findings show through neuroanatomical and biochemical analysis of hippocampal tissue from aged mice that underwent cognitive testing that Cav-1 overexpression significantly enhanced dendritic arborization of CA1 hippocampal neurons in the stratum radiatum of aged mice, results similar to that seen in the adult mice. Furthermore, Cav-1 overexpression in aged animals enhanced hippocampal Cav-1 expression and MLR formation (as indicated by CT-B binding to GM1 gangliosides), and TrkB localization to MLR fractions. These findings in aged animals show that neuron-targeted overexpression of Cav-1 enhances MLR formation, TrkB recruitment to MLRs and structural plasticity of hippocampal neurons, which perhaps produces neuritogenesis and synapse maintenance/stabilization.
Certain cognitive abilities such as executive function, episodic memory, working memory, short-term recollection, the speed of processing new information, and spatial memory that are dependent on the hippocampus are greatly compromised with age (27, 59). These age-related changes render the older brain more susceptible to other forms of neurodegenerative disorders like Alzheimer's disease (26, 27, 60). Several studies have suggested that decreases in synapses and total neuronal loss within regions of the hippocampus more closely correlate with hippocampal dependent cognitive impairment (26, 39). Contextual memory is multisensory and hippocampal-dependent, and involves a combination of spatial, temporal, interoceptive (e.g., hormonal, hunger, stress), cognitive (i.e., understanding the encoding), and social (i.e., understanding the world around us) processing. Therefore, we performed a fear-conditioning paradigm on aged mice (20 month) and found that Cav-1 overexpresssion in the hippocampus significantly enhanced hippocampal-dependent contextual fear memory, but not cued fear memory. The latter is predominantly regulated by the amygdala and medial prefrontal cortex (39). A minor limitation of the present study is that a behavioral test was used that did not demonstrate an aging-induced deficit in hippocampal dependent memory (no difference between adult and aged mice in context and cued freezing behavior). Therefore we speculate that Cav-1 overexpression could potentially improve behaviors that are impaired in aged subjects. Nevertheless, these behavioral findings reaffirm the biochemical and neuroanatomical results and extend the notion that Cav-1 overexpression enhances structural and perhaps functional hippocampal plasticity, and ultimately improves cognitive function.
Restoration of synaptic plasticity and preservation of cognitive function in the aged brain remains a major medical challenge. Understanding how age-related changes affect synaptic membrane protein clustering within neurons may yield therapeutic targets for the purpose of restoring functional MLR, neuronal membrane expansion, and synapse formation that is necessary to promote functional plasticity and reverse age-related behavioral decline. Genetic interventions that re-establish the proper subcellular membrane regions necessary to restore pro-growth and pro-survival signaling have the potential to increase the efficacy of pharmacologic agents designed to improve functional outcomes. The ability of Cav-1 to significantly enhance functional hippocampal neuronal growth and plasticity and improve hippocampal-dependent (i.e., contextual) learning and memory in aged mice holds considerable potential as a novel therapeutic to restore brain function during the aging process.
Supplementary Material
Acknowledgements
The authors thank Airee Kim and Alvaro Ivan Navarro for their assistance with Golgi-Cox analysis. The authors thank McKenzie Fannon for editorial assistance. Work in the authors’ laboratories is supported by Veteran Affairs Merit Award from the Department of Veterans Affairs BX001225 (B. P. Head), BX000783 (D. M. Roth), and BX001963 (H. H. Patel), National Institutes of Health, Bethesda, MD, U.S.A., NS073653 (B. P. Head); HL091071 and HL107200 (H. H. Patel); GM085179 (P. M. Patel) and DA034140 (C. D. Mandyam).
Abbreviations
- AAV9
adeno-associated virus serotype 9
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- CT-B
cholera toxin subunit B
- CS-US
conditioned stimulus-unconditioned stimulus
- CA
cornu ammonis
- DG
dentate gyrus
- MLR
membrane/lipid rafts
- SynCav1
synapsin caveolin-1
- SynRFP
synapsin red fluorescent protein
- TrkB
tropomyosin receptor kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kamiguchi H. The region-specific activities of lipid rafts during axon growth and guidance. J Neurochem. 2006;98:330–335. doi: 10.1111/j.1471-4159.2006.03888.x. [DOI] [PubMed] [Google Scholar]
- 2.Fantini J, Barrantes FJ. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochimica et biophysica acta. 2009;1788:2345–2361. doi: 10.1016/j.bbamem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Da Silva JS, Hasegawa T, Miyagi T, Dotti CG, Abad-Rodriguez J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nature neuroscience. 2005;8:606–615. doi: 10.1038/nn1442. [DOI] [PubMed] [Google Scholar]
- 5.Denny JB. Molecular Mechanisms, Biological Actions, and Neuropharmacology of the Growth-Associated Protein GAP-43. Curr Neuropharmacol. 2006;4:293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guirland C, Zheng JQ. Membrane lipid rafts and their role in axon guidance. Adv Exp Med Biol. 2007;621:144–155. doi: 10.1007/978-0-387-76715-4_11. [DOI] [PubMed] [Google Scholar]
- 7.Willmann R, Pun S, Stallmach L, Sadasivam G, Santos AF, Caroni P, et al. Cholesterol and lipid microdomains stabilize the postsynapse at the neuromuscular junction. The EMBO journal. 2006;25:4050–4060. doi: 10.1038/sj.emboj.7601288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, et al. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- 9.Head BP, Hu Y, Finley JC, Saldana MD, Bonds JA, Miyanohara A, et al. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. The Journal of biological chemistry. 2011;286:33310–33321. doi: 10.1074/jbc.M111.255976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, et al. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. The Journal of biological chemistry. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 11.Grande-Garcia A, del Pozo MA. Caveolin-1 in cell polarization and directional migration. Eur J Cell Biol. 2008;87:641–647. doi: 10.1016/j.ejcb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 12.de Kreuk BJ, Nethe M, Fernandez-Borja M, Anthony EC, Hensbergen PJ, Deelder AM, et al. The F-BAR domain protein PACSIN2 associates with Rac1 and regulates cell spreading and migration. J Cell Sci. 2011;124:2375–2388. doi: 10.1242/jcs.080630. [DOI] [PubMed] [Google Scholar]
- 13.Del Pozo MA, Schwartz MA. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. doi: 10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Nakai Y, Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. The Journal of cell biology. 2002;159:1097–1108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. The Journal of cell biology. 2002;157:521–532. doi: 10.1083/jcb.200109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annual review of biochemistry. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Butz S, Ying Y, Anderson RG. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. The Journal of biological chemistry. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 21.Huang CS, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, et al. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. The Journal of biological chemistry. 1999;274:36707–36714. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- 22.Peiro S, Comella JX, Enrich C, Martin-Zanca D, Rocamora N. PC12 cells have caveolae that contain TrkA. Caveolae-disrupting drugs inhibit nerve growth factor-induced, but not epidermal growth factor-induced, MAPK phosphorylation. The Journal of biological chemistry. 2000;275:37846–37852. doi: 10.1074/jbc.M000487200. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. The EMBO journal. 2003;22:1790–1800. doi: 10.1093/emboj/cdg177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, et al. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. The Journal of cell biology. 2004;167:1205–1215. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira DB, Chao MV. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4859–4869. doi: 10.1523/JNEUROSCI.4587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leal SL, Yassa MA. Perturbations of neural circuitry in aging, mild cognitive impairment, and Alzheimer's disease. Ageing research reviews. 2013;12:823–831. doi: 10.1016/j.arr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Alzheimer's Disease Neuroimaging I. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Progress in neurobiology. 2014;117C:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison JH, Baxter MG. Synaptic health. JAMA psychiatry. 2014;71:835–837. doi: 10.1001/jamapsychiatry.2014.380. [DOI] [PubMed] [Google Scholar]
- 29.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 30.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Progress in neurobiology. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 31.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature reviews Neuroscience. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Experimental neurology. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 2010;5:e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titus DJ, Furones C, Kang Y, Atkins CM. Age-dependent alterations in cAMP signaling contribute to synaptic plasticity deficits following traumatic brain injury. Neuroscience. 2013;231:182–194. doi: 10.1016/j.neuroscience.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niesman IR, Schilling JM, Shapiro LA, Kellerhals SE, Bonds JA, Kleschevnikov AM, et al. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. Journal of neuroinflammation. 2014;11:39. doi: 10.1186/1742-2094-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain structure & function. 2015;220:1705–1720. doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. The European journal of neuroscience. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 38.Gresack JE, Risbrough VB, Scott CN, Coste S, Stenzel-Poore M, Geyer MA, et al. Isolation rearing-induced deficits in contextual fear learning do not require CRF(2) receptors. Behavioural brain research. 2010;209:80–84. doi: 10.1016/j.bbr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alto LT, Havton LA, Conner JM, Hollis ER, 2nd, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nature neuroscience. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nature neuroscience. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santiago JM, Torrado AI, Arocho LC, Rosas OR, Rodriguez AE, Toro FK, et al. Expression profile of flotillin-2 and its pathophysiological role after spinal cord injury. Journal of molecular neuroscience : MN. 2013;49:347–359. doi: 10.1007/s12031-012-9873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch JC, Solis GP, Bodrikov V, Michel U, Haralampieva D, Shypitsyna A, et al. Upregulation of reggie-1/flotillin-2 promotes axon regeneration in the rat optic nerve in vivo and neurite growth in vitro. Neurobiology of disease. 2013;51:168–176. doi: 10.1016/j.nbd.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Trovo L, Van Veldhoven PP, Martin MG, Dotti CG. Sphingomyelin upregulation in mature neurons contributes to TrkB activity by Rac1 endocytosis. Journal of cell science. 2011;124:1308–1315. doi: 10.1242/jcs.078766. [DOI] [PubMed] [Google Scholar]
- 45.McKerracher L, Winton MJ. Nogo on the go. Neuron. 2002;36:345–348. doi: 10.1016/s0896-6273(02)01018-8. [DOI] [PubMed] [Google Scholar]
- 46.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annual review of neuroscience. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 47.Brown DA, London E. Functions of lipid rafts in biological membranes. Annual review of cell and developmental biology. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 48.Simons K, Toomre D. Lipid rafts and signal transduction. Nature reviews Molecular cell biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 49.Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr. Lipid rafts in neuronal signaling and function. Trends in neurosciences. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- 50.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 52.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J. The Hippocampus Book. Oxford University Press, Inc.; New York, New York: 2007. [Google Scholar]
- 54.Henley JM, Wilkinson KA. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues in clinical neuroscience. 2013;15:11–27. doi: 10.31887/DCNS.2013.15.1/jhenley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin M, Dotti CG, Ledesma MD. Brain cholesterol in normal and pathological aging. Biochimica et biophysica acta. 2010;1801:934–944. doi: 10.1016/j.bbalip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Ledesma MD, Martin MG, Dotti CG. Lipid changes in the aged brain: effect on synaptic function and neuronal survival. Progress in lipid research. 2012;51:23–35. doi: 10.1016/j.plipres.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Jiang L, Fang J, Moore DS, Gogichaeva NV, Galeva NA, Michaelis ML, et al. Age-associated changes in synaptic lipid raft proteins revealed by two-dimensional fluorescence difference gel electrophoresis. Neurobiology of aging. 2010;31:2146–2159. doi: 10.1016/j.neurobiolaging.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conte V, Raghupathi R, Watson DJ, Fujimoto S, Royo NC, Marklund N, et al. TrkB gene transfer does not alter hippocampal neuronal loss and cognitive deficits following traumatic brain injury in mice. Restorative neurology and neuroscience. 2008;26:45–56. [PMC free article] [PubMed] [Google Scholar]
- 59.Remy F, Mirrashed F, Campbell B, Richter W. Mental calculation impairment in Alzheimer's disease: a functional magnetic resonance imaging study. Neuroscience letters. 2004;358:25–28. doi: 10.1016/j.neulet.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 60.Yankner BA, Lu T, Loerch P. The aging brain. Annual review of pathology. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.