Abstract
Background
Psychosocial stress is thought to play a key role in the acceleration of immunological aging. This study investigated the relationship between lifetime and past-year history of post-traumatic stress disorder (PTSD) and the distribution of T cell phenotypes thought to be characteristic of immunological aging.
Methods
Data were from 85 individuals who participated in the community-based Detroit Neighborhood Health Study. Immune markers assessed included the CD4:CD8 ratio, the ratio of late-differentiated effector (CCR7-CD45RA+CD27-CD28-) to naïve (CCR7+CD45RA+CD27+CD28+) T cells, the percentage of KLRG1-expressing cells, and the percentage of CD57-expressing cells.
Results
In models adjusted for age, gender, race/ethnicity, education, smoking status, and medication use, we found that past-year PTSD was associated with statistically significant differences in the CD8+ T cell population, including a higher ratio of late-differentiated effector to naïve T cells, a higher percentage of KLRG1+ cells, and a higher percentage of CD57+ cells. The percentage of CD57+ cells in the CD4 subset was also significantly higher and the CD4:CD8 ratio significantly lower among individuals who had experienced past-year PTSD. Lifetime PTSD was also associated with differences in several parameters of immune aging.
Conclusions
PTSD is associated with an aged immune phenotype and should be evaluated as a potential catalyzer of accelerated immunological aging in future studies.
Keywords: Aging, Detroit, Immunity, Immunosenescence, Post traumatic stress disorder, T cells
1. Introduction
Several studies have shown that the T cell repertoire differs greatly between older and younger individuals. Most notably, older individuals tend to have a greater abundance of late-stage differentiated memory T cells and fewer naïve T cells (Bisset et al., 2004; Provinciali et al., 2009; Xu et al., 1993). Psychosocial stress is a key modulator of activity of the neuroendocrine system, and in particular, the hypothalamic–pituitary–adrenal axis and the sympathetic–adrenal–medullary systems, which are known to affect the immune system and contribute to immune aging (Effros, 2011; Pace and Heim, 2011). Moreover, stress has been demonstrated to play a key role in T cell homeostasis (Nakata, 2012; Scanlan et al., 1998).
Post-traumatic stress disorder (PTSD) is a specific stress-related physiological and psychological response that can occur after a traumatic event that elicits fear, helplessness or horror following the threat of injury or death (American Psychiatric Association, 2013). Importantly, PTSD has been implicated in a number of autoimmune comorbidities such as rheumatoid arthritis, psoriasis and asthma (Boscarino, 2004; Boscarino et al., 2010; Qureshi et al., 2009; Shiratori and Samuelson, 2012), suggesting that the immune system is affected by PTSD. Indeed, increases in serum or mitogen-induced levels of inflammatory cytokines including IL-1β, IL-6, TNF-α and dysregulation of the complement cascade have been observed among PTSD patients (Gill et al., 2008; Gola et al., 2013; Hovhannisyan et al., 2010; Spivak et al., 1997; Tucker et al., 2010; von Känel et al., 2010, 2007). These immunological changes appear to be controlled at the epigenetic level (Rusiecki et al., 2013; Smith et al., 2011; Uddin et al., 2010b). Additionally, shortened leukocyte telomere lengths, enhanced T cell responses, a decrease in the population of naïve CD8+ T-cells, and an increase in central memory and effector CD8+ T cells have been observed among PTSD patients (Ladwig et al., 2013; Pace and Heim, 2011; Sommershof et al., 2009). Taken together, PTSD may play a key role in the acceleration of immune aging, broadly defined as immunosenesence.
To the best of our knowledge, no studies have assessed the association between PTSD and the distribution of T cell phenotypes thought to be characteristic of immunological aging using data drawn from a community-based sample. In the present study, we address this gap in the literature using data from a community-based sample of adults who participated in the Detroit Neighborhood Health Study. Specifically, we examined the association between PTSD and the distribution of T cell phenotypes as measured by late-stage differentiated poorly or non-proliferative effector (E, CCR7-CD45RA+CD27-CD28-) to naïve (N, CCR7+CD45RA+CD27+CD28+) T cell ratio (E:N ratio), the percentage of KLRG1-expressing cells, and the percentage of CD57-expressing cells in both the CD4+ and CD8+ T-cell subsets, as well as the CD4:CD8 T-cell ratio. (Pawelec et al., 2009b; Wikby et al., 2005b) We hypothesized that individuals who had experienced PTSD would have a higher E:N ratio, a greater percentage of KLRG1 and CD57 expressing cells, and a lower CD4:CD8 ratio compared to trauma-exposed individuals who had not experienced PTSD.
2. Materials and methods
2.1. Study population
The data for this study were derived from the 2008–2009 Detroit Neighborhood Health Study (DNHS), a community-based longitudinal study of PTSD and other mental health outcomes among adults aged 18 or older living in Detroit. Participants were selected through a probability sample of households within Detroit city limits and one adult from each household was allowed to respond. Consenting participants were administered a 40-min telephone survey using the Composite International Diagnostic Interview which included mental and physical health assessments, and then asked to provide blood specimens by venipuncture or blood spot. Of the 1547 respondents who participated in the first wave of the survey (2008–2009), 612 volunteered blood specimens. Of these 612 subjects with blood specimens, a balanced sample of 100 respondents was selected based on socioeconomic position (SEP), PTSD, and age. For the selection of this sample, SEP was dichotomized based on high school completion or less versus some college or more. PTSD was dichotomized based upon the PTSD checklist score described below of <30 or ≥30 (Weathers and Ford, 1996). Approximately equal proportions of individuals 18–49 years of age and 50 years of age or older were selected within each unique combination of PTSD and SEP. The sample was limited to only participants that had viable immunological cell phenotype data, resulting in a final sample of 85 subjects spanning 19–83 years of age. The DNHS was approved by the institutional review boards at the University of Michigan and the University of North Carolina at Chapel Hill.
2.2. Ascertainment of PTSD status
PTSD symptoms were assessed during the baseline phone interview. As described in previous work (Uddin et al., 2010b), PTSD history was assessed via a 17-item checklist (PCL-C) (Weathers and Ford, 1996), a self-report measure of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) symptoms of PTSD (American Psychiatric Association, 1994), and using additional questions about duration, timing, and impairment or disability due to the symptoms. Two PTSD exposures were assessed, including lifetime PTSD and past year PTSD. If the participant met all six DSM-IV criteria for PTSD (American Psychiatric Association, 1994) for either of two traumatic events over their lifetime, the participant was considered a lifetime PTSD case. Lifetime PTSD cases who reported experiencing PTSD symptoms in the prior year were additionally classified as past year PTSD cases. This measure was validated in the DNHS sample by a trained counselor during randomly selected in-person reappraisals among 51 DNHS survey participants using the Clinician-Administered PTSD Scale for DSM-IV. Results of validation showed that the measure had a sensitivity (SE) of 0.24, specificity (SP) of 0.97, positive predictive value (PPV) of 0.80, negative predictive value (NPV) 0.72, and an area under the ROC curve (AUC) of 0.76.
2.3. Quantification of T-cell phenotypes
T cell subsets from frozen peripheral blood mononuclear cells (PBMC) as described previously (Weckle et al., 2015). Briefly, PBMCs were purified from whole blood by centrifugation through a Ficoll gradient-containing tube (BD Vacutainer CPT). PBMCs were isolated within two hours of blood draw. Cells were frozen at a controlled rate using a freezing medium of 10% Dimethyl Sulfoxide (DMSO)/20% Fetal Bovine Serum (FBS)/70% (Roswell Park Memorial Institute 1640). Frozen samples were stored at −80 °C and after 24 h were transferred to a cryobox and put in a liquid nitrogen tank. The samples were shipped on dry ice and analyzed within a year of the collection date using 10-color flow cytometry methods described previously (Derhovanessian et al., 2013) by the Tübingen Ageing and Tumor Immunology Group at the University of Tübingen, Germany. All staining steps were performed in PFEA buffer (PBS, 2% FCS, 2 mM EDTA and 0.01% Azide). Cryopreserved PBMCs were thawed and treated with human immunoglobulin, GAMUNEX and ethidium monoazide (EMA) for 10 min on ice to block Fc receptors on the cells and label non-viable cells. Cells were then stained with a primary anti-KLRG-1 antibody (kindly provided by Prof. HP Pircher, Freiburg, Germany) for 20 min at 4°C followed by staining with Pacific-Orange-conjugated goat-anti-mouse secondary antibody for another 20 min on ice. Mouse serum was added for 15 min to block non-specific binding to anti-mouse secondary antibody, followed by addition of directly-conjugated monoclonal antibodies (mAb), CD3-AlexaFluor 700, CD4-PerCP, CD8-APC-Cy7, CD27-APC, CD28-PE, CD45RA-Pacific Blue, CCR7-PE-Cy7, and CD57-FITC. After 20 min incubation on ice in the dark, cells were washed twice and measured immediately on a BD-LSR-II flow cytometer with FACSDiva software. The spectral overlap between all channels was calculated automatically by the BD FACSDiva software after measuring negative and single-color controls. DNHS samples were compared to PBMCs from the same healthy donor to detect any technical bias in measurement.
Flow cytometry data were analyzed using FlowJo software (Tree Star, Portland, OR) and T cell subsets were characterized by surface expression as described previously (Derhovanessian et al., 2010). T cell data consisted of percentages of T cells (CD3+) out of total lymphocytes, and were characterized as CD4+ (CD3+CD4+CD8-) or CD8+ (CD3+CD8+CD4-). CD4+ and CD8+ T cells were further analyzed based on surface expression of markers to determine the percentage of naïve T cells (N, CCR7+CD45RA+CD27+CD28+) and the percentage of late-stage differentiated poorly or non-proliferative effector cells (E, CCR-CD45RA+CD27-CD28-, also known as TEMRA cells) (Romero et al., 2007). In addition, we quantified the percentage of KLRG1+ and CD57+ cells within the CD4 and CD8 T cells subsets. These data were used to generate seven out-comes for the analysis, including three ratio measures: (1) CD4:CD8 ratio, (2) E:N ratio among CD4+ T cells, (3) E:N ratio among CD8+ T cells, and four percentage measures: (4) percentage of KLRG1+ cells among CD4+ T cells, (5) percentage of KLRG1+ cells among CD8+ T cells, (6) percentage of CD57+ cells among CD4+ T cells, and (7) percentage of CD57+ cells among CD8+ T cells. All of the T cell outcomes were treated as continuous variables and the three ratio outcomes (CD4:CD8 ratio, CD4 E:N ratio, and CD8 E:N ratio) were natural log-transformed to approximate a normal distribution.
2.4. Assessment of covariates
Covariates hypothesized to be potential confounders of the association between PTSD and markers of immune aging included age, gender, race/ethnicity, SEP, cigarette use, and medication use. These factors have been shown to predict other measures of immune status and may also be correlated with PTSD. Age was treated continuously and divided by 10 in order to produce estimates based on a 10-year increase in age for ease of interpretation. Individuals self-reported their race/ethnicity and this variable was dichotomized as Black/African American or other. SEP was defined based on educational attainment and dichotomized as: high school completion or less versus some college or more. Lifetime history of cigarette use was self-reported and individuals were categorized as ever versus never smokers. Medications were assessed at the time of the blood draw, during which clinicians recorded the name, dosage, and frequency of all prescription and over-the-counter medications taken by the participant in the past month. Medication names were entered into a database and classified according to the Centers for Disease Control and Prevention Ambulatory Care Drug Database System (Centers for Disease Control and Prevention, 2005). We created groups of drugs for treatment categories that could be correlated with immune function (i.e., antimicrobial agents, cardiovascular-renal drugs, central nervous system medications, metabolic and nutrient agents, hormones and agents affecting hormonal mechanisms, immunologic agents, oncolytics, and drugs used for relief of pain). For analytical purposes, medication usage was dichotomized as currently taking any medication that may impact immune function or not.
2.5. Statistical analysis
All statistical analyses were carried out using SAS version 9.3 (SAS Institute, Inc., Cary, NC). Descriptive statistics were used to describe the overall sociodemographic and clinical characteristics of the study populations. Counts and proportions were estimated to assess the distribution of categorical variables and medians with interquartile ranges (IQR) were estimated for continuous variables. We also assessed the age-adjusted associations between participant characteristics and the three T-cell ratio outcome measures (CD4:CD8 ratio, CD4 E:N ratio, and CD8 E:N ratio) using ordinary least-squares (OLS) linear regression, as well as the age-adjusted associations between participant characteristics and the four T-cell percentage outcome measures (CD4%KLRG1+, CD4%CD57+, CD8%KLRG1+, and CD8%CD57+) using beta regression in order to accommodate the skewed outcome distributions, with the best fit parameterization of the precision model for each participant characteristic.
Next, we estimated the associations between past year and lifetime history of PTSD and each of the T-cell outcome measures, separately. OLS linear regression was used to estimate the association between the two PTSD measures and ratio outcomes and beta regression to estimate the association between PTSD measures and percentage outcomes. These models were first adjusted for age, gender, race/ethnicity, and education and then additionally adjusted for smoking status and medication use. Age was used as the only precision parameter for the beta regression models. For all analyses, estimates with a P value <0.05 were considered statistically significant.
We conducted two secondary analyses. In the first, we additionally adjusted the associations between past year and lifetime history of PTSD and each of the T-cell outcome measures for cytomegalovirus (CMV) seropositivity, as CMV is thought to be a primary contributor to age-related declines in T-cell mediated immunity (Derhovanessian et al., 2011; Hadrup et al., 2006; Khan et al., 2002; Pawelec, 2014; Pawelec and Derhovanessian, 2011a; Pawelec et al., 2009a) and PTSD has been linked to increases in CMV IgG antibody levels (Uddin et al., 2010a). CMV serostatus was assessed in frozen serum samples, which were shipped on dry ice to the Stanley Neurovirology Laboratory of the Johns Hopkins University School of Medicine in Baltimore, Maryland. The presence and quantity of serum IgG antibodies to CMV were tested via solid phase enzyme-linked immunosorbent assays (ELISAs), as described previously (Dickerson et al., 2003). Briefly, diluted aliquots of serum were reacted with antigen bound to a solid-phase surface. Quantitation of virus-specific IgG was determined by reaction of bound antibodies with enzyme labeled anti-human IgG and enzyme substrate; optical densities were read by spectrophotometric instrumentation. For each sample, the antibody levels were expressed as the ratio of the optical density of a test sample to that of a standard sample assayed in each test run. Individuals were categorized as seropositive for CMV if their OD ratio value was ≥1.0.
In the next secondary analysis, we examined whether PTSD symptom severity showed consistent associations with the T-cell outcomes. In this analysis, a variable for PTSD symptom severity was created by summing participant scores (1–5, with 1 indicating no symptoms) for each of 17 questions that assessed the severity of psychological symptom associated with the trauma that led to their PTSD, as has been done in previous studies (Sipahi et al., 2014). Thus, the scores for each individual could range from 17 to 85.
3. Results
Participants' sociodemographic and clinical characteristics by PTSD status is shown in Table 1. Overall, participants were a median of 44 years of age (IQR: 35–54 years) and 81.2% were Black or African American. Females, who made up 62.4% of the study population, were slightly older than males (median age 47 versus 39 years). Slightly less than half (48.2%) of participants had more than a high school education. Of the 85 participants, 22.4% ever experienced PTSD in their lifetime and 16.5% reported experiencing PTSD in the past year. Compared to individuals who had never experienced PTSD, individuals who had experienced PTSD in the past year were slightly less likely to identify as Black or African American and slightly more likely to be female and to have smoked cigarettes.
Table 1.
Sociodemographic and clinical characteristics of 85 participants in the Detroit Neighborhood Health Study.
| Overall (N=85) |
PTSD status | |||
|---|---|---|---|---|
|
| ||||
| No PTSD (N = 66) |
Lifetime PTSD (N=19) |
Past year PTSD (N= 14) |
||
| Age in years, median (IQR) | 44 (35–54) | 44 (35–54) | 44 (33–58) | 47 (33–58) |
| Gender, N (%) | ||||
| Female | 53 (62.4) | 41 (62.1) | 12 (63.2) | 10 (71.4) |
| Male | 32 (37.7) | 25 (37.9) | 7 (36.8) | 4 (28.6) |
| Race/ethnicity, N (%) | ||||
| Black | 69 (81.2) | 55 (83.3) | 14 (73.7) | 10 (71.4) |
| Non-black | 16 (18.8) | 11 (16.7) | 5 (26.3) | 4 (28.6) |
| Education, N (%) | ||||
| ≤High school | 44 (51.8) | 33 (50.0) | 11 (57.9) | 7 (50.0) |
| >High school | 41 (48.2) | 33 (50.0) | 8 (42.1) | 7 (50.0) |
| Medication usea, N (%) | ||||
| Yes | 35 (42.7) | 28 (43.7) | 7 (38.9) | 5 (38.5) |
| No | 47 (57.3) | 36 (56.3) | 11 (61.1) | 8 (61.5) |
| Cigarette use in lifetime, N (%) | ||||
| Yes | 51 (60.0) | 37 (56.1) | 14 (73.7) | 9 (64.3) |
| No | 34 (40.0) | 29 (43.9) | 5 (26.3) | 5 (35.7) |
| PBMC count (Millions of cells), median (IQR) | 21.00 (17.60–26.80) | 21.52 (17.32–26.25) | 20.75 (17.85–29.35) | 20.90 (18.10–29.35) |
Abbreviations: PTSD, posttraumatic stress disorder; E:N ratio, ratio of end-stage non-proliferative effector cells (E; CCR7-CD45RA+CD27-CD28-orTEMRA) to naïve T-cells (N; CCR7+CD45RA+CD27+CD28+).
Medications included antimicrobial agents, cardiovascular-renal drugs, central nervous system medications, metabolic and nutrient agents, hormones and agents affecting hormonal mechanisms, immunologic agents, oncolytics, and drugs used for relief of pain. 3 individuals were missing data on medication use.
Table 2 shows the age-adjusted association between participants' sociodemographic and clinical characteristics and each T-cell outcome. Increasing age was significantly associated with a higher E:N ratio, a higher percentage of KLRG1+ cells, and a higher percentage of CD57+ cells in both the CD4 and CD8 T cell subsets. Females had a significantly lower E:N ratio in the CD8 T cell subset only. Self-identification as Black or African American was also associated with a more aged phenotype for all of the T cell outcomes, but the associations were not statistically significant. The distribution of each T cell outcome by PTSD status is shown in Fig. 1.
Table 2.
Age adjusted associations between participant characteristics and the distribution of T-cell phenotypes among 85 participants in the Detroit Neighborhood Health Study.
| β (95% confidence intervals) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CD4 | CD8 | ||||||
|
|
|
||||||
| CD4:CD8 ratioa | E:N ratioa | %KLRG1b | %CD57b | E:N ratioa | %KLRG1b | %CD57b | |
| Each 10-year increase in age | 0.00 (−0.05, 0.06) | 0.56 (0.16, 0.95)* | 0.14 (0.03, 0.25)* | 0.17 (0.05, 0.28)* | 0.55 (0.33, 0.77)* | 0.21 (0.12, 0.31)* | 0.20 (0.08, 0.31)* |
| Females versus males | 0.15 (−0.04, 0.34) | 0.23 (−1.13, 1.59) | −0.12 (−0.50, 0.26) | 0.01 (−0.39, 0.42) | −0.74 (−1.48, −0.002)* | −0.22 (−0.56, 0.12) | −0.32 (−0.70, 0.07) |
| Black/African American versus other | −0.11 (−0.35, 0.12) | 0.81 (−0.85, 2.46) | 0.09 (−0.38, 0.56) | 0.44 (−0.06, 0.94) | 0.22 (−0.71,1.15) | 0.23 (−0.11, 0.56) | 0.27 (−0.20, 0.75) |
| ≤High school education versus >High school | −0.02 (−0.21, 0.16) | −0.49 (−1.78, 0.79) | −0.19 (−0.56, 0.17) | −0.16 (−0.54, 0.22) | 0.27 (−0.45, 0.98) | 0.13 (−0.20, 0.46) | 0.14 (−0.23, 0.51) |
| Medication use versus no medication usec | 0.05 (−0.15, 0.25) | −0.53 (−1.94, 0.87) | −0.32 (−0.77, 0.13) | −0.20 (−0.62, 0.22) | −0.36 (−1.16, 0.45) | −0.08 (−0.44, 0.29) | −0.13 (−0.54, 0.28) |
| Cigarette use versus no cigarette use | −0.08 (−0.26, 0.11) | 0.72 (−0.59, 2.03) | 0.13 (−0.25, 0.50) | 0.13 (−0.26, 0.52) | 0.20 (−0.54, 0.93) | 0.00 (−0.34, 0.34) | 0.07 (−0.31, 0.45) |
| Lifetime PTSD versus no lifetime PTSD | −0.27 (−0.48, −0.05)* | 1.55 (0.04, 3.05)* | 0.56 (0.00,1.13)* | 0.65 (0.03,1.27)* | 0.92 (0.08, 1.76)* | 0.34 (−0.05, 0.73) | 0.55 (0.06, 1.05)* |
| Past-year PTSD versus no past-year PTSD | −0.36 (−0.60, −0.13)* | 1.91 (0.23,3.60)* | 0.77 (0.16,1.38)* | 0.47 (−0.01, 0.96) | 1.39 (0.47, 2.31)* | 0.55 (0.12, 0.98)* | 0.67 (0.20, 1.15)* |
Abbreviations: PTSD, posttraumatic stress disorder; E:N ratio, ratio of end-stage non-proliferative effector cells (E; CCR7-CD45RA+CD27-CD28-orTEMRA) to naïve T-cells (N; CCR7+CD45RA+CD27+CD28+).
p value <0.05.
Estimates are in log ratio units.
Estimates are in log odds.
Medications included antimicrobial agents, cardiovascular-renal drugs, central nervous system medications, metabolic and nutrient agents, hormones and agents affecting hormonal mechanisms, immunologic agents, oncolytics, and drugs used for relief of pain. 3 individuals were missing data on medication use.
Fig. 1.
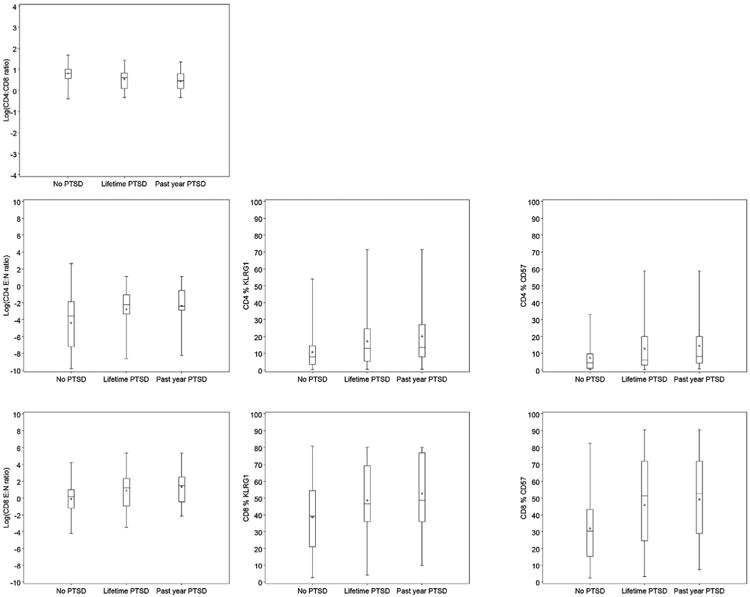
Distribution of T cell phenotypes by PTSD status among 85 participants in the Detroit Neighborhood Health Study.
The covariate-adjusted associations between past year PTSD and each T cell outcome are shown in Fig. 2. Adjusting for sociodemographics, smoking, and medication use (Model 2), we observed that individuals who had experienced PTSD in the past year had a 1.52 (95% CI: 0.56, 2.48) log-unit increase in the E:N ratio, a 0.32 (95% CI: 0.12, 0.52) unit increase in the percentage of KLRG1-expressing cells, and a 0.44 (95% CI: 0.17, 0.72) unit increase in the percentage of CD57-expressing cells in the CD8+T cell subset compared to individuals who had not experienced PTSD in the past year. Individuals who had experienced PTSD in the past year also showed similar increases in the CD4+ T cell subset, but the difference was only statistically significant for the percentage of CD57-expressing cells after adjusting for the full set of covariates. The covariate-adjusted associations between lifetime PTSD and each T cell outcome are shown in Fig. 3. The associations for lifetime PTSD were similar to those for past-year PTSD, but only the associations with the CD8 E:N ratio was statistically significant. Moreover, both lifetime (β: −0.32 [95% CI: −0.53, −0.10]) and past-year PTSD (β: −0.45 [95% CI: −0.68, −0.21]) were significantly associated with a decrease in CD4:CD8 ratio.
Fig. 2.
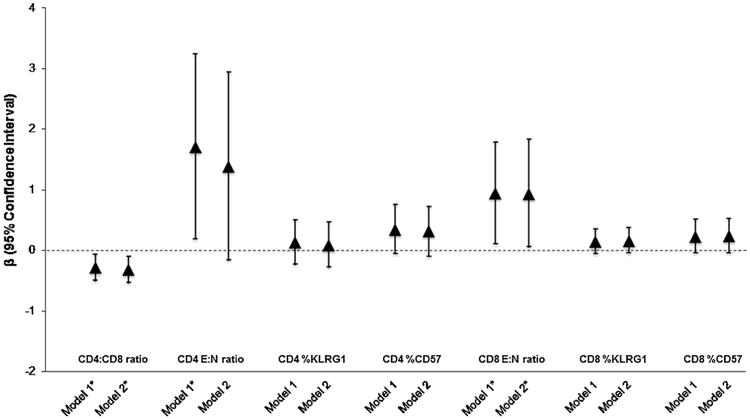
Covariate-adjusted associations between Lifetime PTSD and the distribution of T cell phenotypes among 85 participants in the Detroit Neighborhood Health Study. Abbreviations: PTSD, posttraumatic stress disorder; E:N ratio, ratio of end-stage non-proliferative effector cells (E; CCR7-CD45RA+CD27-CD28-orTEMRA) to naïve T-cells (N; CCR7+CD45RA+CD27+CD28 +).
*P value <0.05.
aModel 1: Association between PTSD and T-cell outcome adjusted for age, gender, race/ethnicity, and education.
bModel 2: Association between PTSD and T-cell outcome additionally adjusted for smoking status and medication use. 3 individuals missing data on medication use were excluded from the model.
Fig. 3.
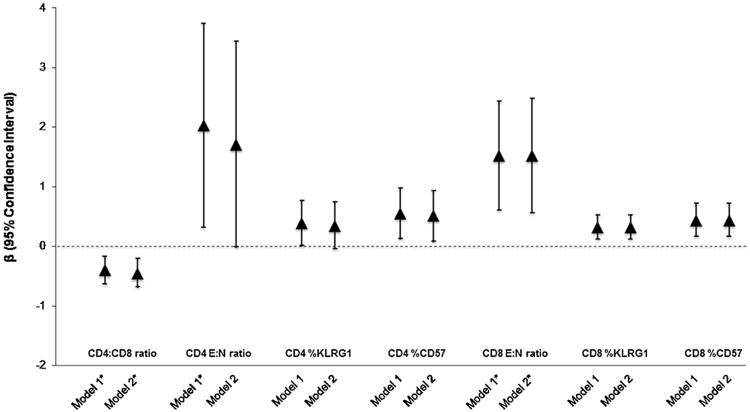
Covariate-adjusted associations between Past-Year PTSD and the distribution of T cell phenotypes among 85 participants in the Detroit Neighborhood Health Study. Abbreviations: PTSD, posttraumatic stress disorder; E:N ratio, ratio of end-stage non-proliferative effector cells (E; CCR7-CD45RA+CD27-CD28-orTEMRA) to naïve T-cells (N; CCR7+CD45RA+CD27+CD28 +).
*P value <0.05.
aModel 1: Association between PTSD and T-cell outcome adjusted for agegender, race/ethnicity, and education.
bModel 2: Association between PTSD and T-cell outcome additionally adjusted for smoking status and medication use. 3 individuals missing data on medication use were excluded from the model.
Supplemental Table 1 shows the covariate-adjusted associations between both past year and lifetime PTSD and each T cell outcome, additionally adjusting for cytomegalovirus seropositivity (see Models 3). Statistical significance remained unchanged for all associations, except that the association between lifetime PTSD and the CD8 E:N ratio was no longer statistically significant. Supplemental Table 2 shows the results for the analysis of PTSD symptom severity and the T cell outcomes, which were not statistically significant.
4. Discussion
This is the first study to examine the association between PTSD and immune aging using data drawn from a community-based study of individuals spanning a broad age range (19–83 years of age). We found that past year PTSD was significantly associated with a decrease in CD4:CD8 ratio, an increase in CD8 E:N ratio, increases in the populations of KLRG1- and CD57-expressing CD8+ T cells cells, and an increase in CD57+ CD4+ T cells even after adjusting for age, demographics, smoking, and medication use. Consistent associations were observed for lifetime PTSD, with statistically significant associations observed for the CD4:CD8 and CD8 E:N ratios. Taken together, our results suggest that exposure to PTSD may influence immunological aging.
Our findings are consistent with prior studies conducted in clinical populations that have demonstrated that PTSD patients have a lower percentage of naïve CD8+ T cells, a higher percentage of memory (CD45RA-) CD8+ T cells, and a lower percentage of regulatory (CD4+CD25+FoxP3+) T cells compared to controls (Morath et al., 2014; Sommershof et al., 2009). While participants in these studies were refugees with a history of war and torture experience residing in Germany, ours is the first to study the association between PTSD and immune aging in a US community-based sample spanning a broad age range (Morath et al., 2014; Sommershof et al., 2009). Although we did not assess regulatory T cells as was done in in the previous studies, we did assess the ratio of CD4:CD8 T cells, as well as the percentage of KLRG1- and CD57-expressing cells in the CD4+ and CD8+ T cell subsets. CD4:CD8 ratio inversion is a marker of altered immune function associated with increased morbidity and mortality (Ferguson et al., 1995). KLRG1 and CD57 are surface receptors that deliver negative signals to the cells bearing them and are commonly considered as markers of the latest stage of T cell differentiation and potentially true senescence (Brenchley et al., 2003; Derhovanessian et al., 2010; Voehringer et al., 2002). Our focus on late-stage differentiated T cells (i.e., effector or TEMRA CCR7-CD45RA+CD27-CD28-), based on the studies of Romero et al. (2007), allowed us to obtain a more complete picture of the immune phenotype of study participants than previous studies. Thus, in addition to supporting previously reported findings pertaining to the accumulation of end stage memory T cells and depletion of the naïve T cell pool among those with PTSD, we also show decreased CD4:CD8 ratio and increases in KLRG1- and CD57-expressing CD8+ cells among those with past year PTSD, suggesting an aged immune system phenotype among those with recent PTSD.
In the present paper, we focus on the distribution of T cell phenotypes that research suggests are key biomarkers of immunosenescence (Lang et al., 2013; Pawelec, 2012). Immunosenescence has been an intensive area of research because it has been shown to adversely affect not just immune response to novel infection, but also susceptibility to and morbidity associated with a range of health conditions (Lang et al., 2013; Pawelec and Derhovanessian, 2011b; Wikby et al., 2005a, 2006). Indeed, older individuals have an increased incidence of infectious diseases, autoimmune disorders, immunodeficiencies, allergies, and cancer compared to younger individuals. Older individuals also respond less effectively to interventions that target the immune system, such as immunizations and cancer immunotherapy. However, while the T cell outcomes assessed in this observational study provide key information on general immune processes and aging, it should be noted that none of these markers provide specific information on the functionality of the immune system. Future studies of PTSD in relation to immune system aging that additionally incorporate linkages to functionality are warranted. It should also be noted that, in this paper, we have focused on the most informative phenotypes of the many different permutations possible using polychromatic flow cytometry and presented the most informative results. Larger planned studies will be better able to distinguish whether other differentiation stages are more or less associated with the outcomes measured.
Another limitation of the present study is that we were unable to ascertain timing in regards to exposure to early life stress and PTSD onset. Although we did not have specific information on early life stress, we did have information on early life trauma exposure and these events were captured in our PTSD exposure assessment. We also did not examine the potential influence of comorbid mental health conditions given our limited sample size. As almost half (47.4%) of study participants who met the criteria for lifetime PTSD also met the criteria for major depressive disorder or generalized anxiety disorder, future studies should assess how these potentially interrelated pathways impact the immunological outcomes examined here.
In our analysis, we differentiated between associations resulting from past year PTSD and lifetime PTSD, revealing that the impact of PTSD on the immune system may be greatest in the period directly following episodes of PTSD symptoms. Indeed, Sommershof et al. (2009) demonstrated a dose–response-like effect of trauma on T cell outcomes whereby a trauma-exposed non-PTSD group displayed a phenotype intermediate to the PTSD and control groups. Alternatively, it is possible that alterations to the T-cell repertoire resulting from trauma exposure may reverse over time. However, an intervention study testing the impact of narrative exposure therapy on PTSD symptoms and T cell outcomes found no impact on naïve or memory T cell percentages although PTSD symptoms were alleviated in the intervention group (Morath et al., 2014). We found that most T cell changes associated with experiencing PTSD in the past year were also associated with ever experiencing PTSD, although the associations were not as strong and in some instances not statistically significant. An important limitation of the present study is that the small sample size (n = 85) may have precluded detection of statistically significant results for some associations. Nonetheless, these results support the conclusion that recent PTSD symptoms are more robustly associated with concurrent signs of an aged immune system than lifetime PTSD. While it is possible that lifetime PTSD may play a smaller role in immune system aging, further prospective studies are needed to identify whether lifetime PTSD symptoms lead to life long immune aged profiles or whether the effects wane over time.
Overall, the present study adds to the mounting evidence from the literature that PTSD is associated with a phenotype of accelerated immune system aging (Lohr et al., 2015). The exact mechanisms underlying these associations remain unclear, but it is likely that the psychosocial stress associated with PTSD has strong influences on the neuroendocrine system, which may directly and indirectly contribute to immune aging, for example through the hypothalamic–pituitary–adrenal axis and the sympathetic–adrenal–medullary systems (Effros, 2011; Pace and Heim, 2011). Moreover, evidence suggests that individuals who experience PTSD demonstrate a distinct network of immune-system related genes (Breen et al., 2015) and future studies in this area may help uncover whether lower methylation in immune-system related genes or higher expression in those genes may be driving the T cell phenoytpoes observed in the present study. More research of mechanisms underlying the association between PTSD and immune system aging are warranted and future studies assessing the outcome of PTSD-specific therapies should assess whether the detrimental effects of PTSD on the immune system can be reversed.
Supplementary Material
Acknowledgments
The Detroit Neighborhood Health Study, which provided data for this secondary analysis, was funded by the National Institutes of Health (grant numbers: DA22720 [DNHS], DA022720-S1 [DNHS PhenX], and RC1MH088283 [DNHS Epigenetics]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would also like to acknowledge the Stanley Medical Research Institute for Cytomegalovirus testing.
Role of the funding source: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2016.01.024
Contributors: Due to the highly interdisciplinary nature of the research study, the submitted paper has a total of 11 authors. All authors have approved the final version of the submitted manuscript. The contribution of each author is detailed below:
Dr. Aiello is the PI of the parent project from which the data were obtained. She oversaw data collection, analysis, and interpretation of data, contributed to drafting the manuscript, and critically revised the manuscript for important intellectual content.
Drs. Dowd, Jayabalasingham, Feinstein, and Simanek contributed to the study design, led implementation of epidemiological data analysis, contributed to drafting the manuscript, and critically revised the manuscript for important intellectual content.
Ms. Cheng contributed to the implementation of epidemiological data analysis, contributed to drafting the manuscript, and critically revised the manuscript for important intellectual content.
Dr. Pawelec helped select the immunological biomarkers assessed in this study, conducted all laboratory analyses and interpreted the results, and critically revised the manuscript for important intellectual content.
Dr. Galea was the original PI of the parent project from which the data for this paper were obtained. He was integral in the collection of socioeconomic data, contributed to the study design and interpretation of data, and critically revised the manuscript for important intellectual content.
Dr. Wildman contributed to the collection and processing of the biological samples required for the study and critically revised the manuscript for important intellectual content.
Drs. Uddin and Karestan contributed to the acquisition of biological samples and interpretation of data, contributed to drafting the manuscript and critically revised the manuscript for important intellectual content.
Contributor Information
Allison E. Aiello, Email: aaiello@email.unc.edu.
Jennifer B. Dowd, Email: jdowd@hunter.cuny.edu.
Bamini Jayabalasingham, Email: baminij@gmail.com.
Lydia Feinstein, Email: lfeinst@email.unc.edu.
Monica Uddin, Email: muddin@illinois.edu.
Amanda M. Simanek, Email: simaneka@uwm.edu.
Caroline K. Cheng, Email: kaiyi@med.umich.edu.
Sandro Galea, Email: sgalea@bu.edu.
Derek E. Wildman, Email: wildmand@illinois.edu.
Karestan Koenen, Email: kkoenen@hsph.harvard.edu.
Graham Pawelec, Email: graham.pawelec@uni-tuebingen.de.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), fourth ed. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V), fourth ed. American Psychiatric Press; Arlington, VA: 2013. [Google Scholar]
- Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann NY Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72:481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, Risbrough VB, Baker DG, O'Connor DT, Nievergelt CM, Woelk CH. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. 2015;20:1538–1545. doi: 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Ambulatory Care Drug Database System. 2005 < http://www2.cdc.gov/drugs/applicationnav1.asphttp://www2.cdc.gov/drugs/applicationnav1.asp>.
- Derhovanessian E, Maier AB, Beck R, Jahn G, Hähnel K, Slagboom PE, de Craen AJM, Westendorp RGJ, Pawelec G. Hallmark features of immunosenescence are absent in familial longevity. J Immunol (Baltimore, Md:1950) 2010;185:4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31:685–690. doi: 10.1016/j.vaccine.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46:135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995;50:B378–382. doi: 10.1093/gerona/50a.6.b378. [DOI] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Hovhannisyan LP, Mkrtchyan GM, Sukiasian SH, Boyajyan AS. Alterations in the complement cascade in post-traumatic stress disorder Allergy, asthma, and clinical immunology. J Can Soc Allergy Clin Immunol. 2010;6:3. doi: 10.1186/1710-1492-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, Codd V, Hafner S, Albrecht E, Illig T, Samani NJ, Wichmann HE, Gieger C, Peters A. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PO, Govind S, Aspinall R. Reversing T cell immunosenescence: why, who, and how. Age. 2013;35:609–620. doi: 10.1007/s11357-012-9393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, Thorp SR, Jeste DV. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23:709–725. doi: 10.1016/j.jagp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morath J, Gola H, Sommershof A, Hamuni G, Kolassa S, Catani C, Adenauer H, Ruf-Leuschner M, Schauer M, Elbert T, Groettrup M, Kolassa IT. The effect of trauma-focused therapy on the altered T cell distribution in individuals with PTSD: evidence from a randomized controlled trial. J Psychiatr Res. 2014;54:1–10. doi: 10.1016/j.jpsychires.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Nakata A. Psychosocial job stress and immunity: a systematic review. In: Yan Q, editor. Psychoneuroimmunology. Humana Press; 2012. pp. 39–75. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Hallmarks of human immunosenescence: adaptation or dysregulation? Immun Ageing. 2012;9 doi: 10.1186/1742-4933-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2014;54:1–5. doi: 10.1016/j.exger.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011a;157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011b;157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009a;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009b;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Moresi R, Donnini A, Lisa RM. Reference values forCD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerontology. 2009;55:314–321. doi: 10.1159/000199451. [DOI] [PubMed] [Google Scholar]
- Qureshi SU, Pyne JM, Magruder KM, Schulz PE, Kunik ME. The link between post-traumatic stress disorder and physical comorbidities: a systematic review. Psychiatr Q. 2009;80:87–97. doi: 10.1007/s11126-009-9096-4. [DOI] [PubMed] [Google Scholar]
- Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+T lymphocytes. J Immunol (Baltimore, Md:1950) 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, Byrne C, Galdzicki Z, Srikantan V, Chen L, Poulin M, Yan L, Baccarelli A. PTSD and DNA methylation in select immune function gene promoter regions: a repeated measures case-control study of U.S. Military Service members. Front Psychiatry. 2013;4:56. doi: 10.3389/fpsyt.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan JM, Vitaliano PP, Ochs H, Savage MV, Borson S. CD4 and CD8 counts are associated with interactions of gender and psychosocial stress. Psychosom Med. 1998;60:644–653. doi: 10.1097/00006842-199809000-00023. [DOI] [PubMed] [Google Scholar]
- Shiratori Y, Samuelson KW. Relationship between posttraumatic stress disorder and asthma among New York area residents exposed to the World Trade Center disaster. J Psychosom Res. 2012;73:122–125. doi: 10.1016/j.jpsychores.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Sipahi L, Wildman DE, Aiello AE, Koenen KC, Galea S, Abbas A, Uddin M. Longitudinal epigenetic variation of DNA methyltransferase genes is associated with vulnerability to post-traumatic stress disorder. Psychol Med. 2014;44:3165–3179. doi: 10.1017/S0033291714000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT. Substantial reduction of naïve and regulatory T cells following traumatic stress. Brain Behav Immun. 2009;23:1117–1124. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Tucker P, Jeon-Slaughter H, Pfefferbaum B, Khan Q, Davis NJ. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. Am J Disaster Med. 2010;5:113–125. doi: 10.5055/ajdm.2010.0013. [DOI] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010a;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010b;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- von Känel R, Begre S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17:39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Weathers F, Ford J. Psychometric review of PTSD checklist (PCL-C, PCL-S, PCL-M, PCL-R) In: Stamm B, editor. Measurement of Stress, Trauma, and Adaptation. Sidran Press; Lutherville, MD: 1996. [Google Scholar]
- Weckle A, Aiello AE, Uddin M, Galea S, Coulburn RM, Soliven R, Meier H, Wildman DE. Rapid fractionation and isolation of whole blood components in samples obtained from a community-based setting. J Vis Exp. 2015 doi: 10.3791/52227. (November (105)), http://dx.doi.org/10.3791/52227. [DOI] [PMC free article] [PubMed]
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Löfgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005a;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Löfgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005b;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Löfgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Xu X, Beckman I, Ahern M, Bradley J. A comprehensive analysis of peripheral blood lymphocytes in healthy aged humans by flow cytometry. Immunol Cell Biol. 1993;71(Pt 6):549–557. doi: 10.1038/icb.1993.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


