Abstract
Forty-three 1,5-diheteroaryl-1,4-pentadien-3-ones were designed as potential curcumin mimics, structurally featuring a central five-carbon dienone linker and two identical nitrogen-containing aromatic rings. They were synthesized using a Horner–Wadsworth–Emmons reaction as the critical step and evaluated for their cytotoxicity and antiproliferative activities toward both androgen-insensitive and androgen-sensitive prostate cancer cell lines and an aggressive cervical cancer cell line. Most of the synthesized compounds showed distinctly better in vitro potency than curcumin in the four cancer cell lines. The structure–activity data acquired from the study validated (1E,4E)-1,5-dihereroaryl-1,4-pentadien-3-ones as an excellent scaffold for in-depth development for clinical treatment of prostate and cervical cancers. 1-Alkyl-1H-imidazol-2-yl, ortho pyridyl, 1-alkyl-1H-benzo[d]imidazole-2-yl, 4-bromo-1-methyl-1H-pyrazol-3-yl, thiazol-2-yl, and 2-methyl-4-(trifluoromethyl)thiazol-5-yl were identified as optimal heteroaromatic rings for the promising in vitro potency. (1E,4E)-1,5-Bis(2-methyl-4-(trifluoromethyl)thiazol-5-yl)penta-1,4-dien-3-one, featuring thiazole rings and trifluoromethyl groups, was established as the optimal lead compound because of its good in vitro potency and attractive in vivo pharmacokinetic profiles.
Graphical abstract
INTRODUCTION
The significant difference in the rate of prostate cancer incidence between Western (120 per 100 000 in Northern America) and East Asian countries (less than 10 per 100 000 in Asia)1 and the soared risk of prostate cancer in the first generation of Asian men relocating to the United States2 imply the potential of Asian traditional food in preventing prostate cancer. Turmeric, the rhizomes of Curcuma longa, represents a typical Asian diet that has benefited people as a yellow spice used as curry ingredient and as a traditional Indian medicine for centuries. Scientists have identified curcumin (1, Figure 1) as the major chemical component responsible for the biological activity of turmeric. Not only having been demonstrated to have capability to prevent prostate cancer,3 curcumin has also been confirmed in 2000 by both in vitro cell culture systems and in vivo mice models to have potential in treating prostate cancer.4 Furthermore, its high safety profile in humans has been validated by the U.S. Food and Drug Administration (FDA).2,5 However, curcumin has difficulty in reaching blood system and its action targets, which has been verified by a phase I human clinical trial.6 This constitutes the critical problems of curcumin that prevent itself from becoming a clinical chemotherapeutic but has already triggered extensive research in search for curcumin mimics with improved potency and/or better pharmacokinetic profiles for the potential clinical treatment of cancers.3,7–16 Curcumin analogue 2, differing from curcumin by replacing the seven-carbon β-diketone linker of curcumin with a five-carbon dienone linear linker, showed 5- to 5.5-fold increase in cytotoxic potency relative to curcumin toward PC-3 prostate cancer cell line.17 Our previous preliminary data showed that 1,5-diheteroarylpenta-1,4-diene-3-ones, represented by compounds 3–5, is an optimal scaffold for developing curcumin mimics as potential anticancer agents due to their superior in vitro potency (up to 60 times better than curcumin) and potential pharmacokinetic profiles.16,18 This scaffold of curcumin mimics16,18 is characteristic of two identical terminal five- or six-membered heteroaromatic rings and a central linear dienone linker. They consistently exhibit enhanced cytotoxic potency than curcumin in androgen-insensitive prostate cancer cell lines (PC-3 and DU-145) but no apparent toxicity against MCF-10A normal mammary epithelial cells. Moreover, they might afford better bioavailability due to the existence of two basic nitrogen-containing heteroaromatic rings. It is therefore very meaningful to launch in-depth investigation of this class of curcumin mimics as potential drug candidates for the treatment of prostate and cervical cancers. Herein, we reported the synthesis and biological evaluation of forty-one new and two known 1,5-diheteroaryl-1,4-pentadien-3-ones, as well as their structure–activity relationship and pharmacokinetic studies.
Figure 1.
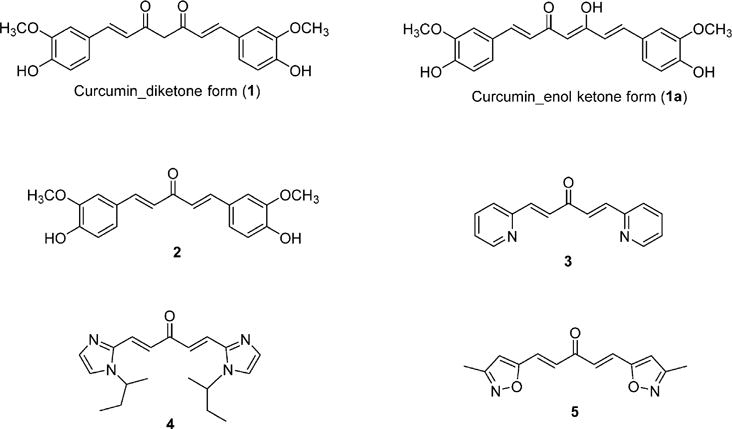
Structures of curcumin and its representative mimics.
It is worth noting that dienone moiety was flagged by Baell and Holloway as May Be PAINS (pan assay interference compounds)19 because the dienone moiety might covalently interact with proteins, which might lead to its nonselective cytotoxicity through interaction with one or more unidentified cellular proteins important for cell viability. However, as suggested by Baell,20 “with more medicinal chemistry, compound utility can be judged to be firmly in the positive based on clear SAR and optimization to low or mid nanomolar levels of activity.” We therefore consider that it is rational to retain compounds containing dienone motif that were apparently clean in our studies based on the following points: (i) our target compounds selectively exhibit cytotoxicity toward cancer cell lines but no apparent cytotoxicity against normal mammary epithelial cells;18 (ii) the structure–activity relationship studies indicate that our optimal compounds are over 100-fold more potent than compounds 17, 33, and 34 in in vitro cytotoxicity toward prostate and cervical cancer cells, and (iii) optimization of our target compounds has resulted in nanomolar level of cytotoxicity.
RESULTS AND DISCUSSION
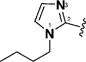
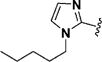
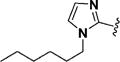
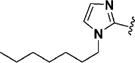
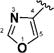
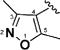
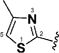
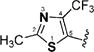
Encouraged by the potency conferred by the linear dienone linker and the basic aromatic rings,18 we set out to explore the effect of various heteroaromatic rings on the cytotoxicity of 1,5-diheteroarylpenta-1,4-dien-3-ones against prostate and cervical cancer cell lines. Consequently, forty-three compounds (6–48, Table 1 and Scheme 1) containing two identical terminal five-membered, six-membered, or bulky heteroaromatic rings and a central dienone linker have been designed and synthesized as curcumin mimics for cytotoxic and antiproliferative evaluation.
Table 1.
Structures of Heteroaromatic rings (BHR)
| Compd | BHR | Compd | BHR | Compd | BHR |
|---|---|---|---|---|---|
|
| |||||
| 6 & 49 |
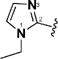
|
7 & 50 |
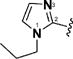
|
8 & 51 |
|
| 9 & 52 |
|
10 & 53 |
|
11 & 54 |
|
| 12 & 55 |
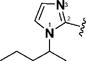
|
13 & 56 |
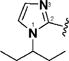
|
14 & 57 |
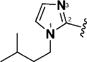
|
| 15 & 58 |
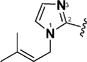
|
16 & 59 |
|
17 & 60 |
|
| 18 & 61 |
|
19 & 62 |
|
20 & 63 |
|
| 21 & 64 |
|
22 & 65 |
|
23 & 66 |
|
| 24 & 67 |
|
25 & 95 |
|
26 & 69 |
|
| 27 & 70 |
|
28 & 71 |
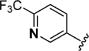
|
29 & 72 |
|
| 30 & 73 |
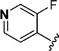
|
31 & 74 |
|
32 & 75 |
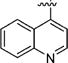
|
| 33 & 76 |
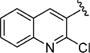
|
34 & 77 |
|
35 & 78 |
|
| 36 & 79 |
|
37 & 80 |
|
38 & 81 |
|
| 39 & 82 |
|
40 & 83 |
|
41 & 84 |
|
| 42 & 85 |
|
43 & 86 |
|
44 & 87 |
|
| 45 & 88 |
|
46 & 89 |
|
47 & 90 |
|
| 48 & 91 |
|
||||
Scheme 1.
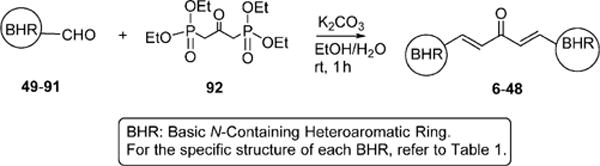
Synthesis of Curcumin-Based Compounds via a Horner–Wadsworth–Emmons Reaction
Chemistry
The designed forty-three 1,5-diheteroarylpenta-1,4-dien-3-ones (6–48) have been synthesized. All of them are new compounds except for compounds 27 and 29 that were included for systematic structure–activity relationship studies. According to the procedure described in the literature,21 the potassium carbonate-mediated double aldol condensation of the appropriate aromatic aldehyde with acetone was employed to successfully synthesize eleven 1,5-diheteroarylpenta-1,4-dien-3-ones in our previous study.18 However, most of target compounds for the current study were hard to obtain through the base-catalyzed aldol condensation. After various explorations, we eventually succeeded in synthesizing compounds 6–48 in good yields through the Hornor–Wadsworth–Emmons reaction of 1,3-bis(diethylphosphonato)acetone with the appropriate aromatic aldehyde22 (Scheme 1). The key reagent 1,3-bis(diethylphosphonato)acetone (92) for the synthesis of 6–48 was readily prepared from carbazic acid methyl ester, 1,3-dichloroacetone, and triethyl phosphite according to the procedure described in the literature.23 The aromatic carboxaldehydes (59–77) were available from commercial sources. 1-Alkyl-1H-imidazole-2-carbaldehydes (49–58) were synthesized from 1H-imidazole-2-carbaldyhyde (93) using potassium carbonate as base (Scheme 2) according to the procedure described in the literature.24 As illustrated in Schemes 3 and 4, benzo[d]thiazole-2-carbaldehyde (78) and 1-alkyl-1H-benzo[d]imidazole-2-carbaldehydes (79–91) were obtained by oxidation of the corresponding primary alcohols 95 and 98–110 with Dess–Martin reagent.25–27 (Benzo[d]-thiazole-2-yl)methanol (95) was synthesized by refluxing 2-aminothiophenol (94) with glycolic acid in hydrochloric acid (4 M) (Scheme 3). 1-Alkyl-1H-benzo[d]imidazole-2-yl)methanols (98–110) were achieved by N-alkylation of (1H-benzo[d]-imidazole-2-yl)methanol (97) with the appropriate alkyl halide using potassium carbonate as base and DMF as solvent (Scheme 4). (1H-Benzo[d]imidazole-2-yl)methanol (97) was obtained from benzene-1,2-diamine (96) by refluxing with glycolic acid in hydrochloric acid (4 M).
Scheme 2.
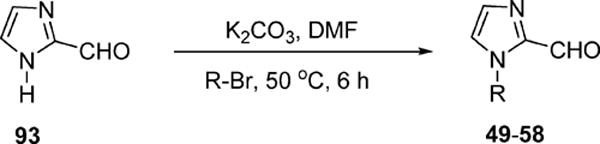
Synthesis of 1-Alkyl-1H-imidazole-2-carbaldehydes (49–58)
Scheme 3.
Synthesis of Benzo[d]thiazole-2-carbaldehyde (78)
Scheme 4.
Synthesis of 1-Alkyl-1H-benzo[d]imidazole-2-carbaldehydes (79–91)
Cytotoxicity toward Prostate and Cervical Cancer Cell Lines
The in vitro cytotoxicity of 1,5-diheteroarylpenta-1,4-dien-3-ones (6–48) was determined using trypan blue dye exclusion assay (TB) against a panel of cancer cell lines (PC-3, DU-145, LNCaP, and HeLa). Both PC-3 and DU145 cell lines are androgen-insensitive metastatic prostate cancer cells that cannot express prostate-specific antigen,28,29 while LNCaP cell line is androgen-sensitive and has capability to express prostate-specific antigen.30 They are the most common cell-based models for in vitro assessment of potency and efficacy of antiprostate cancer agents. Curcumin and DMSO were used as positive and negative control, respectively.
As shown in Table 2, with few exceptions, exposure of the cancer cells to most of the synthesized 1,5-diheteroarylpenta-1,4-dien-3-ones at 1 and 10 μM concentrations significantly decreases the viability of four cell lines. Among the series of compounds tested, thirty-three (6–15, 19, 21–26, 28–30, and 36–48) out of forty-three compounds displayed markedly improved ability to inhibit the growth of four cancer cell lines at both concentrations, as compared with curcumin. Seven compounds (16, 18, 20, 27, 31, 32, and 35) appeared to be as effective (or slightly more effective) as curcumin. Compounds 17, 33, and 34 were almost inactive even at 10 μM concentration. The IC50 values for fifteen compounds (10, 12–14, 19, 24, 26, 32, 36, and 43–48) in HeLa cells and for seven compounds (32, 36, and 43–47) in PC-3 cells were measured, which exhibit over 11–95 times higher cytotoxic potency than curcumin (Tables 3 and 4).
Table 2.
Cytotoxicity of 1,5-Diheteroarylpenta-1,4-dien-3-ones
| compd | inhibitory rate (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PC-3a
|
DU145b
|
LNCaPc
|
HeLad
|
|||||
| 10 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | |
| curcumin | 55.07 | 2.45 | 58.49 | 7.25 | 65.45 | 28.78 | 50.30 | 13.75 |
| 6 | 94.34 | 76.97 | 84.97 | 45.75 | 80.90 | 53.63 | 95.13 | 92.70 |
| 7 | 97.39 | 84.35 | 86.93 | 50.02 | 78.17 | 47.27 | 97.56 | 94.59 |
| 8 | 93.91 | 91.30 | 84.97 | 65.70 | 80.90 | 77.26 | 96.48 | 97.83 |
| 9 | 90.87 | 91.30 | 83.66 | 63.74 | 91.80 | 50.90 | 96.75 | 97.02 |
| 10 | 84.59 | 87.50 | 93.39 | 79.25 | 72.49 | 41.66 | 97.13 | 96.69 |
| 11 | 92.17 | 85.22 | 88.56 | 35.93 | 85.44 | 52.72 | 98.91 | 89.73 |
| 12 | 82.83 | 92.42 | 89.08 | 91.70 | 85.70 | 77.13 | 97.25 | 95.73 |
| 13 | 93.94 | 89.90 | 87.77 | 83.41 | 88.56 | 61.42 | 94.81 | 97.25 |
| 14 | 90.91 | 92.93 | 94.32 | 84.28 | 88.56 | 67.13 | 95.73 | 96.03 |
| 15 | 85.52 | 71.98 | 90.00 | 79.55 | 83.86 | 51.60 | 96.34 | 96.95 |
| 16 | 59.40 | 0.00 | 47.18 | 19.34 | 58.32 | 18.52 | 84.08 | 18.27 |
| 17 | 27.15 | 0.00 | 0.01 | 0.00 | 38.70 | 6.45 | 20.72 | 0.00 |
| 18 | 70.76 | 0.00 | 84.25 | 31.48 | 52.77 | 9.26 | 92.69 | 32.36 |
| 19 | 86.92 | 80.85 | 87.27 | 67.29 | 83.86 | 61.28 | 99.08 | 96.34 |
| 20 | 87.39 | 9.37 | 85.91 | 0.01 | 41.93 | 25.80 | 97.56 | 16.15 |
| 21 | 94.55 | 50.03 | 85.96 | 27.25 | 50.72 | 31.88 | 99.18 | 69.12 |
| 22 | 89.60 | 69.82 | 85.11 | 54.07 | 68.11 | 59.07 | 97.83 | 68.30 |
| 23 | 89.60 | 19.33 | 90.21 | 23.85 | 46.37 | 14.49 | 98.64 | 22.75 |
| 24 | 89.47 | 84.22 | 90.32 | 54.86 | 77.14 | 64.39 | 93.67 | 76.59 |
| 25 | 91.92 | 13.16 | 82.97 | 21.42 | 64.99 | 45.71 | 89.63 | 14.63 |
| 26 | 84.85 | 73.75 | 93.45 | 69.88 | 84.99 | 77.85 | 96.64 | 97.25 |
| 27 | 86.71 | 8.05 | 77.66 | 4.76 | 53.70 | 15.74 | 97.65 | 26.62 |
| 28 | 91.59 | 37.89 | 75.47 | 0.01 | 68.80 | 22.58 | 97.86 | 33.82 |
| 29 | 87.77 | 31.97 | 89.74 | 31.85 | 78.69 | 29.62 | 95.04 | 40.19 |
| 30 | 81.92 | 57.48 | 90.11 | 42.13 | 78.69 | 26.85 | 98.17 | 50.37 |
| 31 | 83.79 | 0.90 | 86.53 | 23.48 | 66.95 | 9.61 | 96.52 | 21.19 |
| 32 | 60.96 | 4.20 | 48.42 | 4.03 | 74.10 | 48.21 | 96.52 | 79.75 |
| 33 | 1.64 | 0.00 | 6.22 | 6.22 | 14.28 | 11.61 | 21.51 | 6.64 |
| 34 | 25.73 | 14.40 | 35.23 | 13.46 | 23.43 | 34.37 | 48.97 | 22.91 |
| 35 | 69.81 | 6.29 | 46.80 | 18.16 | 69.52 | 21.87 | 96.18 | 21.34 |
| 36 | 78.84 | 81.09 | 90.83 | 55.32 | 67.96 | 45.31 | 97.97 | 95.50 |
| 37 | 86.14 | 84.66 | 81.95 | 64.60 | 91.19 | 74.71 | 98.87 | 92.09 |
| 38 | 85.15 | 89.60 | 79.87 | 67.37 | 79.11 | 73.11 | 98.87 | 95.48 |
| 39 | 83.17 | 88.62 | 87.15 | 64.95 | 85.70 | 68.12 | 97.74 | 97.17 |
| 40 | 81.69 | 89.60 | 89.58 | 60.78 | 90.09 | 32.96 | 97.45 | 98.02 |
| 41 | 82.82 | 62.00 | 91.13 | 79.85 | 78.21 | 60.46 | 96.99 | 96.29 |
| 42 | 88.12 | 72.79 | 86.11 | 36.46 | 74.71 | 42.07 | 97.45 | 90.40 |
| 43 | 83.79 | 79.29 | 84.82 | 92.26 | 88.27 | 62.49 | 97.53 | 96.63 |
| 44 | 84.19 | 88.46 | 85.77 | 84.19 | 79.42 | 59.26 | 89.25 | 93.12 |
| 45 | 84.69 | 91.44 | 87.11 | 89.11 | 77.33 | 70.30 | 97.08 | 95.28 |
| 46 | 87.61 | 69.67 | 88.54 | 75.00 | 67.32 | 59.25 | 94.17 | 90.66 |
| 47 | 91.02 | 71.97 | 81.43 | 79.60 | 70.55 | 45.95 | 97.34 | 90.66 |
| 48 | 83.34 | 58.49 | 80.24 | 79.40 | 72.97 | 54.00 | 96.64 | 96.29 |
Human androgen-insensitive prostate cancer cell line.
Human androgen-insensitive prostate cancer cell line.
Human androgen-sensitive prostate cancer cell line.
Human aggressive cervical cancer cell line.
Table 3.
Antiproliferative Activity and Cytotoxicity of Selected 1,5-Diheteroarylpenta-1,4-dien-3-ones
| compd | IC50 (μM)a
|
|||||
|---|---|---|---|---|---|---|
| PC-3b
|
DU145,c WST-1 | LNCaP,d WST-1 | HeLae
|
|||
| WST-1 | TB | WST-1 | TB | |||
| curcumin | 25.43 ± 2.15 | 12.36 ± 0.04 | 26.23 ± 0.65 | 13.61 ± 2.69 | 12.11 ± 0.67 | 10.46 ± 1.97 |
| 6 | 1.07 ± 0.38 | 0.91 ± 0.08 | 0.59 ± 0.10 | 0.52 ± 0.25 | ||
| 7 | 1.53 ± 0.05 | 0.66 ± 0.14 | 0.78 ± 0.12 | 0.40 ± 0.05 | ||
| 8 | 0.68 ± 0.08 | 0.37 ± 0.02 | 0.49 ± 0.03 | 0.23 ± 0.04 | ||
| 9 | 0.71 ± 0.06 | 0.55 ± 0.14 | 0.59 ± 0.03 | 0.35 ± 0.04 | ||
| 10 | 0.90 ± 0.07 | 0.85 ± 0.14 | 1.13 ± 0.07 | 0.65 ± 0.02 | 0.59 ± 0.13 | |
| 12 | 0.31 ± 0.06 | 0.29 ± 0.05 | 0.24 ± 0.05 | 0.19 ± 0.08 | 0.15 ± 0.13 | |
| 13 | 0.69 ± 0.07 | 0.62 ± 0.13 | 0.58 ± 0.17 | 0.36 ± 0.02 | 0.25 ± 0.05 | |
| 14 | 0.71 ± 0.06 | 0.71 ± 0.11 | 0.57 ± 0.17 | 0.41 ± 0.02 | 0.23 ± 0.04 | |
| 15 | 0.60 ± 0.05 | 0.53 ± 0.12 | 0.39 ± 0.11 | 0.33 ± 0.03 | ||
| 19 | 0.51 ± 0.06 | 0.45 ± 0.13 | 0.31 ± 0.14 | 0.14 ± 0.10 | 0.21 ± 0.03 | |
| 22 | 0.84 ± 0.11 | 0.96 ± 0.02 | 0.46 ± 0.25 | 0.67 ± 0.05 | ||
| 24 | 0.71 ± 0.04 | 1.14 ± 0.00 | 0.60 ± 0.05 | 0.47 ± 0.26 | 0.92 ± 0.17 | |
| 26 | 0.47 ± 0.08 | 0.47 ± 0.16 | 0.23 ± 0.17 | 0.20 ± 0.01 | 0.25 ± 0.05 | |
| 30 | 0.73 ± 0.20 | 1.51 ± 0.28 | 0.67 ± 0.31 | 0.71 ± 0.15 | ||
| 32 | 0.47 ± 0.13 | 0.41 ± 0.04 | 0.66 ± 0.20 | 0.41 ± 0.13 | 0.42 ± 0.20 | 0.48 ± 0.15 |
| 36 | 0.21 ± 0.03 | 0.39 ± 0.13 | 0.34 ± 0.08 | 0.27 ± 0.06 | 0.14 ± 0.04 | 0.43 ± 0.20 |
| 37 | 0.52 ± 0.01 | 0.42 ± 0.13 | 0.38 ± 0.08 | 0.23 ± 0.01 | ||
| 38 | 0.34 ± 0.04 | 0.45 ± 0.22 | 0.52 ± 0.12 | 0.17 ± 0.01 | ||
| 39 | 0.40 ± 0.04 | 0.51 ± 0.14 | 0.58 ± 0.15 | 0.20 ± 0.04 | ||
| 40 | 0.37 ± 0.04 | 0.70 ± 0.22 | 0.86 ± 0.20 | 0.32 ± 0.04 | ||
| 41 | 0.64 ± 0.22 | 1.83 ± 0.33 | 1.16 ± 0.23 | 0.59 ± 0.10 | ||
| 43 | 0.23 ± 0.02 | 0.14 ± 0.01 | 0.53 ± 0.10 | 0.45 ± 0.02 | 0.16 ± 0.02 | 0.14 ± 0.05 |
| 44 | 0.22 ± 0.01 | 0.21 ± 0.07 | 0.43 ± 0.03 | 0.38 ± 0.07 | 0.14 ± 0.02 | 0.15 ± 0.02 |
| 45 | 0.34 ± 0.10 | 0.24 ± 0.06 | 0.83 ± 0.14 | 0.79 ± 0.10 | 0.23 ± 0.02 | 0.23 ± 0.07 |
| 46 | 0.30 ± 0.06 | 0.70 ± 0.24 | 0.89 ± 0.04 | 0.70 ± 0.08 | 0.27 ± 0.03 | 0.69 ± 0.13 |
| 47 | 0.26 ± 0.03 | 0.93 ± 0.22 | 0.83 ± 0.15 | 0.54 ± 0.12 | 0.18 ± 0.02 | 0.93 ± 0.29 |
| 48 | 0.79 ± 0.20 | >2 | 0.92 ± 0.07 | 0.54 ± 0.12 | 0.84 ± 0.26 | |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay (WST-1) or trypan blue exclusion assay (TB) after 3 days exposure.
Human androgen-insensitive prostate cancer cell line.
Human androgen-insensitive prostate cancer cell line.
Human androgen-sensitive prostate cancer cell line.
Human aggressive cervical cancer cell line.
Table 4.
Relative Potency of Curcumin Mimics
| compd | IC50(curcumin)/IC50(compd)a
|
|||||
|---|---|---|---|---|---|---|
| PC-3b
|
DU145,c WST-1 | LNCaP,d WST-1 | HeLae
|
|||
| WST-1 | TB | WST-1 | TB | |||
| curcumin | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | 24 | 29 | 23 | 23 | ||
| 7 | 17 | 40 | 17 | 30 | ||
| 8 | 37 | 71 | 28 | 53 | ||
| 9 | 36 | 48 | 23 | 35 | ||
| 10 | 28 | 31 | 12 | 19 | ||
| 12 | 82 | 90 | 57 | 64 | 75 | |
| 13 | 37 | 42 | 24 | 34 | 42 | |
| 14 | 36 | 37 | 24 | 30 | 46 | |
| 15 | 42 | 49 | 35 | 37 | ||
| 19 | 50 | 58 | 44 | 87 | 51 | |
| 22 | 30 | 27 | 30 | 18 | ||
| 24 | 36 | 23 | 23 | 26 | 11 | |
| 26 | 54 | 56 | 59 | 61 | 43 | |
| 30 | 35 | 17 | 20 | 17 | ||
| 32 | 54 | 30 | 40 | 33 | 29 | 22 |
| 36 | 121 | 32 | 77 | 50 | 87 | 24 |
| 37 | 49 | 63 | 36 | 53 | ||
| 38 | 75 | 66 | 26 | 71 | ||
| 39 | 64 | 51 | 24 | 61 | ||
| 40 | 69 | 38 | 16 | 38 | ||
| 41 | 40 | 14 | 12 | 21 | ||
| 43 | 111 | 90 | 50 | 30 | 76 | 75 |
| 44 | 116 | 58 | 61 | 36 | 87 | 95 |
| 45 | 75 | 53 | 32 | 17 | 53 | 45 |
| 46 | 85 | 18 | 30 | 19 | 45 | 15 |
| 47 | 98 | 13 | 32 | 25 | 67 | 11 |
| 48 | 32 | 15 | 22 | 13 | ||
The relative potency of curcumin mimics obtained by dividing the IC50 value of curcumin by that of each curcumin mimic.
Human androgen-insensitive prostate cancer cell line.
Human androgen-insensitive prostate cancer cell line.
Human androgen-sensitive prostate cancer cell line.
Human aggressive cervical cancer cell line.
Antiproliferative Activity toward Prostate and Cervical Cancer Cell Lines
To further determine the in vitro anticancer activity of the synthesized curcumin mimics, those compounds with inhibitory rate greater than 45% at 1 μM toward the four cancer cell lines were selected for further evaluation of their antiproliferative effects using WST-1 cell proliferation assay according to the manufacturer’s instruction. To acquire enough data for structure–activity relationship studies, compounds 10, 30, and 40 were also included on the list even they do not fully meet the criteria. The assay is based on the cleavage of the water-soluble tetrazolium salt WST-1 to formazan catalyzed by the cellular mitochondrial dehydrogenases. The amount of formazan dye yielded directly correlates to the number of live cells in the culture. According to preliminary cytotoxic potency by trypan blue dye exclusion assay, twenty-seven compounds (6–10, 12–15, 19, 22, 24, 26, 30, 32, 36–41, and 43–48) were selected for WST-1 cell proliferation assay using the procedure as described in the Experimental Section in three prostate cancer cell lines and one cervical cancer cell line and their IC50 values were determined. Curcumin was used as a positive control for comparison in the parallel experiments, and the results were summarized in Tables 3 and 4. All twenty-seven 1,5-diheteroarylpenta-1,4-dien-3-ones were appraised as promising antiprostate and anticervical cancer agents by comparing their IC50 values with that of curcumin (Table 4). They are 13–121 times, 12–59 times, and 11–95 times, respectively, more potent than curcumin in two androgen-insensitive human prostate cancer cell lines (PC-3 and DU145), the androgen-sensitive human cancer cell line (LNCaP), and the human cervical cancer cell line (HeLa). This validates the promising scaffold containing two identical terminal basic heteroaromatic rings and a central linear dienone linker as novel curcumin mimics with promising cytotoxic and antiproliferative effects against cancer cells.
Structure–Activity Relationships
The below structure–activity relationships of curcumin mimics can be summed up based on the in vitro cell-based experimental data that were derived from efforts focused on the terminal heteroaromatic ring modifications of 1,5-diheteroarylpenta-1,4-dien-3-ones.
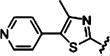
In prostate and cervical cancer cell models, among fortythree (1E,4E)-1,5-diheteroarylpenta-1,4-dien-3-ones, only compounds 17, 33, and 34 did not show increased or similar cytotoxicity against prostate and cervical cancer cell lines, as compared with curcumin (Table 2). This suggests that (1E,4E)-1,5-diheteroarylpenta-1,4-dien-3-ones represent the optimal scaffold for curcumin mimics with substantially improved cytotoxicity and antiproliferative effect.
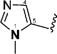
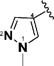
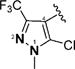
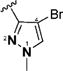
1-Alkyl-1H-imidazol-2-yl in compounds 6–15 represent the optimal terminal five-membered heteroaromatic rings for the potency in four cancer cell lines. The pentan-2-yl in compound 12, as well as the isobutyl in (1E,4E)-1,5-bis(1-isobutyl-1H-imidazol-2-yl)penta-1,4-dien-3-one and the sec-butyl in compound 4,18 serves as the most favorable alkyl group on the nitrogen atom of 1H-imidazol-2-yl for the activity. Other five-membered heteroaromatic rings beneficial to the potency include 4-bromo-1-methyl-1H-pyrazol-3-yl in compound 19, 4-methylthiazol-2-yl in compound 22, and 2-methyl-4-(trifluoromethyl)thiazol-5-yl in 24.
Regarding six-membered heteroaromatic rings, it has been reported that ortho pyridyl generally increases the cytotoxic and antiproliferative potency by 7–60 times.16 The substituent at the meta position is crucial for the potency because compound 26 with an electron donating methyl group at meta position showed better inhibition, while the corresponding analogue 25 with an electron withdrawing bromo group at meta position did not show significantly enhanced activity. No apparent effect can generally be bestowed by meta and para pyridyl (e.g., 27–29). The enhanced activity of para pyridyl analogue 30 can be attributed to the electron withdrawing group (F) at ortho position. Collectively, the structure–activity relationships acquired for the six-membered analogues revealed that promising activity requires the presence of a nitrogen atom or a substituent at ortho position displaying electron-withdrawing properties.
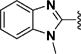
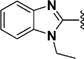
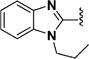
Among 18 curcumin mimics (31–48) with bulky heteroaromatic rings, only those having 1-alkyl-1H-benzo[d]imidazole-2-yl as heteroaromatic rings (e.g., compounds 36–48) possessed the enhanced potency. Methyl (in 36), isopropyl (in 43), and isobutyl (in 44) represent the optimal alkyl group on the nitrogen atom for increased potency.
In Vivo Pharmacokinetic Studies
One of the major challenges that this study seeks to address is to improve the pharmaceutical profile of curcumin, known for its poor bioavailability following oral administration.6,31–33 To this end we have modified the curcumin structure to temper its metabolic instability and water solubility by introducing a shorter dienone linker and replacing the six-membered phenol terminal rings with nitrogen-containing heteroaromatic rings that vary in size and polarity. To evaluate if the curcumin analogues with markedly improved anticancer activities could also possess greater bioavailability, we chose two most promising mimics, 14 and 24, for pharmacokinetic studies. The two compounds were among the most potent mimics tested in this study, but in particular, compound 24 was selected mainly because it contains thiazole rings and trifluoromethyl groups, two structural moieties generally expected to confer attractive pharmacokinetic profiles. C57 mice were administered with 14, 24, or curcumin via oral gavage at a single dose of 1 mg/kg, and blood samples were collected at 30 min and 1, 2, 4, and 24 h after the oral administration. Plasma was prepared from the blood samples and was analyzed by HPLC–MS/MS for determination of drug concentrations as described in the Experimental Section. Summarized in Table 5 are the plasma concentrations of 14, 24, and curcumin at different sampling time points. Compound 14 exhibited slightly improved oral bioavailability showing 2- to 10-fold increase in plasma concentration than curcumin at 30 min, 4 h, and 24 h after treatment. Compound 24, however, showed significantly increased oral bioavailability with 58-, 21-, 28-, 533-, and 653-fold higher plasma concentration than curcumin at 30 min, 1 h, 4 h, and 24 h after drug administration, respectively. With the peak concentration reaching 61.92 ng/mL, compound 24 affords an AUC value of 892.77 ng mL−1 h−1 versus 2.43 ng mL−1 h−1 for curcumin, clearly demonstrating its markedly improved therapeutic potential. Our pharmacokinetic results of curcumin are consistent with previous reports where plasma curcumin levels remain at low (ng/mL) levels in patients, which is insufficient to yield the anticancer benefits of curcumin.34 Even at a dose 12 000 mg/day, the peak plasma concentration of curcumin was only 57 ng/mL,35 a level that is considered to be therapeutically effective. Thus, the dose of 1 mg/mL of compound 24 used in mice, when extrapolated to humans, would correspond to a daily dose of 70 mg for a person of 155 lb that will afford a peak plasma concentration of 62 ng/kg, exceeding the level achieved by the high dose of 12 000 mg/day. Considering that compound 24 is 11–36 times more potent than curcumin in the tested cancer cell lines, it is therefore reasonable to conclude that the excellent bioavailability of 24, as shown in its high peak concentration and AUC value, will provide the therapeutic efficacy necessary to be cytotoxic to cancer cells.
Table 5.
24 h Mouse-Plasma Concentrations of Curcumin, 14, and 24
| time | concentration in mouse plasma (ng/mL)
|
|||
|---|---|---|---|---|
| curcumin | 14 | 24 | ||
| 30 min | 0.04 | 0.41 | 2.46 | |
| 1 h | 0.33 | 0.71 | 6.73 | |
| 2 h | 0.57 | 0.38 | 15.94 | |
| 4 h | 0.13 | 0.28 | 61.92 | |
| 1 day | 0.03 | 0.05 | 18.09 | |
|
| ||||
| curcumin | 14 | 24 | ||
|
| ||||
| tmax (h) | 2 | 1 | 4 | |
| Cmax (ng/mL) | 0.57 | 0.71 | 61.90 | |
| area under curve (AUC) | (ng mL−1 h−1) | 2.43 | 5.04 | 892.77 |
CONCLUSION
In a continuing study of curcumin mimics as potential drug candidates to treat prostate cancer, we designed and synthesized forty-one new and two known (1E,4E)-1,5-diheteroarylpenta-1,4-dien-3-ones. These curcumin mimics were tested for cytotoxicity against androgen-sensitive LNCaP and androgen-insensitive PC-3 and DU-145 human prostate cancer cell lines, as well as HeLa human cervical cancer cells. Thirty-two compounds possessed significantly improved cytotoxicity in the four cancer cell lines. Twenty-six compounds were selected for the evaluation of their antiproliferative activities in these cancer cell lines. Compared to curcumin, the twenty-six analogues are 12- to 121-fold more potent in inhibiting cancer cell proliferation. The acquired structure–activity relationship data indicated (i) that (1E,4E)-1,5-diheteroarylpenta-1,4-dien-3-ones represent the optimal scaffold for curcumin mimics with substantially improved cytotoxicity and antiproliferative effect in prostate and cervical cancer cell models and (ii) 1-alkyl-1H-imidazol-2-yl, ortho pyridyl, 1-alkyl-1H-benzo[d]imidazole-2-yl, 4-bromo-1-methyl-1H-pyrazol-3-yl, thiazol-2-yl, and 2-methyl-4-(trifluoromethyl)-thiazol-5-yl serve as optimal heteroaromatic rings for increased in vitro potency. Pharmacokinetic studies in mice identified compound 24 as a promising lead compound that is currently under further evaluation as a potential clinical trial candidate for the treatment of prostate and cervical cancers. This study established robust structure–activity relationships (SARs) of (1E,4E)-1,5-diheteroarylpenta-1,4-dien-3-ones, which will guide future designs of new curcumin mimics with the hope to further improve the therapeutic index for the treatment of cancer.
EXPERIMENTAL SECTION
General Procedures
HRMS results were obtained on an Orbitrap mass spectrometer with electrospray ionization (ESI). NMR spectra were obtained on a Bruker Fourier 300 spectrometer or an Agilent-Varian 400 spectrometer in CDCl3 or DMSO-d6. The chemical shifts are given in ppm referenced to the respective solvent peak, and coupling constants are reported in Hz. Anhydrous THF and dichloromethane were purified by PureSolv MD 7 solvent purification system from Innovative Technologies (MB-SPS-800). All other reagents and solvents were purchased from commercial sources and were used without further purification. Silica gel column chromatography was performed using silica gel (32–63 μm). Preparative thin-layer chromatography (PTLC) separations were carried out on 1000 μm AnalTech thin layer chromatography plates (lot no. 13401). Curcumin was synthesized by Claisen–Schmidt condensation of aromatic aldehyde with acetylacetone according to the procedure described in the literature.36 1,3-Bis(diethylphosphonato)acetone was synthesized using the procedure illustrated in the literature.23 The purities of biologically tested compounds are ≥95% as determined by HPLC. Specifically, the major peak accounted for ≥95% of the combined total peak area when monitored by a diode array detector (DAD) at 325 ± 100 nm. The HPLC analyses were performed on an Agilent Hewlett-Packard 1100 series HPLC DAD system using a 5 μm C18 reversed phase column (4.6 mm × 250 mm) and a diode array detector.
General Procedure for the Synthesis of 1-Alkyl-1H-imidazole-2-carbaldehyde.24
To a solution of 1H-imidazole-2-carbaldehyde (13 mmol) and potassium carbonate (16 mmol) in DMF (13 mL) was added alkyl bromide (16 mmol), and the reaction mixture was stirred at 50 °C for 6 h. The inorganic solids were removed by filtration, and the filtrate was diluted with water and extracted with diethyl ether. The combined organic extracts were dried over anhydrous magnesium sulfate, and the volatile components were evaporated under vacuum to give the respective product.
1-Ethyl-1H-imidazole-2-carbaldehyde (49)
Yellow oil, 60% yield. 1H NMR (300 MHz, CDCl3): δ 9.67 (s, 1H), 7.15 (s, 1H), 7.09 (s, 1H), 4.32 (q, J = 7.2 Hz, 2H), 1.31 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 181.8, 143.0, 131.5, 125.6, 42.8, 16.2.
1-Propyl-1H-imidazole-2-carbaldehyde (50)
Yellow oil, 64% yield. 1H NMR (300 MHz, CDCl3): δ 9.77 (s, 1H), 7.24 (s, 1H), 7.13 (s, 1H), 4.33 (t, J = 7.2 Hz, 2H), 1.86−1.69 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 182.1, 143.4, 131.6, 126.4, 49.4, 24.4, 10.9.
1-Butyl-1H-imidazole-2-carbaldehyde (51)
Yellow oil, 94% yield. 1H NMR (300 MHz, CDCl3): δ 9.71 (s, 1H), 7.18 (s, 1H), 7.09 (s, 1H), 4.30 (t, J = 7.3 Hz, 2H), 1.73−1.59 (m, 2H), 1.31−1.16 (m, 2H), 0.84 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 181.9, 143.2, 131.4, 126.3, 47.5, 33.0, 19.5, 13.5.
1-Pentyl-1H-imidazole-2-carbaldehyde (52)
Yellow oil, 73% yield. 1H NMR (300 MHz, CDCl3): δ 9.74 (s, 1H), 7.21 (s, 1H), 7.11 (s, 1H), 4.32 (t, J = 7.3 Hz, 2H), 1.79−1.64 (m, 2H), 1.35−1.17 (m, 4H), 0.82 (t, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 182.0, 143.3, 131.5, 126.3, 47.8, 30.7, 28.5, 22.2, 13.9.
1-Hexyl-1H-imidazole-2-carbaldehyde (53)
Yellow oil, 98% yield. 1H NMR (300 MHz, CDCl3): δ 9.77 (s, 1H), 7.24 (s, 1H), 7.13 (s, 1H), 4.35 (t, J = 7.3 Hz, 2H), 1.76−1.69 (m, 2H), 1.31−1.23 (m, 6H), 0.84 (t, J = 5.9 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 182.1, 143.4, 131.6, 126.3, 47.9, 31.3, 31.1, 26.1, 22.5, 14.0.
1-Heptyl-1H-imidazole-2-carbaldehyde (54)
Yellow oil, 73% yield. 1H NMR (300 MHz, CDCl3): δ 9.80 (s, 1H), 7.26 (s, 1H), 7.13 (s, 1H), 4.36 (t, J = 7.2 Hz, 2H), 1.82−1.65 (m, 2H), 1.30−1.15 (m, 8H), 0.84 (t, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 182.0, 143.3, 131.4, 126.3, 48.0, 31.7, 31.1, 28.9, 26.5, 22.6, 14.1.
1-(Pentan-2-yl)-1H-imidazole-2-carbaldehyde (55)
Yellow oil, 99% yield. 1H NMR (300 MHz, CDCl3): δ 9.81 (s, 1H), 7.29 (s, 1H), 7.28 (s, 1H), 5.48−5.36 (m, 1H), 1.80−1.62 (m, 2H), 1.43 (d, J = 6.7 Hz, 3H), 1.27−1.07 (m, 2H), 0.87 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 182.5, 143.3, 132.2, 122.3, 52.6, 39.9, 22.0, 19.2, 13.8.
1-(Pentan-3-yl)-1H-imidazole-2-carbaldehyde (56)
Yellow oil, 99% yield. 1H NMR (300 MHz, CDCl3): δ 9.76 (s, 1H), 7.27 (s, 1H), 7.20 (s, 1H), 5.24−5.04 (m, 1H), 1.88−1.54 (m, 4H), 0.71 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 182.5, 144.3, 132.3, 122.3, 59.8, 28.9, 10.2.
1-(Isopentyl)-1H-imidazole-2-carbaldehyde (57)
Yellow oil, 99% yield. 1H NMR (300 MHz, CDCl3): δ 9.73 (s, 1H), 7.20 (s, 1H), 7.11 (s, 1H), 4.33 (t, J = 7.4 Hz, 2H), 1.63−1.49 (m, 3H), 0.88 (d, J = 6.3 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 181.9, 143.3, 131.5, 126.1, 46.2, 39.9, 25.6, 22.3.
1-(3-Methylbut-2-en-1-yl)-1H-imidazole-2-carbaldehyde (58)
Yellow oil, 55% yield. 1H NMR (300 MHz, CDCl3): δ 9.81 (d, J = 1.0 Hz, 1H), 7.26 (s, 1H), 7.16 (s, 1H), 5.37−5.32 (m, 1H), 5.00 (d, J = 7.2 Hz, 2H), 1.773 (s, 3H), 1.770 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 182.2, 143.2, 138.8, 131.6, 125.5, 118.4, 45.3, 25.7, 18.1.
Synthesis of Benzo[d]thiazol-2-ylmethanol (95).27
To a mixture of 2-aminobenzenethiol (0.25 g, 2 mmol) and glycolic acid (0.46 g, 6 mmol) was added HCl (4 N, 6 mL). The reaction was allowed to proceed with refluxing at 100 °C for 6 h prior to being quenched with saturated aqueous sodium bicarbonate. The solution was extracted with ethyl acetate (20 mL × 3). The combined extracts was evaporated in vacuo to give the crude product, which was subjected to the PTLC purification using DCM/MeOH (100/2, v/v) as eluent to yield the title compound benzo[d]thiazol-2-ylmethanol in 15% yield. 1H NMR (300 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.46 (t, J = 8.0 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 5.08 (s, 2H), 4.73 (br s, 1H). 13C NMR (75 MHz, CDCl3) δ 173.6, 152.7, 134.6, 126.3, 125.2, 122.6, 121.9, 62.3.
Synthesis of Benzo[d]thiazole-2-carbaldehyde (78)
To a solution of benzo[d]thiazol-2-ylmethanol (47 mg, 0.28 mmol) in methylene chloride (3 mL) was added DMP (132 mg, 0.31 mmol), and the reaction was allowed to proceed with stirring at cold room (4 °C) for 1 h prior to being quenched with saturated aqueous sodium thiosulfate solution (1 mL). The subsequent mixture was extracted with methylene chloride (5 mL × 3). The combined organic extracts were dried over anhydrous magnesium sulfate and concentrated. The crude product obtained was purified by PTLC, eluting with DCM/MeOH (100/1, v/v) to yield the title product benzo[d]thiazole-2-carbaldehyde in 56% yield. 1H NMR (300 MHz, CDCl3) δ 10.19 (s, 1H), 8.28 (d, J = 8.0 Hz, 2H), 8.02 (d, J = 8.0 Hz, 2H), 7.68−7.53 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 186.6, 165.5, 153.7, 136.5, 128.6, 127.5, 125.9, 122.8.
Synthesis of (1H-Benzo[d]imidazole-2-yl)methanol (97).25
To a mixture of benzene-1,2-diamine (1.08 g, 10 mmol) and glycolic acid (2.28 g, 30 mmol) was added HCl (4 N, 30 mL). The reaction was allowed to proceed with refluxing at 100 °C for 6 h prior to being quenched with saturated aqueous sodium bicarbonate. The white solid were collected by filtration in 58% yield. 1H NMR (300 MHz, DMSO-d6) δ 12.35 (br s, 1H), 7.50 (dd, J = 5.6, 3.2 Hz, 2H), 7.13 (dd, J = 5.6, 3.2 Hz, 2H), 5.78 (br s, 1H), 4.71 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 155.2, 138.7, 121.4, 114.9, 57.8.
General Procedure for the Synthesis of (1-Alkyl-1H-benzo-[d]imidazole-2-yl)methanol
To a solution of (1H-benzo[d]-imidazole-2-yl)methanol (148 mg, 1 mmol) in DMF (2 mL) were added the appropriate alkyl bromide (2 mmol) and potassium carbonate (690 mg, 5 mmol), and the reaction mixture was stirred at room temperature for 24 h prior to being diluted with methylene chloride (8 mL) and sodium chloride (8 mL). The organic layer was separated, and the aqueous layer was extracted with methylene chloride (8 mL × 2). The combined organic layers were dried over anhydrous magnesium sulfate and concentrated in vacuo to provide a crude product, which was subjected to PTLC purification using DCM/MeOH (100/5, v/v) as eluent to yield the title compound.
(1-Methyl-1H-benzo[d]imidazole-2-yl)methanol (98)
Yellow oil, 52% yield. 1H NMR (300 MHz, CDCl3) δ 7.76−7.64 (m, 1H), 7.34−7.26 (m, 3H), 4.92 (s, 2H), 3.85 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 154.0, 141.2, 135.8, 123.1, 122.5, 119.1, 109.5, 56.8, 30.0.
(1-Ethyl-1H-benzo[d]imidazol-2-yl)methanol (99)
Yellow oil, 33% yield. 1H NMR (300 MHz, CDCl3) δ 7.69 (d, J = 6.3 Hz, 1H), 7.41−7.15 (m, 3H), 4.89 (s, 2H), 4.31 (q, J = 7.1 Hz, 2H), 1.47 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 153.7, 141.6, 134.8, 123.0, 122.3, 119.4, 109.7, 56.7, 38.9, 15.3.
(1-Propyl-1H-benzo[d]imidazol-2-yl)methanol (100)
Yellow oil, 52% yield. 1H NMR (400 MHz, CDCl3) δ 7.51 (dd, J = 6.2, 4.0 Hz, 1H), 7.18−6.99 (m, 3H), 6.74 (br s, 1H), 4.73 (s, 2H), 4.00 (t, J = 7.3 Hz, 2H), 1.73−1.64 (m, 2H), 0.78 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 154.0, 141.3, 134.9, 122.6, 122.0, 119.0, 109.8, 56.3, 45.3, 23.1, 11.3.
(1-Butyl-1H-benzo[d]imidazol-2-yl)methanol (101)
Yellow oil, 73% yield. 1H NMR (400 MHz, CDCl3) δ 7.52−7.46 (m, 1H), 7.15 (br s, 1H), 7.09−6.98 (m, 3H), 4.70 (s, 2H), 3.96 (t, J = 7.5 Hz, 2H), 1.61−1.53 (m, 2H), 1.18−1.12 (m, 2H), 0.78 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 153.8, 141.2, 134.7, 122.5, 121.8, 118.8, 109.6, 56.2, 43.5, 31.7, 19.9, 13.5.
(1-Pentyl-1H-benzo[d]imidazol-2-yl)methanol (102)
Yellow oil, 62% yield. 1H NMR (300 MHz, CDCl3) δ 7.72 (dd, J = 5.8, 2.6 Hz, 1H), 7.41−7.18 (m, 3H), 6.14 (br s, 1H), 4.94 (s, 2H), 4.25 (t, J = 7.5 Hz, 2H), 1.98−1.76 (m, 2H), 1.53−1.29 (m, 4H), 0.95 (t, J = 6.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 153.9, 141.4, 134.9, 122.7, 122.1, 119.1, 109.8, 56.4, 43.9, 29.6, 29.0, 22.3, 13.9.
(1-n-Hexyl-1H-benzo[d]imidazole-2-yl)methanol (103)
Yellow oil, 91% yield. 1H NMR (300 MHz, CDCl3) δ 7.71−7.62 (m, 1H), 7.30−7.18 (m, 3H), 4.88 (s, 2H), 4.19 (t, J = 7.5 Hz, 2H), 1.83−1.76 (m, 2H), 1.47−1.15 (m, 6H), 0.89 (t, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 154.0, 141.4, 135.0, 122.7, 122.1, 119.1, 109.8, 56.4, 44.0, 31.4, 29.9, 26.6, 22.5, 14.0.
(1-Heptyl-1H-benzo[d]imidazol-2-yl)methanol (104)
Yellow oil, 58% yield. 1H NMR (300 MHz, CDCl3) δ 7.65 (dd, J = 5.9, 2.5 Hz, 1H), 7.31−7.15 (m, 3H), 4.87 (s, 2H), 4.16 (t, J = 7.5 Hz, 2H), 1.89−1.70 (m, 2H), 1.39−1.19 (m, 8H), 0.88 (t, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 153.8, 141.3, 134.9, 122.6, 122.0, 119.0, 109.7, 56.4, 43.9, 31.6, 29.8, 28.8, 26.8, 22.5, 14.0.
(1-Isopropyl-1H-benzo[d]imidazole-2-yl)methanol (105)
Yellow oil, 29% yield. 1H NMR (300 MHz, CDCl3) δ 7.70−7.59 (m, 1H), 7.57−7.40 (m, 1H), 7.20−7.17 (m, 2H), 5.02−4.93 (m, 1H), 4.87 (s, 2H), 1.62 (d, J = 6.9 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 153.6, 142.0, 133.4, 122.4, 121.8, 119.5, 112.0, 56.9, 48.3, 21.4.
(1-Isobutyl-1H-benzo[d]imidazole-2-yl)methanol (106)
Yellow oil, 56% yield. 1H NMR (300 MHz, CDCl3) δ 7.67−7.63 (m, 1H), 7.26−7.16 (m, 3H), 4.88 (s, 2H), 4.00 (d, J = 7.7 Hz, 2H), 2.30−2.21 (m, 1H), 0.92 (d, J = 6.7 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 154.4, 141.4, 135.3, 122.7, 122.1, 119.1, 110.2, 56.5, 51.2, 29.2, 20.2.
(1-sec-Butyl-1H-benzo[d]imidazole-2-yl)methanol (107)
Yellow oil, 36% yield. 1H NMR (300 MHz, CDCl3) δ 7.76−7.65 (m, 1H), 7.55−7.46 (m, 1H), 7.25−7.21 (m, 2H), 4.91 (s, 2H), 4.62−4.48 (m, 1H), 2.22−2.12 (m, 1H), 2.03−1.88 (m, 1H), 1.64 (d, J = 6.9 Hz, 3H), 0.82 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 154.2, 141.9, 133.3, 122.4, 121.9, 119.5, 112.0, 56.9, 54.3, 28.1, 19.7, 11.2.
(1-(Pentan-2-yl)-1H-benzo[d]imidazole-2-yl)methanol (108)
Yellow oil, 68% yield. 1H NMR (300 MHz, CDCl3) δ 7.69−7.58 (m, 1H), 7.53−7.39 (m, 1H), 7.18−7.15 (m, 2H), 4.90 (s, 2H), 4.83−4.67 (m, 1H), 2.12−2.07 (m, 1H), 1.88−1.77 (m, 1H), 1.58 (d, J = 6.8 Hz, 3H), 1.07−0.95 (m, 1H), 1.13−0.91 (m, 1H), 0.82 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 154.0, 141.9, 133.3, 122.3, 121.7, 119.4, 111.9, 56.8, 52.6, 37.1, 19.9, 19.8, 13.7.
(1-(Pentan-3-yl)-1H-benzo[d]imidazole-2-yl)methanol (109)
Yellow oil, 37% yield. 1H NMR (400 MHz, CDCl3) δ 7.71−7.67 (m, 1H), 7.47−7.43 (m, 1H), 7.24−7.17 (m, 2H), 4.89 (s, 2H), 4.29−4.21 (m, 1H), 2.20−2.08 (m, 2H), 2.00−1.90 (m, 2H), 0.78 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 155.0, 141.9, 133.2, 122.3, 121.8, 119.5, 112.0, 60.5, 57.0, 26.7, 11.0.
(1-Isopentyl-1H-benzo[d]imidazole-2-yl)methanol (110)
Yellow oil, 67% yield. 1H NMR (300 MHz, CDCl3) δ 7.65−7.59 (m, 1H), 7.22−7.06 (m, 3H), 4.82 (s, 2H), 4.06 (t, J = 7.2 Hz, 2H), 1.68−1.50 (m, 3H), 0.91 (d, J = 5.7 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 153.6, 141.2, 134.6, 122.4, 121.7, 118.8, 109.4, 56.1, 42.0, 38.3, 25.7, 22.1.
General Procedure for the Synthesis of 1-Alkyl-1H-benzo-[d]imidazole-2-carbaldehyde
To a solution of (1-alkyl-1H-benzo-[d]imidazole-2-yl)methanol (1 mmol) in methylene chloride (10 mL) was added DMP (466 mg, 1.1 mmol), and the reaction was allowed to proceed with stirring at cold room (4 °C) for 1 h prior to being quenched with saturated aqueous sodium thiosulfate solution (3 mL). The subsequent mixture was extracted with methylene chloride (10 mL × 3). The combined organic extracts were dried over anhydrous magnesium sulfate and concentrated. The crude product obtained was purified by PTLC eluting with DCM/MeOH (100/5, v/v) to yield the title product.
1-Methyl-1H-benzo[d]imidazole-2-carbaldehyde (79)
Yellow oil, 83% yield. 1H NMR (300 MHz, CDCl3) δ 10.11 (s, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.56−7.32 (m, 3H), 4.15 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 185.2, 146.3, 142.9, 137.1, 127.0, 124.2, 122.5, 110.6, 31.5.
1-Ethyl-1H-benzo[d]imidazole-2-carbaldehyde (80)
Yellow oil, 68% yield. 1H NMR (300 MHz, CDCl3) δ 10.07 (s, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.48−7.31 (m, 3H), 4.60 (q, J = 6.8 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 184.9, 145.7, 142.9, 136.0, 126.8, 124.1, 122.4, 110.7, 39.8, 15.5.
1-Propyl-1H-benzo[d]imidazole-2-carbaldehyde (81)
Yellow oil, 46% yield. 1H NMR (300 MHz, CDCl3) δ 10.06 (s, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.45−7.29 (m, 3H), 4.51 (t, J = 7.3 Hz, 2H), 1.90−1.73 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 184.8, 146.0, 142.8, 136.5, 126.7, 124.0, 122.4, 111.0, 46.2, 23.6, 11.2.
1-Butyl-1H-benzo[d]imidazole-2-carbaldehyde (82)
Yellow oil, 47% yield. 1H NMR (300 MHz, CDCl3) δ 10.01 (s, 1H), 7.83 (d, J = 8.1 Hz, 1H), 7.40−7.21 (m, 3H), 4.47 (t, J = 7.3 Hz, 2H), 1.72−1.65 (m, 2H), 1.31−1.23 (m, 2H), 0.85 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 184.6, 145.8, 142.6, 136.2, 126.5, 123.8, 122.2, 110.8, 44.4, 32.3, 19.8, 13.6.
1-Pentyl-1H-benzo[d]imidazole-2-carbaldehyde (83)
Yellow oil, 85% yield. 1H NMR (300 MHz, CDCl3) δ 10.03 (s, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.44−7.24 (m, 3H), 4.58−4.38 (m, 2H), 1.74 (quin, J = 7.4 Hz, 2H), 1.28−1.25 (m, 4H), 0.81 (t, J = 6.7 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 184.9, 145.7, 142.9, 136.0, 126.8, 124.1, 122.4, 110.7, 39.8, 15.5.
1-n-Hexyl-1H-benzo[d]imidazole-2-carbaldehyde (84)
Yellow oil, 42% yield. 1H NMR (400 MHz, CDCl3) δ 10.06 (s, 1H), 7.87 (dt, J = 8.2, 0.8 Hz, 1H), 7.44−7.37 (m, 2H), 7.35−7.29 (m, 1H), 4.52 (t, J = 5.6 Hz, 2H), 1.81−1.72 (m, 2H), 1.30−1.22 (m, 6H), 0.82 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 184.6, 145.8, 142.6, 136.2, 126.5, 123.8, 122.2, 110.8, 44.6, 31.2, 30.1, 26.2, 22.3, 13.8.
1-Heptyl-1H-benzo[d]imidazole-2-carbaldehyde (85)
Yellow oil, 75% yield. 1H NMR (300 MHz, CDCl3) δ 10.06 (s, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.43−7.26 (m, 3H), 4.52 (t, J = 7.4 Hz, 2H), 1.79−1.73 (m, 2H), 1.27−1.21 (m, 8H), 0.81 (t, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 184.8, 146.0, 142.8, 136.4, 126.7, 123.9, 122.4, 111.0, 44.8, 31.6, 30.4, 28.9, 26.7, 22.5, 14.0.
1-Isopropyl-1H-benzo[d]imidazole-2-carbaldehyde (86)
Yellow oil, 91% yield. 1H NMR (300 MHz, CDCl3) δ 10.09 (s, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.46−7.31 (m, 2H), 5.90−5.81 (m, 1H), 1.65 (d, J = 7.0 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 185.2, 145.9, 143.6, 135.3, 126.4, 123.8, 122.8, 113.6, 48.4, 21.6.
1-Isobutyl-1H-benzo[d]imidazole-2-carbaldehyde (87)
Yellow oil, 80% yield. 1H NMR (400 MHz, CDCl3) δ 10.01 (s, 1H), 7.82 (dt, J = 8.1, 1.0 Hz, 1H), 7.49−7.13 (m, 3H), 4.28 (d, J = 7.5 Hz, 2H), 2.19−2.04 (m, 1H), 0.82 (d, J = 6.7 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 184.8, 146.2, 142.7, 136.7, 126.7, 123.9, 122.3, 111.4, 51.7, 29.8, 20.0.
1-sec-Butyl-1H-benzo[d]imidazole-2-carbaldehyde (88)
Yellow oil, 58% yield. 1H NMR (400 MHz, CDCl3) δ 10.11 (s, 1H), 7.94−7.91 (m, 1H), 7.65−7.60 (m, 1H), 7.43−7.33 (m, 2H), 5.68−5.57 (m, 1H), 2.21−2.10 (m, 1H), 2.00−1.90 (m, 1H), 1.65 (d, J = 7.0 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 185.3, 146.6, 143.7, 135.4, 126.4, 123.9, 122.8, 113.7, 54.3, 28.5, 20.0, 11.1.
1-(Pentan-2-yl)-1H-benzo[d]imidazole-2-carbaldehyde (89)
Yellow oil, 82% yield. 1H NMR (300 MHz, CDCl3) δ 10.09 (s, 1H), 7.91 (dd, J = 6.9, 1.7 Hz, 1H), 7.61 (d, J = 7.4 Hz, 1H), 7.44−7.30 (m, 2H), 6.01−5.38 (m, 1H), 2.21−2.02 (m, 1H), 1.94−1.72 (m, 1H), 1.61 (d, J = 7.0 Hz, 3H), 1.30−1.10 (m, 1H), 1.05−0.93 (m, 1H), 0.81 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 185.3, 146.4, 143.7, 135.4, 126.4, 124.0, 122.8, 113.7, 52.5, 37.4, 20.2, 19.8, 13.7.
1-(Pentan-3-yl)-1H-benzo[d]imidazole-2-carbaldehyde (90)
Yellow oil, 57% yield. 1H NMR (400 MHz, CDCl3) δ 10.11 (s, 1H), 7.95−7.89 (m, 1H), 7.58 (d, J = 7.0 Hz, 1H), 7.40−7.32 (m, 2H), 5.79−5.25 (m, 1H), 2.14 (m, 2H), 2.06−1.84 (m, 2H), 0.72 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 185.4, 147.3, 143.8, 135.2, 126.4, 123.9, 122.8, 113.9, 60.5, 27.0, 11.0.
1-Isopentyl-1H-benzo[d]imidazole-2-carbaldehyde (91)
Yellow oil, 68% yield. 1H NMR (300 MHz, CDCl3) δ 9.96 (s, 1H), 7.77 (d, J = 8.1 Hz, 1H), 7.30−7.28 (overlapped, 2H), 7.26−7.18 (m, 1H), 4.40 (t, J = 7.4 Hz, 2H), 1.56−1.50 (m, 3H), 0.87 (d, J = 6.1 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 184.4, 145.6, 142.6, 136.0, 126.4, 123.7, 122.1, 110.6, 43.0, 38.8, 25.8, 22.2.
General Procedure for the Synthesis (1E,4E)-1,5-Diarylpenta-1,4-dien-3-one.22
A 25 mL flask was charged with 1,3-bis(diethylphosphonato)acetone (0.50 g, 1.51 mmol) and the appropriate heteroaromatic carboxaldehyde (3.02 mmol). A solution of potassium carbonate (2.80 g, 20.3 mmol) in water (2.5 mL) and ethanol (1.5 mL) were added, and the biphasic mixture was stirred rapidly at room temperature for 1 h to overnight. The reaction mixture was extracted with ethyl acetate (10 mL × 3). The combined extracts were dried over anhydrous magnesium sulfate and concentrated. The residue was purified over preparative thin layer chromatography, eluting with dichloromethane/methanol (100:5, v/v), hexanes/ethyl acetate (50:50, v/v), and/or ethyl acetate/methanol (9:1, v/v) to give the respective product.
(1E,4E)-1,5-Bis(1-ethyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (6)
This compound was prepared in 91% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.55 (d, J = 15.2 Hz, 2H), 7.43 (d, J = 15.2 Hz, 2H), 7.17 (s, 2H), 7.04 (s, 2H), 4.10 (q, J = 7.3 Hz, 4H), 1.42 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 188.2, 142.8, 130.9, 127.3, 126.8, 122.2, 41.2, 16.8. IR (KBr): 3104, 2978, 1648, 1616, 1588, 1477, 1441 cm−1. HRMS (ESI), m/z calculated for C15H19N4O [M + H]+: 271.1559. Found: 271.1558. HPLC purity 97.2% (20 min run of 45−80% CH3CN in H2O with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-propyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (7)
This compound was prepared in 83% yield as a yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.59 (d, J = 15.3 Hz, 2H), 7.52 (d, J = 15.3 Hz, 2H), 7.20 (s, 2H), 7.04 (d, J = 0.8 Hz, 2H), 4.05 (t, J = 7.2 Hz, 4H), 1.88−1.74 (m, 4H), 0.93 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 188.2, 143.1, 130.3, 127.8, 126.7, 123.0, 48.1, 24.9, 11.2. IR (KBr): 3105, 2964, 2933, 2875, 1647, 1616, 1589, 1508, 1474, 1443, 1281, 1092 cm−1. HRMS (ESI), m/z calculated for C17H22N4O [M + H]+: 299.1872. Found: 299.1871. HPLC purity 96.8% (20 min run of 20−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-butyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (8)
This compound was prepared in 95% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.57 (d, J = 15.2 Hz, 2H), 7.47 (d, J = 15.2 Hz, 2H), 7.18 (s, 2H), 7.02 (s, 2H), 4.06 (t, J = 7.2 Hz, 4H), 1.81−1.67 (m, 4H), 1.39, 1.26 (m, 4H), 0.91 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 188.2, 143.0, 130.6, 127.4, 126.8, 122.9, 46.1, 33.5, 19.8, 13.6. IR (KBr): 3104, 2955, 2929, 2859, 1648, 1616, 1589, 1474, 1444, 1091 cm−1. HRMS (ESI), m/z calculated for C19H27N4O [M + H]+: 327.2185. Found: 327.2183. HPLC purity 96.4% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-pentyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (9)
This compound was prepared in 80% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.54 (d, J = 15.3 Hz, 2H), 7.43 (d, J = 15.3 Hz, 2H), 7.14 (s, 2H), 7.00 (s, 2H), 4.02 (t, J = 7.2 Hz, 4H), 1.79−1.65 (m, 4H), 1.33−1.17 (m, 8H), 0.83 (t, J = 6.8 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 188.2, 142.9, 130.5, 127.3, 126.7, 122.9, 46.3, 31.1, 28.6, 22.2, 13.9. IR (KBr): 2929, 2859, 1649, 1616, 1588, 1474, 1444, 1134, 1090 cm−1. HRMS (ESI), m/z calculated for C21H30N4O [M + H]+: 355.2498. Found: 355.2497. HPLC purity 96.4% (25 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-hexyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (10)
This compound was prepared in 74% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.58 (d, J = 15.2 Hz, 2H), 7.47 (d, J = 15.2 Hz, 2H), 7.19 (s, 2H), 7.03 (s, 2H), 4.06 (t, J = 7.3 Hz, 4H), 1.34−1.24 (m, 12H), 0.86 (t, J = 6.5 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 188.2, 143.1, 130.7, 127.3, 126.9, 122.9, 46.4, 31.5, 31.3, 26.3, 22.5, 14.0. IR (KBr): 3105, 2954, 2927, 2857, 1649, 1617, 1445, 1274, 1096 cm−1. HRMS (ESI), m/z calculated for C23H35N4O [M + H]+: 383.2811. Found: 383.2821. HPLC purity 95.3% (25 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-heptyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (11)
This compound was prepared in 91% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.52 (d, J = 15.2 Hz, 2H), 7.41 (d, J = 15.2 Hz, 2H), 7.13 (s, 2H), 6.98 (s, 2H), 4.00 (t, J = 7.2 Hz, 4H), 1.78−1.59 (m, 4H), 1.27−1.12 (m, 16H), 0.79 (t, J = 6.6 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 188.0, 142.9, 130.5, 127.3, 126.7, 122.8, 46.3, 31.5, 31.4, 28.7, 26.5, 22.5, 14.0. IR (KBr): 3104, 2953, 2925, 2855, 1650, 1617, 1590, 1507, 1474, 1445, 1274, 1093 cm−1. HRMS (ESI), m/z calculated for C25H39N4O [M + H]+: 411.3124. Found: 411.3126. HPLC purity 96.9% (25 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-(pentan-2-yl)-1H-imidazol-2-yl)penta-1,4-dien-3-one (12)
This compound was prepared in 90% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.61 (d, J = 15.2 Hz, 2H), 7.49 (d, J = 15.2 Hz, 2H), 7.22 (s, 2H), 7.08 (s, 2H), 4.53−4.42 (m, 2H), 1.72 (q, J = 7.6 Hz, 4H), 1.44 (d, J = 6.7 Hz, 6H), 1.27−1.08 (m, 4H), 0.86 (t, J = 7.2 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 188.3, 143.0, 131.1, 127.6, 126.9, 118.9, 51.9, 40.0, 22.4, 19.4, 13.8. IR (KBr): 3104, 2958, 2931, 2872, 1647, 1641, 1588, 1454, 1422, 1270, 1173, 1091 cm−1. HRMS (ESI), m/z calculated for C21H31N4O [M + H]+: 355.2498. Found: 355.2507. HPLC purity 97.5% (25 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-(pentan-3-yl)-1H-imidazol-2-yl)penta-1,4-dien-3-one (13)
This compound was prepared in 99% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.56 (d, J = 15.2 Hz, 2H), 7.50 (d, J = 15.2 Hz, 2H), 7.24 (s, 2H), 7.03 (s, 2H), 4.18−4.08 (m, 2H), 1.91−1.78 (m, 4H), 1.76−1.63 (m, 4H), 0.77 (t, J = 6.6 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 188.4, 144.1, 131.3, 127.6, 127.0, 118.9, 59.7, 29.2, 10.6. IR (KBr): 3104, 2966, 2934, 2877, 1648, 1615, 1591, 1456, 1293, 1174, 1092 cm−1. HRMS (ESI), m/z calculated for C21H31N4O [M + H]+: 355.2498. Found: 355.2505. HPLC purity 97.2% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-isopentyl-1H-imidazol-2-yl)penta-1,4-dien-3-one (14)
This compound was prepared in 99% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.56 (d, J = 15.2 Hz, 2H), 7.42 (d, J = 15.2 Hz, 2H), 7.17 (s, 2H), 7.02 (s, 2H), 4.05 (t, J = 7.1 Hz, 4H), 1.68−1.54 (m, 6H), 0.93 (d, J = 6.2 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 188.1, 143.0, 130.8, 127.4, 126.9, 122.8, 44.7, 40.4, 25.7, 22.3. IR (KBr): 3105, 2955, 2929, 2869, 1649, 1617, 1448, 1276, 1173, 1096 cm−1. HRMS (ESI), m/z calculated for C21H31N4O [M + H]+: 355.2498. Found: 355.2503. HPLC purity 96.3% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-(3-methylbut-2-en-1-yl)-1H-imidazol-2-yl)-penta-1,4-dien-3-one (15)
This compound was prepared in 60% yield as a yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.60 (d, J = 15.3 Hz, 2H), 7.45 (d, J = 15.3 Hz, 2H), 7.18 (s, 2H), 7.03 (s, 2H), 5.29 (t, J = 7.0 Hz, 2H), 4.65 (d, J = 7.0 Hz, 4H), 1.80 (s, 6H), 1.78 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 188.3, 143.1, 138.5, 130.6, 127.5, 127.1, 122.6, 118.7, 44.3, 25.8, 18.2. IR (KBr): 3107, 2971, 2915, 1649, 1615, 1643, 1377, 1273, 1108 cm−1. HRMS (ESI), m/z calculated for C21H27N4O [M + H]+: 351.2185. Found: 351.2184. HPLC purity 95.0% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-methyl-1H-imidazol-5-yl)penta-1,4-dien-3-one (16)
This compound was prepared in 62% yield as a yellow solid; mp 186−188 °C. 1H NMR (300 MHz, CDCl3): δ 7.59 (d, J = 15.6 Hz, 2H), 7.57 (s, 2H), 7.55 (s, 2H), 6.84 (d, J = 15.6 Hz, 2H), 3.75 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 187.3, 141.4, 144.1, 132.9, 129.5, 123.8, 32.4. IR (KBr): 3109, 1644, 1612, 1578, 1492, 1389, 1278, 1227, 1196, 1123 cm−1. HRMS (ESI), m/z calculated for C13H15N4O [M + H]+: 243.1246. Found: 243.1247. HPLC purity 100.0% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-methyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (17)
This compound was prepared in 60% yield as a yellow solid; mp 150−152 °C. 1H NMR (300 MHz, CDCl3): δ 7.75 (s, 2H), 7.59 (s, 2H), 7.58 (d, J = 15.9 Hz, 2H), 6.75 (d, J = 15.9 Hz, 2H), 3.92 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 188.8, 139.0, 133.5, 130.9, 123.7, 119.2, 39.3. IR (KBr): 3106, 2940, 1647, 1615, 1547, 1484, 1447, 1412, 1396, 1263, 1158, 1098 cm−1. HRMS (ESI), m/z calculated for C13H15N4O [M + H]+: 243.1246. Found: 243.1249. HPLC purity 96.1% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(5-chloro-1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)penta-1,4-dien-3-one (18)
This compound was prepared in 82% yield as a yellow solid; mp 156−158 °C. 1H NMR (300 MHz, CDCl3): δ 7.55 (d, J = 16.2 Hz, 2H), 7.11 (d, J = 16.2 Hz, 2H), 3.95 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 188.4, 140.3 (q, JCF = 37.5 Hz), 130.1, 129.1, 127.1, 120.8 (q, JCF = 268.5 Hz), 112.8, 37.4. IR (KBr): 2964, 1674, 1620, 1589, 1525, 1484, 1411, 1337, 1302, 1110 cm−1. HRMS (ESI), m/z calculated for C15H10Cl2F6N4O [M + H]+: 447.0214. Found: 447.0218. HPLC purity 99.5% (30 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(4-bromo-1-methyl-1H-pyrazol-3-yl)penta-1,4-dien-3-one (19)
This compound was prepared in 88% yield as a yellow solid; mp 150−152 °C. 1H NMR (300 MHz, CDCl3): δ 7.64 (d, J = 16.1 Hz, 2H), 7.43 (s, 2H), 7.40 (d, J = 16.1 Hz, 2H), 3.93 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 189.1, 145.5, 132.02, 131.97, 126.7, 96.2, 40.1. IR (KBr): 3127, 2943, 1667, 1624, 1586, 1460, 1425, 1398, 1347, 1254, 1143, 1101 cm−1. HRMS (ESI), m/z calculated for C13H13Br2N4O [M + H]+: 398.9456. Found: 398.9463. HPLC purity 99.8% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(oxazol-4-yl)penta-1,4-dien-3-one (20)
This compound was prepared in 74% yield as a yellow solid; mp 128−130 °C. 1H NMR (300 MHz, CDCl3): δ 7.92 (s, 2H), 7.87 (s, 2H), 7.58 (d, J = 15.5 Hz, 2H), 7.26 (d, J = 15.5 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 188.6, 151.8, 140.1, 137.4, 130.7, 127.4. IR (KBr): 3123, 1677, 1636, 1594, 1553, 1523, 1335, 1315, 1207, 1082 cm−1. HRMS (ESI), m/z calculated for C11H9N2O3 [M + H]+: 217.0613. Found: 217.0612. HPLC purity 96.4% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(3,5-dimethylisoxazol-4-yl)penta-1,4-dien-3-one (21)
This compound was prepared in 97% yield as a yellow solid; mp 108−110 °C. 1H NMR (300 MHz, CDCl3): δ 7.54 (d, J = 16.1 Hz, 2H), 6.69 (d, J = 16.1 Hz, 2H), 2.56 (s, 6H), 2.45 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 187.6, 170.7, 158.6, 131.8, 125.1, 112.0, 12.2, 12.0. IR (KBr): 2927, 1642, 1579, 1426, 1323, 1266, 1101 cm−1. HRMS (ESI), m/z calculated for C15H17N2O3 [M + H]+: 273.1239. Found: 273.1237. HPLC purity 96.0% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(4-methylthiazol-2-yl)penta-1,4-dien-3-one (22)
This compound was prepared in 72% yield as a yellow solid; mp 118−120 °C. 1H NMR (300 MHz, CDCl3): δ 7.77 (d, J = 15.7 Hz, 2H), 7.34 (d, J = 15.7 Hz, 2H), 7.06 (s, 2H), 2.52 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 187.9, 163.1, 155.6, 134.7, 128.5, 117.2, 17.2. IR (KBr): 2920, 2855, 1650, 1613, 1591, 1506, 1438, 1320, 1110 cm−1. HRMS (ESI), m/z calculated for C13H13N2OS2 [M + H]+: 277.0469. Found: 277.0473. HPLC purity 99.6% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Di(thiazol-5-yl)penta-1,4-dien-3-one (23)
This compound was prepared in 72% yield as a light yellow solid; mp 153−155 °C. 1H NMR (300 MHz, CDCl3): δ 8.84 (s, 2H), 8.11 (s, 2H), 7.90 (d, J = 15.6 Hz, 2H), 6.82 (d, J = 15.6 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 186.7, 155.0, 147.3, 135.6, 132.8, 127.7. IR (KBr): 3074, 1645, 1614, 1585, 1498, 1387, 1318, 1174 cm−1. HRMS (ESI), m/z calculated for C11H9N2OS2 [M + H]+: 249.0156. Found: 249.0157. HPLC purity 100.0% (20 min run of 20−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(2-methyl-4-(trifluoromethyl)thiazol-5-yl)-penta-1,4-dien-3-one (24)
This compound was prepared in 68% yield as a yellow solid; mp 138−140 °C. 1H NMR (300 MHz, CDCl3): δ 7.92 (d, J = 15.5 Hz, 2H), 6.72 (d, J = 15.5 Hz, 2H), 2.76 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 186.1, 168.1, 143.8 (q, JCF = 35.3 Hz), 136.0, 130.5, 130.0, 120.8 (q, JCF = 270.8 Hz), 19.8. IR (KBr): 1655, 1611, 1483, 1364, 1318, 1159, 1124 cm−1. HRMS (ESI), m/z calculated for C15H11F6N2OS2 [M + H]+: 413.0217. Found: 413.0217. HPLC purity 96.2% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(6-bromopyridin-2-yl)penta-1,4-dien-3-one (25)
This compound was prepared in 84% yield as a gray yellow solid; mp 208−210 °C. 1H NMR (300 MHz, CDCl3): δ 7.64 (d, J = 16.0 Hz, 2H), 7.61 (d, J = 16.0 Hz, 2H), 7.58 (d, J = 7.4 Hz, 2H), 7.48 (d, J = 7.4 Hz, 2H), 7.43 (t, J = 7.2 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 189.1, 154.4, 142.9, 140.6, 139.2, 130.1, 129.0, 123.8. IR (KBr): 2923, 1695, 1657, 1579, 1550, 1434, 1408, 1119 cm−1. HRMS (ESI), m/z calculated for C15H11Br2N2O [M + H]+: 392.9238. Found: 392.9247. HPLC purity 97.7% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(6-methylpyridin-2-yl)penta-1,4-dien-3-one (26)
This compound was prepared in 98% yield as a gray yellow solid; mp 208−210 °C. 1H NMR (300 MHz, CDCl3): δ 7.74 (d, J = 15.7 Hz, 2H), 7.62 (t, J = 7.7 Hz, 2H), 7.60 (d, J = 15.7 Hz, 2H), 7.32 (d, J = 7.6 Hz, 2H), 7.15 (d, J = 7.7 Hz, 2H), 2.61 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 189.9, 159.1, 152.7, 142.6, 137.0, 128.7, 124.3, 121.9, 24.7. IR (KBr): 3056, 2921, 1654, 1600, 1449, 1367, 1183 cm−1. HRMS (ESI), m/z calculated for C15H11Br2N2O [M + H]+: 265.1341. Found: 265.1344. HPLC purity 97.6% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Di(pyridin-3-yl)penta-1,4-dien-3-one (27)
This compound was prepared in 58% yield as a light yellow oil. 1H NMR (400 MHz, CDCl3): δ 8.85 (d, J = 2.2 Hz, 2H), 8.64 (dd, J = 4.8, 1.6 Hz, 2H), 7.93 (dt, J = 8.0, 1.9 Hz, 2H), 7.74 (d, J = 16.0 Hz, 2H), 7.37 (dd, J = 8.0, 4.8 Hz, 2H), 7.14 (d, J = 16.0 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 187.8, 151.4, 150.1, 140.3, 134.8, 130.6, 127.0, 124.0. IR (KBr): 3033, 2925, 1700, 1654, 1624, 1585, 1478, 1416, 1342, 1199, 1101, 1025 cm−1. HRMS (ESI), m/z calculated for C15H13N2O [M + H]+: 237.1028. Found: 237.1031. HPLC purity 97.9% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(6-(trifluoromethyl)pyridin-3-yl)penta-1,4-dien-3-one (28)
This compound was prepared in 97% yield as a light yellow solid; mp 186−188 °C. 1H NMR (300 MHz, CDCl3): δ 8.95 (s, 2H), 8.10 (d, J = 7.8 Hz, 2H), 7.79 (d, J = 16.0 Hz, 2H), 7.76 (d, J = 7.8 Hz, 2H), 7.21 (d, J = 16.0 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 186.9, 149.8, 149.3 (q, JCF = 35.2 Hz), 138.8, 136.1, 128.6, 121.3 (q, JCF = 272.2 Hz), 120.7. IR (KBr): 2923, 1704, 1660, 1600, 1573, 1397, 1337, 1247, 1177, 1086 cm−1. HRMS (ESI), m/z calculated for C17H11F6N2O [M + H]+: 373.0776. Found: 373.0784. HPLC purity 95.7% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Di(pyridin-4-yl)penta-1,4-dien-3-one (29)
This compound was prepared in 72% yield as a yellow solid; mp 118−120 °C. 1H NMR (400 MHz, CDCl3): δ 8.68 (d, J = 4.5 Hz, 2H), 8.67 (d, J = 4.5 Hz, 2H), 7.63 (d, J = 16.0 Hz, 2H), 7.44 (d, J = 4.5 Hz, 2H), 7.43 (d, J = 4.5 Hz, 2H), 7.19 (d, J = 16.0 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 187.9, 150.8, 141.8, 141.2, 128.8, 122.1. IR (KBr): 3027, 1658, 1591, 1549, 1412, 1342, 1299, 1179, 1100 cm−1. HRMS (ESI), m/z calculated for C15 H12N2O [M + H]+: 237.1028. Found: 237.1033. HPLC purity 95.9% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(3-fluoropyridin-4-yl)penta-1,4-dien-3-one (30)
This compound was prepared in 81% yield as a yellow solid; mp 104−106 °C. 1H NMR (300 MHz, CDCl3): δ 8.58 (s, 2H), 8.51 (d, J = 5.0 Hz, 2H), 7.78 (d, J = 16.1 Hz, 2H), 7.50 (t, J = 5.7 Hz, 2H), 7.31 (d, J = 16.1 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 187.7, 157.5 (d, JCF = 261 Hz), 146.3, 139.6 (d, JCF = 22.5 Hz), 134.2, 131.1, 129.4, 122.4. IR (KBr): 3032, 1676, 1625, 1590, 1548, 1487, 1413, 1323, 1214, 1196, 1096 cm−1. HRMS (ESI), m/z calculated for C15H11F2N2O [M + H]+: 273.0839. Found: 273.0842. HPLC purity 97.9% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Di(quinolin-2-yl)penta-1,4-dien-3-one (31)
This compound was prepared in 88% yield as a yellow solid; mp 162−165 °C. 1H NMR (300 MHz, CDCl3): δ 8.20 (d, J = 8.5 Hz, 2H), 8.15 (d, J = 8.5 Hz, 2H), 7.98 (d, J = 15.9 Hz, 2H), 7.84−7.68 (m, 8H), 7.57 (t, J = 7.6 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 189.7, 153.6, 148.5, 143.2, 137.0, 130.3, 130.0, 128.3, 127.7, 127.6, 121.0. IR (KBr): 3059, 1651, 1589, 1503, 1428, 1345, 1301, 1192 cm−1. HRMS (ESI), m/z calculated for C23H17N2O [M + H]+: 337.1341. Found: 337.1342. HPLC purity 98.4% (25 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Di(quinolin-4-yl)penta-1,4-dien-3-one (32)
This compound was prepared in 63% yield as a yellow solid; mp 150−152 °C. 1H NMR (300 MHz, CDCl3): δ 8.92 (d, J = 4.5 Hz, 2H), 8.46 (d, J = 15.7 Hz, 2H), 8.15 (d, J = 8.6 Hz, 2H), 8.12 (d, J = 8.6 Hz, 2H), 7.79 (t, J = 7.7 Hz, 2H), 7.61−7.58 (overlapped, 4H), 7.31 (d, J = 15.7 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 187.6, 150.1, 148.7, 140.4, 138.8, 130.9, 130.4, 130.2, 127.7, 126.4, 123.4, 118.3. IR (KBr): 3060, 1675, 1622, 1595, 1563, 1505, 1462, 1387, 1316, 1114, 1076 cm−1. HRMS (ESI), m/z calculated for C23H17N2O [M + H]+: 337.1341. Found: 337.1347. HPLC purity 99.0% (30 min run of 20−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(2-chloroquinolin-3-yl)penta-1,4-dien-3-one (33)
This compound was prepared in 99% yield as a yellow solid; mp 220 °C (decomposed). 1H NMR (300 MHz, CDCl3): δ 9.14 (s, 2H), 8.12 (d, J = 8.1 Hz, 4H), 8.11 (d, J = 15.9 Hz, 2H), 8.00 (d, J = 8.1 Hz, 2H), 7.74 (t, J = 7.5 Hz, 2H), 7.61 (d, J = 15.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6 + CDCl3): δ 187.6 149.4, 147.3, 137.6, 137.4, 132.0, 129.6, 128.7, 127.9, 127.7, 127.1, 126.9. IR (KBr): 3055, 1655, 1614, 1592, 1580, 1392, 1375, 1342, 1294, 1191, 1133, 1045 cm−1. HRMS (ESI), m/z calculated for C23H15Cl2N2O [M + H]+: 405.0561. Found: 405.0577. HPLC purity 96.7% (30 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(4-methyl-5-(pyridin-4-yl)thiazol-2-yl)penta-1,4-dien-3-one (34)
This compound was prepared in 75% yield as a yellow solid; mp 225 °C (decomposed). 1H NMR (300 MHz, CDCl3): δ 8.72 (d, J = 5.9 Hz, 4H), 7.89 (d, J = 15.3 Hz, 2H), 7.79 (d, J = 5.9 Hz, 4H), 6.73 (d, J = 15.3 Hz, 2H), 2.64 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 186.4, 164.7, 158.7, 150.9, 139.7, 132.5, 131.2, 127.4, 120.4, 16.1. IR (KBr): 3029, 3007, 1671, 1583, 1381, 1314, 1272, 1097 cm−1. HRMS (ESI), m/z calculated for C23H18N4OS2 [M + H]+: 431.1000. Found: 431.1005. HPLC purity 95.7% (30 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(benzo[d]thiazol-2-yl)penta-1,4-dien-3-one (35)
This compound was prepared in 81% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 8.12 (d, J = 8.0 Hz, 2H), 7.99−7.93 (overlapped, 2H), 7.95 (d, J = 15.8 Hz, 2H), 7.58−7.44 (overlapped, 4H), 7. 46 (d, J = 15.8 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 187.3, 163.7, 154.2, 136.1, 135.6, 131.3, 127.1, 126.9, 124.3, 122.0. IR (KBr): 3047, 2924, 1668, 1647, 1624, 1583, 1475, 1311, 1162, 1093 cm−1. HRMS (ESI), m/z calculated for C19H12N2OS2 [M + H]+: 349.0469. Found: 349.0473. HPLC purity 98.9% (40 min run of 45−65% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-methyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (36)
This compound was prepared in 81% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.94−7.81 (m, 6H), 7.38−7.34 (m, 6H), 3.96 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 187.7, 148.4, 143.4, 136.6, 131.4, 127.9, 124.4, 123.7, 120.5, 110.0, 30.3. IR (KBr): 1676, 1633, 1614, 1599, 1484, 1461, 1403, 1336, 1305, 1093 cm−1. HRMS (ESI), m/z calculated for C21H18N4O [M + H]+: 243.1559. Found: 243.1570. HPLC purity 97.3% (15 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-ethyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (37)
This compound was prepared in 69% yield as a yellow solid; mp 166−168 °C. 1H NMR (300 MHz, CDCl3): δ 7.88 (d, J = 15.3 Hz, 2H), 7.88−7.80 (overlapped, 2H), 7.80 (d, J = 15.3 Hz, 2H), 7.48−7.28 (m, 6H), 4.40 (q, J = 7.0 Hz, 4H), 1.49 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 187.7, 147.6, 143.6, 135.6, 131.5, 127.8, 124.3, 123.7, 120.6, 110.0, 38.8, 16.1. IR (KBr): 3062, 2979, 1654, 1625, 1600, 1410, 1331, 1187, 1094 cm−1. HRMS (ESI), m/z calculated for C23H22N4O [M + H]+: 371.1872. Found: 371.1872. HPLC purity 97.2% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-propyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (38)
This compound was prepared in 99% yield as a yellow solid; mp 109−112 °C. 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 15.3 Hz, 2H), 7.87−7.79 (overlapped, 2H), 7.79 (d, J = 15.3 Hz, 2H), 7.42−7.30 (m, 6H), 4.31 (t, J = 7.1 Hz, 4H), 1.98−1.87 (m, 4H), 0.98 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.8, 148.1, 143.45, 136.1, 131.5, 128.0, 124.3, 123.6, 120.6, 110.2, 45.4, 24.1, 11.5. IR (KBr): 3061, 2964, 2932, 2875, 1653, 1624, 1599, 1444, 1406, 1330, 1183, 1093 cm−1. HRMS (ESI), m/z calculated for C25H26N4O [M + H]+: 399.2185. Found: 399.2185. HPLC purity 99.7% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-butyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (39)
This compound was prepared in 92% yield as a yellow solid; mp 128−130 °C. 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 15.4 Hz, 2H), 7.87−7.79 (overlapped, 2H), 7.79 (d, J = 15.4 Hz, 2H), 7.42−7.30 (m, 6H), 4.33 (t, J = 7.2 Hz, 4H), 1.91−1.78 (m, 4H), 1.42−1.33 (m, 4H), 0.96 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.7, 148.0, 143.5, 136.0, 131.5, 128.0, 124.3, 123.6, 120.6, 110.2, 43.8, 32.9, 20.3, 13.8. IR (KBr): 3061, 2957, 2930, 2872, 1654, 1624, 1596, 1464, 1177, 1092 cm−1. HRMS (ESI), m/z calculated for C27H30N4O [M + H]+: 427.2498. Found: 427.2499. HPLC purity 99.7% (25 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-pentyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (40)
This compound was prepared in 34% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 15.3 Hz, 2H), 7.87−7.78 (overlapped, 2H), 7.78 (d, J = 15.3 Hz, 2H), 7.43−7.28 (m, 6H), 4.31 (t, J = 7.3 Hz, 4H), 1.93−1.75 (m, 4H), 1.41−1.26 (m, 8H), 0.88 (t, J = 6.6 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.7, 148.0, 143.4, 136.0, 131.4, 127.9, 124.3, 123.6, 120.5, 110.2, 43.9, 30.5, 29.0, 22.4, 14.0. IR (KBr): 3051, 2956, 2929, 2858, 1655, 1624, 1598, 1445, 1406, 1330, 1092 cm−1. HRMS (ESI), m/z calculated for C29H34N4O [M + H]+: 455.2811. Found: 455.2812. HPLC purity 97.0% (40 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-hexyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (41)
This compound was prepared in 66% yield as a yellow oil. 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 15.2 Hz, 2H), 7.84−7.80 (overlapped, 2H), 7.79 (d, J = 15.2 Hz, 2H), 7.42−7.28 (m, 6H), 4.31 (t, J = 7.3 Hz, 4H), 1.90−1.80 (m, 4H), 1.41−1.25 (m, 12H), 0.86 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.5, 148.0, 143.5, 136.0, 131.5, 127.9, 124.3, 123.6, 120.6, 110.2, 44.0, 31.5, 30.8, 26.7, 22.6, 14.1. IR (KBr): 2954, 2928, 2857, 1654, 1625, 1603, 1466, 1409, 1331, 1183, 1094 cm−1. HRMS (ESI), m/z calculated for C31H39N4O [M + H]+: 483.3124. Found: 483.3130. HPLC purity 96.1% (25 min run of 65−90% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-heptyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (42)
This compound was prepared in 85% yield as a yellow solid; mp 171−173 °C. 1H NMR (300 MHz, CDCl3): δ 7.88 (d, J = 15.3 Hz, 2H), 7.85−7.80 (overlapped, 2H), 7.80 (d, J = 15.3 Hz, 2H), 7.42−7.30 (m, 6H), 4.33 (t, J = 7.3 Hz, 4H), 1.91−1.80 (m, 4H), 1.45−1.15 (m, 16H), 0.86 (t, J = 6.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.7, 148.0, 143.5, 136.0, 131.5, 128.0, 124.3, 123.6, 120.6, 110.2, 44.0, 31.7, 30.8, 29.0, 27.0, 22.7, 14.2. IR (KBr): 3062, 2926, 2855, 1654, 1625, 1601, 1407, 1331, 1181, 1093 cm−1. HRMS (ESI), m/z calculated for C33H42N4O [M + H]+: 511.3437. Found: 511.3431. HPLC purity 100.0% (40 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
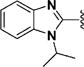
(1E,4E)-1,5-Bis(1-isopropyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (43)
This compound was prepared in 63% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 15.8 Hz, 2H), 7.85 (d, J = 15.8 Hz, 2H), 7.81 (d, J = 5.2 Hz, 2H), 7.62−7.52 (m, 2H), 7.31−7.28 (m, 4H), 5.06−4.97 (m, 2H), 1.71 (d, J = 6.8 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 187.8, 147.6, 144.0, 134.5, 131.8, 128.5, 123.9, 123.3, 120.8, 112.2, 48.2, 22.0. IR (KBr): 3053, 2977, 2935, 1653, 1621, 1608, 1456, 1385, 1184, 1104 cm−1. HRMS (ESI), m/z calculated for C25H26N4O [M + H]+: 399.2185. Found: 399.2192. HPLC purity 96.0% (20 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
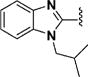
(1E,4E)-1,5-Bis(1-isobutyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (44)
This compound was prepared in 85% yield as a yellow solid; mp 113−115 °C. 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 15.2 Hz, 2H), 7.83−7.81 (overlapped, 2H), 7.79 (d, J = 15.2 Hz, 2H), 7.41−7.31 (m, 6H), 4.14 (d, J = 7.5 Hz, 4H), 2.31−2.18 (m, 2H), 0.98 (d, J = 6.6 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 187.7, 148.4, 143.4, 136.4, 131.5, 128.2, 124.3, 123.6, 120.6, 110.5, 51.2, 30.2, 20.4. IR (KBr): 2961, 2931, 2872, 1654, 1625, 1601, 1468, 1369, 1331, 1299, 1186, 1094 cm−1. HRMS (ESI), m/z calculated for C27H31N4O [M + H]+: 427.2498. Found: 427.2510. HPLC purity 99.2% (40 min run of 45−65% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
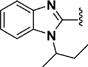
(1E,4E)-1,5-Bis(1-(sec-butyl)-1H-benzo[d]imidazol-2-yl)-penta-1,4-dien-3-one (45)
This compound was prepared in 98% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.98−7.74 (overlapped, 6H), 7.56−7.53 (m, 2H), 7.37−7.27 (m, 4H), 4.74−4.66 (m, 2H), 2.23−2.15 (m, 2H), 2.10−1.94 (m, 2H), 1.71 (d, J = 6.9 Hz, 6H), 0.80 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.7, 148.3, 143.9, 134.6, 131.9, 128.5, 123.9, 123.3, 120.8, 112.2, 54.4, 28.7, 20.4, 11.3. IR (KBr): 3052, 2967, 2933, 2875, 1652, 1620, 1596, 1454, 1407 cm−1. HRMS (ESI), m/z calculated for C27H30N4O [M + H]+: 427.2498. Found: 427.2506. HPLC purity 97.7% (30 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
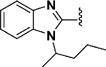
(1E,4E)-1,5-Bis(1-(pentan-2-yl)-1H-benzo[d]imidazol-2-yl)-penta-1,4-dien-3-one (46)
This compound was prepared in 76% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 8.05−7.80 (overlapped, 6H), 7.59−7.56 (m, 2H), 7.36−7.29 (m, 4H), 4.87−4.83 (m, 2H), 2.25−2.12 (m, 2H), 2.04−1.87 (m, 2H), 1.72 (d, J = 6.9 Hz, 6H), 1.35−1.18 (m, 2H), 1.14−0.99 (m, 2H), 0.86 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 187.6, 148.0, 143.6, 134.5, 132.0, 128.4, 123.9, 123.4, 120.6, 112.2, 52.7, 37.7, 20.6, 20.0, 13.7. IR (KBr): 2958, 2931, 2872, 1653, 1620, 1599, 1454, 1407, 1382 cm−1. HRMS (ESI), m/z calculated for C27H31N4O [M + H]+: 455.2811. Found: 455.2818. HPLC purity 98.0% (30 min run of 65−90% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-(pentan-3-yl)-1H-benzo[d]imidazol-2-yl)-penta-1,4-dien-3-one (47)
This compound was prepared in 88% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.93−7.80 (overlapped, 6H), 7.53 (d, J = 7.2 Hz, 2H), 7.37−7.27 (m, 4H), 4.44−4.38 (m, 2H), 2.29−2.13 (m, 4H), 2.11−1.97 (m, 4H), 0.78 (t, J = 7.3 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 187.7, 149.1, 143.9, 134.6, 131.9, 128.5, 123.8, 123.3, 120.7, 112.2, 60.9, 27.2, 11.2. IR (KBr): 2965, 2933, 2875, 1653, 1609, 1454, 1385 cm−1. HRMS (ESI), m/z calculated for C29H35N4O [M + H]+: 455.2811. Found: 455.2819. HPLC purity 95.1% (40 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
(1E,4E)-1,5-Bis(1-isopentyl-1H-benzo[d]imidazol-2-yl)penta-1,4-dien-3-one (48)
This compound was prepared in 98% yield as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.86 (d, J = 15.2 Hz, 2H), 7.82−7.78 (overlapped, 2H), 7.77 (d, J = 15.2 Hz, 2H), 7.38−7.24 (m, 6H), 4.27 (t, J = 7.3 Hz, 4H), 1.78−1.60 (m, 6H), 1.00 (d, J = 6.1 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 187.5, 147.8, 143.3, 135.8, 131.5, 127.7, 124.2, 123.6, 120.5, 110.0, 42.3, 39.5, 26.0, 22.5. IR (KBr): 2956, 2929, 2870, 1654, 1613, 1458, 1409, 1331 cm−1. HRMS (ESI), m/z calculated for C29H35N4O [M + H]+: 455.2811. Found: 455.2824. HPLC purity 99.4% (40 min run of 45−80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
Cell Culture
All cell lines were initially purchased from American Type Culture Collection (ATCC). The PC-3 and LNCaP prostate cancer cell lines and the HeLa cervical cancer cell line were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cultures were maintained in a high humidity environment supplemented with 5% carbon dioxide at a temperature of 37 °C. The DU-145 prostate cancer cells were routinely cultured in Eagle’s minimum essential medium (EMEM) supplemented with 10% FBS and 1% penicillin/streptomycin.
Trypan Blue Dye Exclusion Assay
PC-3 or DU145 or LNCaP or HeLa cells were plated in 24-well plates at a density of 20 000 each well in 10% FBS RPMI-1640 medium. The cells were then treated with curcumin or with synthesized mimics separately at different doses for 3 days, while equal treatment volumes of DMSO were used as vehicle control. Cell numbers were counted with a cell viability analyzer (VI-Cell XR, Beckman Coulter). The ratio of drug treated viable cell numbers to vehicle treated viable cell numbers was defined as percentage viability, and variation between replicate experiments is not greater than 5%. The inhibitory rates in Table 2 were expressed as cell viability reduction rates (percentage viability of untreated cell culture control–percentage viability of drug-treated cell culture). The IC50 value is the concentration of each compound that inhibits cell growth by 50% under the experimental conditions and is the average from triplicate determinations that were reproducible and statistically significant. For calculating the IC50 values, a linear inhibition was made based on at least five dosages for each compound.
WST-1 Cell Proliferation Assay
PC-3 or DU-145 or LNCaP or HeLa cells were plated in 96-well plates at a density of 3200 each well in 200 μL of culture medium. The cells were then treated with curcumin or with synthesized analogues separately at different doses from 0.1 to 1 μM for 3 days, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in a CO2 incubator at 37 °C for 3 days. Then 10 μL of the premixed WST-1 cell proliferation reagent (Clontech) was added to each well. After mixing gently for 1 min on an orbital shaker, the cells were incubated for additional 3 h at 37 °C. To ensure homogeneous distribution of color, it is important to mix gently on an orbital shaker for 1 min. The absorbance of each well was measured using a microplate-reader (Synergy HT, BioTek) at a wavelength of 430 nm. The IC50 value is the concentration of each compound that inhibits cell proliferation by 50% under the experimental conditions and is the average from triplicate determinations that were reproducible and statistically significant. For calculating the IC50 values, a linear proliferative inhibition was made based on at least five dosages for each compound.
Pharmacokinetic Study.37
Female C57BL/6 mice were used for the pharmacokinetic study of curcumin and compounds 14 and 24. Mice were given oral gavage containing PBS and ethanol-dissolved curcumin, 14, or 24 at a single dose of 1 (mg/kg)/mouse. After oral administration, blood samples were collected from the orbital sinus of the mice at 0.5, 1, 2, 4, and 24 h with each group of five mice subjected to only one sampling. Mice blood was collected with a capillary into 1.5 mL microcentrifuge tubes containing 0.1 mL of 10% EDTA anticoagulant. Plasma was then separated from red cells by centrifugation in a refrigerated centrifuge at 4 °C and transferred to a separate tube. The plasma samples were frozen at −80 °C until analysis. All procedures involving these animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by Xavier University Animal Care and Use Committee. The facilities and laboratory animals program of Xavier University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
High Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC–MS/MS) for Drug Analysis in Plasma Samples
Plasma samples were extracted with chloroform/methanol (2:1) using traditional Folch method for lipid extraction. Methanol (1 mL) and chloroform (2 mL) were added to each plasma sample followed by addition of 5 ng of trans-tamoxifen-13C2,15N to each sample as the internal standard. The mixtures were stored at −20 °C overnight. Next the samples were sonicated for 5 min and centrifuged with a Thermo Scientific Heraeus Megafuge16 centrifuge. The top layer was transferred to another test tube. The bottom layer was washed with 1 mL of chloroform/methanol (2:1), centrifuged, and the top layer was transferred and combined with the previous top layer. Eight-tenth of a milliliter of HPLC grade water was added to the extracts. After vortexing, the mixture was centrifuged. The bottom layer was dried out with nitrogen and resuspended in 100 μL of HPLC grade acetonitrile. An aliquot of 10 μL of sample was injected onto a Hypersil Gold column (50 mm × 2.1 mm; particle size 1.9 μm, Thermo Scientific) on a Dionex Ultimate 3000 UPLC system equipped with a TSQ Vantage triple quadrupole mass spectrometer for analysis. A binary mobile phase (A, water with 0.05% formic acid; B, acetonitrile with 0.05% formic acid) was used to achieve the gradient of initial 30% B for 1 min and then to 80% B at 8 min, to 100% B at 9 min, and returned to 30% B for 4 min. The flow rate was controlled at 0.6 mL/min. The settings of HESI source were as follows: spray voltage (3200 V); vaporizer temperature (365 °C); sheath gas pressure (45 psi); auxiliary gas pressure (10 psi); capillary temperature (330 °C). Nitrogen was used as the sheath gas and auxiliary gas. Argon was used as the collision gas.
Supplementary Material
Acknowledgments
This work was financially supported by California State University (CSU)—Fresno and CSU Program for Education and Research in Biotechnology (CSUPERB) New Investigator Award. Pharmacokinetic studies and HRMS were supported by NIH RCMI program at Xavier University of Louisiana through Grant 2G12MD007595-064 (G.W.) and NIGMS-NIH through Grant 1U54GM104940 (G.W.).
ABBREVIATIONS
- DMP
Dess–Martin periodinane
- FBS
fetal bovine serum
- PANIS
pan assay interference compounds
- TB
trypan blue dye exclusion assay
- PTLC
preparative thin-layer chromatography
Footnotes
Supporting Information
1H NMR and 13C NMR spetra. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.5b00470.
Notes
The authors declare no competing financial interest.
References
- 1.Parkin DM, Brag F, Ferlay J, Pisani P. Global cancer statistics. Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Teiten M-H, Gaascht F, Eifes S, Dicato M, Diederich M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010;5:61–74. doi: 10.1007/s12263-009-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal BB. Prostate cancer and curcumin: add spice to your life. Cancer Biol Ther. 2008;7:1436–1440. doi: 10.4161/cbt.7.9.6659. [DOI] [PubMed] [Google Scholar]
- 4.Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer. I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 5.Toennesen HH, Karlsen J, Mostad A. Structural studies of curcuminoids. I. The crystal structure of curcumin. Acta Chem Scand, Ser B. 1982;B36:475–479. [Google Scholar]
- 6.Garcea G, Jones DJL, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010;30:818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 8.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonn GA, Aronson W, Litwin MS. Impact of diet on prostate cancer: a review. Prostate Cancer Prostatic Dis. 2005;8:304–310. doi: 10.1038/sj.pcan.4500825. [DOI] [PubMed] [Google Scholar]
- 10.Mimeault M, Batra SK. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chin Med. 2011;6:31. doi: 10.1186/1749-8546-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nambiar D, Singh RP. Advances in prostate pancer chemoprevention: a translational perspective. Nutr Cancer. 2013;65(Suppl. 1):12–25. doi: 10.1080/01635581.2013.785006. [DOI] [PubMed] [Google Scholar]
- 12.Hajare RA, Bajad SA, Parwani KS, Chandekar NA, Chandewar AV. Therapeutic potential of curcumin in prostate cancer. Pharma Rev. 2009;7:123–124. [Google Scholar]
- 13.Kurien BT, Scofield RH. Curry spice curcumin and prostate cancer. Mol Nutr Food Res. 2009;53:939–940. doi: 10.1002/mnfr.200990022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cretu E, Trifan A, Vasincu A, Miron A. Plant-derived anticancer agents—curcumin in cancer prevention and treatment. Rev Med-Chir Soc Med Nat Iasi. 2012;116:1223–1229. [PubMed] [Google Scholar]
- 16.Chen Q-H. Curcumin-based anti-prostate cancer agents. Anti-Cancer Agents Med Chem. 2015;15:138–156. doi: 10.2174/1871520615666150116102442. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Hutzen B, Ball S, Foust E, Sobo M, Deangelis S, Pandit B, Friedman L, Li C, Li PK, Fuchs J, Lin J. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation on breast and prostate cancer cells. Cancer Sci. 2009;100:1719–1727. doi: 10.1111/j.1349-7006.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaan N, Zhong Q, Fernandez J, Chen G, Hussain AM, Zheng S, Wang G, Chen Q-H. Design, synthesis, and evaluation of novel heteroaromatic analogs of curcumin as anti-cancer agents. Eur J Med Chem. 2014;75:123–131. doi: 10.1016/j.ejmech.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 20.Baell JB. Screening-based translation of public research encounters painful problems. ACS Med Chem Lett. 2015;6:229–234. doi: 10.1021/acsmedchemlett.5b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao B, Wang Y, Ding K, Neamati N, Long Y-Q. Synthesis of the pyridinyl analogues of dibenzylideneacetone (pyr-dba) via an improved Claisen–Schmidt condensation, displaying diverse biological activities as curcumin analogues. Org Biomol Chem. 2012;10:1239–1245. doi: 10.1039/c1ob06773g. [DOI] [PubMed] [Google Scholar]
- 22.Sehnal P, Taghzouti H, Fairlamb IJS, Jutand A, Lee AF, Whitwood AC. Heteroaromatic analogues of dibenzylideneacetone (dba) and Pd02(het-dba)3 complexes: effect of a thienyl moiety on the reactivity of Pd0(η2-thn-dba)(PPh3)2/Pd0(PPh3)2 (n=1 or 2) and Pd0 (η2-thn-dba)(dppe)/Pd0(dppe) in oxidative addition reactions with iodobenzene. Organometallics. 2009;28:824–829. [Google Scholar]
- 23.Corbel B, Medinger L, Haelters JP, Sturtz G. An efficient synthesis of dialkyl 2-oxoalkanephosphonates and diphenyl-2-oxoalkylphosphine oxides from 1-chloralkyl ketones. Synthesis. 1985:1048–105185. [Google Scholar]
- 24.Seto M, Miyamoto N, Aikawa K, Aramaki Y, Kanzaki N, Iizawa Y, Baba M, Shiraishi M. Orally active CCR5 antagonists as anti-HIV-1 agents. Part 3: synthesis and biological activities of 1-benzazepine derivatives containing a sulfoxide moiety. Bioorg Med Chem. 2005;13:363–386. doi: 10.1016/j.bmc.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Woo HB, Eom YW, Park KS, Ham J, Ahn CM, Lee S. Synthesis of substituted benzimidazolyl curcumin mimics and their anticancer activity. Bioorg Med Chem Lett. 2012;22:933–936. doi: 10.1016/j.bmcl.2011.12.074. [DOI] [PubMed] [Google Scholar]
- 26.Valdez-Padilla D, Rodriguez-Morales S, Hernandez-Campos A, Hernandez-Luis F, Yepez-Mulia L, Tapia-Contreras A, Castillo R. Synthesis and antiprotozoal activity of novel 1-methylbenzimidazole derivatives. Bioorg Med Chem. 2009;17:1724–1730. doi: 10.1016/j.bmc.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 27.Campaigne E, Thompson RL, Van Werth JE. Some heterocyclic aldehyde thiosemicarbazones possing anti-viral activity. J Med Chem. 1959;1:577–599. doi: 10.1021/jm50007a003. [DOI] [PubMed] [Google Scholar]
- 28.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 29.Stone KR, Mickey DD, Wunderli H, Michey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 30.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GGP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 31.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 33.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 34.Kanai M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol. 2014;28:9384–9391. doi: 10.3748/wjg.v20.i28.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complementary Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wichitnithad W, Nimmannit U, Wacharasindhu S, Rojsitthisak P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules. 2011;16:1888–1900. doi: 10.3390/molecules16021888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y-Z, Yang C-W, Hsu H-Y, Qiu Y-Q, Yeh T-K, Chang H-Y, Chao Y-S, Lee S-J. Synthesis and biological evaluation of tylophorine-derived dibenzoquinolines as orally active agents: exploration of the role of tylophorine E ring on biological activity. J Med Chem. 2012;55:10363–10377. doi: 10.1021/jm300705j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


