Orthostatic myoclonus (OM) is a clinical phenomenon in which myoclonus of the lower limbs appears or becomes worse upon standing.1 OM usually affects patients older than 65 years and may be a frequent cause of unsteadiness upon standing in the elderly.2 Although the underlying etiology remains unclear, OM has predominantly been described in association with neurodegenerative diseases.1 We describe a patient with OM in association with antibodies against contactin-associated protein-2 (Caspr2) whose symptoms markedly improved with immunotherapy.
Case report.
A 68-year-old man presented to our department with a slowly progressive 2-year history of severe unsteadiness and involuntary jerks of his legs upon standing, along with coexisting burning pain of his feet and mild cognitive deficits. Symptoms first developed 1 month after a severe thoracic herpes zoster infection. On examination, there was slightly asymmetric (left more than right), non–stimulus sensitive, action myoclonus in the outstretched upper and lower limbs. Leg myoclonus was exacerbated upon standing and improved if the patient leaned forward on a chair. Gait was mildly unsteady with intermittent myoclonic jerks (video on the Neurology® Web site at Neurology.org). Distal allodynia and decreased sensation to pain and temperature were present in the legs.
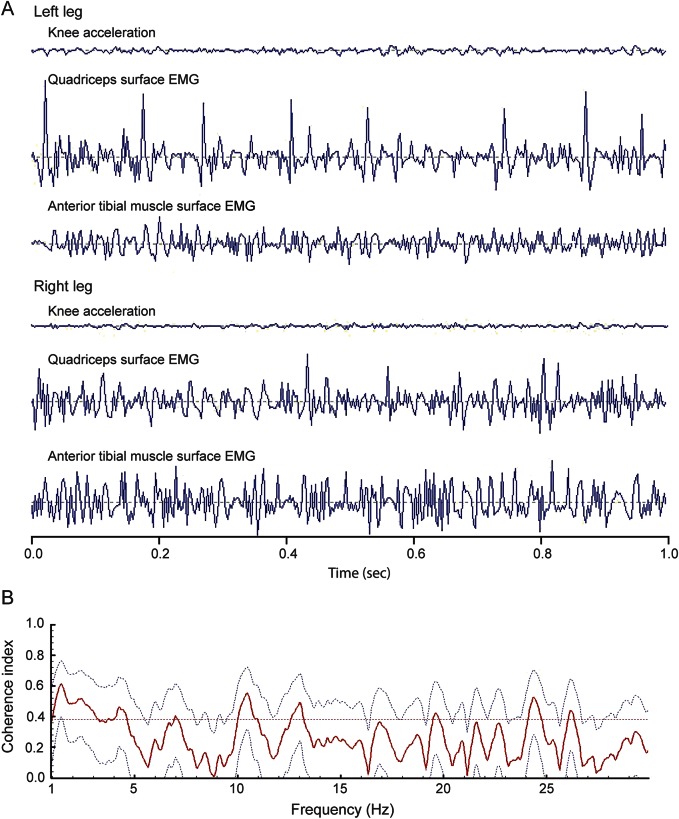
Upon standing, surface EMG of the legs showed short (<50 ms), irregular, high-frequency (10- to 13-Hz) myoclonic bursts without intermittent silent periods (figure 1A). Homologous muscles in the right and left legs did not display significant coherence of bursts (figure 1B). Needle EMG of the vastus lateralis, tibialis anterior, biceps brachii, and abductor digiti minimi muscles were normal and without evidence of neuromyotonic discharges. Routine nerve conduction studies and sensory and motor evoked potentials of the arms and legs were unremarkable. MRI of the brain and spinal cord were normal; there was no evidence of thoracic myelopathy. EEG demonstrated generalized slowing (figure e-1). Autonomic testing showed marked orthostatic hypotension and reduced heartbeat variability due to sympathetic failure. Cognitive testing revealed executive dysfunction, slight impairment of verbal memory, and impaired attention. The CSF revealed mild lymphocytic pleocytosis (10/μL), elevated total protein (516 mg/dL), and no intrathecal synthesis of immunoglobulins. Testing for antineuronal antibodies using rat brain immunohistochemistry and cell-based assays revealed Caspr2 immunoglobulin G antibodies (titer CSF: 1:360; serum: 1:6,400). Radioimmunoassay for voltage-gated potassium channel complex antibodies (VGKC) was positive in CSF (52 pmol/L) and serum (466 pmol/L). No other autoantibodies were detected. Whole-body CT and fluorodeoxyglucose PET did not show evidence of thymoma or other tumors.
Figure 1. Surface EMG recordings show noncoherent short, irregular, high-frequency myoclonus of the legs while standing.
(A) Surface EMG recordings of both quadriceps and anterior tibial muscles and corresponding knee acceleration. Note the short duration (<50 ms), irregular frequency (10–13 Hz), nonsynchronized bursts of myoclonus in all examined muscles. (B) The lack of coherence of EMG bursts over the entire frequency range is demonstrated by Fourier analysis of the right and left quadriceps muscle. Mean coherence index (solid red) and 95% confidence interval (gray dashed lines) are not significantly above 0.4. Coherence index of 1 denotes perfect synchrony, while 0 denotes perfect asynchrony.
Treatment with methylprednisolone (1 g, IV) was started but discontinued the following day due to the development of severe psychosis. The patient then started IV immunoglobulins (IVIg, 2 g/kg body weight), resulting in a few days in near complete resolution of the myoclonus and substantial improvement of his stance (video). The neuropathic pain improved; however, add-on therapy with carbamazepine was necessary. After 6 weeks, the OM recurred and again responded to IVIg. The patient has been maintained on IVIg every 8–12 weeks and remained stable over 12 months of follow-up.
Discussion.
We report a patient with prominent unsteadiness upon standing and involuntary jerks of the lower limbs, whose clinical presentation and EMG findings fulfill the proposed criteria for OM.1 The detection of Caspr2 antibodies and the near complete improvement after IVIg strongly support an autoimmune etiology of the OM in this patient.
Antibodies against Caspr2 were initially reported in patients with encephalitis or peripheral nerve hyperexcitability, or both (Morvan syndrome).3,4 However, the clinical spectrum of Caspr2-associated autoimmunity is still being characterized and chronic pain has been shown to be a frequent accompaniment.5 Our patient had a combination of OM with chronic neuropathic pain and mild cognitive deficits.
An autoimmune etiology has previously been proposed in a few patients with unsteadiness upon standing. Hegde et al.6 reported a patient with slow orthostatic tremor and superimposed myoclonus in association to an antibody against an unknown antigen who had an excellent response to IVIg. In a case series of patients with “isolated generalized polymyoclonus,” 14 of 19 patients had stance impairment and one had myoclonic knee buckling as in our patient.7 Five of these patients had cancer or antineuronal antibodies: 2 collapsin response mediator protein 5 (CRMP5) antibodies, 1 VGKC antibodies, 1 ganglionic acetylcholine receptor antibodies and breast cancer, and 1 breast cancer without antibodies, although testing for recently described neuronal cell surface antibodies, such as Caspr2, was not available.7
Our case suggests that (1) treatable, immune-mediated forms of OM can be identified by assessment of neuronal antibodies, and (2) OM should be added to the spectrum of symptoms associated with Caspr2 antibodies.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank Dr. M. R. Rosenfeld for critical review and editing of the manuscript.
Footnotes
Supplemental data at Neurology.org
Author contributions: Dr. Gövert contributed to design and conceptualization of the study, clinical assessment and data acquisition, as well as drafting and revising the manuscript. Dr. Witt contributed to clinical assessment, data acquisition, and revising the manuscript. Dr. Erro contributed to analysis of the data and revision of the manuscript. He has been partly supported by COST (ref. COST-STSMBM1101-14567). Dr. Hellriegel contributed to data acquisition, analysis, and revision of the manuscript. Dr. Paschen contributed to data acquisition, analysis, and revision of the manuscript. Dr. Martinez-Hernandez contributed to data acquisition, analysis, and revision of the manuscript. Dr. Wandinger contributed to data acquisition, analysis, and revision of the manuscript. Dr. Deuschl contributed to clinical assessment and revision of the manuscript. Dr. Dalmau contributed to analysis, drafting, and revision of the manuscript. Dr. Leypoldt contributed to clinical assessment, data acquisition, analysis, drafting, and revision of the manuscript.
Study funding: Supported in part by Instituto de Salud Carlos III-FEDER, Spain (FIS 14/00203, J.D.), Fundació CELLEX (J.D.), Agència de Gestió d'Ajuts Universitaris i de Recerca (2014SGR93, J.D.) and the US National Institute of Mental Health (MH094741-01).
Disclosure: F. Gövert reports no disclosures in relation to this report. He has received travel support from Ipsen. K. Witt reports no disclosures in relation to this report. He received grants from German Research Council and the German Ministry of Education and Health and receives fees from TEVA, GlaxoSmithKline, Medtronic, and Desitin. R. Erro has been partly supported by COST (ref. COST-STSMBM1101-14567). H. Hellriegel reports no disclosures relevant to the manuscript. S. Paschen reports no disclosures in relation to this report. He received speaker honoraria from Medtronic, Merz, and Ipsen. E. Martinez-Hernandez and K. Wandinger report no disclosures relevant to the manuscript. G. Deuschl reports no disclosures in relation to this report. He received personal fees from Medtronic, Desitin, and UCB, and grants from German Research Council, the German Ministry of Education and Health, and Medtronic; he has a patent pending. J. Dalmau reports receiving royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and licensing fees from Euroimmun for the use of NMDAR and GABAbR as autoantibody tests. F. Leypoldt reports receipt of speaker's honoraria from Grifols, Teva, and Biogen. His institution is involved in commercial antibody testing but he receives no personal reimbursement. Go to Neurology.org for full disclosures.
References
- 1.Glass GA, Ahlskog JE, Matsumoto JY. Orthostatic myoclonus: a contributor to gait decline in selected elderly. Neurology 2007;68:1826–1830. [DOI] [PubMed] [Google Scholar]
- 2.Gasca-Salas C, Arcocha J, Artieda J, Pastor P. Orthostatic myoclonus: an underrecognized cause of unsteadiness? Parkinsonism Relat Disord 2013;19:1013–1017. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 2011;69:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein CJ, Lennon VA, Aston PA, McKeon A, Pittock SJ. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology 2012;79:1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegde M, Glass GA, Dalmau J, Christine CW. A case of slow orthostatic tremor, responsive to intravenous immunoglobulin. Mov Disord 2011;26:1563–1565. [DOI] [PubMed] [Google Scholar]
- 7.McKeon A, Pittock SJ, Glass GA, et al. Whole-body tremulousness: isolated generalized polymyoclonus. Arch Neurol 2007;64:1318–1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.