Abstract
Objective:
To investigate the role of multiple distributed brain networks, including the default mode, fronto-temporo-parietal, and cingulo-opercular networks, which mediate domain-general and task-specific processes during speech production after aphasic stroke.
Methods:
We conducted an observational functional MRI study to investigate the effects of a previous left hemisphere stroke on functional connectivity within and between distributed networks as patients described pictures. Study design included various baseline tasks, and we compared results to those of age-matched healthy participants performing the same tasks. We used independent component and psychophysiological interaction analyses.
Results:
Although activity within individual networks was not predictive of speech production, relative activity between networks was a predictor of both within-scanner and out-of-scanner language performance, over and above that predicted from lesion volume, age, sex, and years of education. Specifically, robust functional imaging predictors were the differential activity between the default mode network and both the left and right fronto-temporo-parietal networks, respectively activated and deactivated during speech. We also observed altered between-network functional connectivity of these networks in patients during speech production.
Conclusions:
Speech production is dependent on complex interactions among widely distributed brain networks, indicating that residual speech production after stroke depends on more than the restoration of local domain-specific functions. Our understanding of the recovery of function following focal lesions is not adequately captured by consideration of ipsilesional or contralesional brain regions taking over lost domain-specific functions, but is perhaps best considered as the interaction between what remains of domain-specific networks and domain-general systems that regulate behavior.
Complex behaviors emerge from distributed functional brain networks in several cortical regions.1–5 Within the same cortical region, multiple functionally distinct but anatomically overlapping components of different brain networks may exist.6–8 Furthermore, while some brain networks are specific to a motor, sensory, or cognitive domain, others are domain-general across a wide range of cognitive tasks.4,8–14 Therefore successful execution of a specific task (such as language) likely depends on interaction among several brain networks, crossing several cognitive domains.
Language is best considered as core language networks regulated by domain-general networks.8,10,15,16 A study of distinct but overlapping networks revealed a left fronto-temporo-parietal network (LFTP) that was active during propositional speech but not other tasks.6 This speech-task-specific network had a spatial distribution similar to that attributed to language function.17–19 Investigators observed right fronto-temporo-parietal network (RFTP) deactivation during speech, but activation for other tasks requiring sustained attention, suggesting a degree of domain-generality. They observed default mode network (DMN) deactivation during all tasks, but mostly during speech. The DMN, a domain-general network, encompasses inferior parietal lobules, ventral medial prefrontal and posterior cingulate cortices, and somewhat more variably the lateral temporal lobes and hippocampi.20 This network reliably demonstrates task-dependent deactivations that typically correlate with task difficulty.9,11,20 However, the DMN contains multiple dissociable but interacting components involved in a variety of functions (semantic memory, episodic memory, decision-making, and internally directed cognition).8,9,13,14 Therefore, activity related to these cognitive functions may not be captured by net deactivation within specific regions of the DMN over the duration of a task.8
The study also identified cingulo-opercular network (CingOper) activation that was specific to a nonverbal decision task, indicating its role in detection and response to salient stimuli.4,21 Activity within components of this network also increases with language tasks that require effort, and may support recovery from aphasia.16,22 CingOper is a domain-general network that can be active as part of a larger “multiple demand” system.4,12
Functional connectivity within and between these networks is disrupted in many pathologic processes.23–26 Although language after a stroke is dependent on residual function within language-specific networks, interactions with other domain-general networks may also play a role.16 In the present study, we investigated propositional speech production after left hemisphere stroke. Here, speech production refers to multiple processes, ranging from concept formation to articulatory plan formation, and specifically excludes pure articulatory deficits such as dysarthria. We hypothesized that the relative balance of activity between domain-general and domain-specific networks would be altered in patients compared to healthy participants by amounts that correlated with measures of speech production.
METHODS
Participants.
We enrolled 53 consecutive patients with left hemisphere infarcts and premorbid fluency in English (aged 62 ± 14 years [mean ± SD], male:female = 1:0.7) (figure e-1 and table e-1 on the Neurology® Web site at Neurology.org). We excluded individuals with prior stroke resulting in aphasia, other neurologic illness, severe receptive aphasia, or inability to comply with fMRI tasks. We included 24 right-handed, age-matched English-speaking healthy participants (controls) (aged 57 ± 11 years; years of education 16 ± 1.8; male:female = 1:4). See the e-Methods for cluster analysis of the patient group.
Standard protocol approvals, registrations, and consents.
The National Research Ethics Service approved the study. All participants gave informed consent prior to participation.
Behavioral assessments.
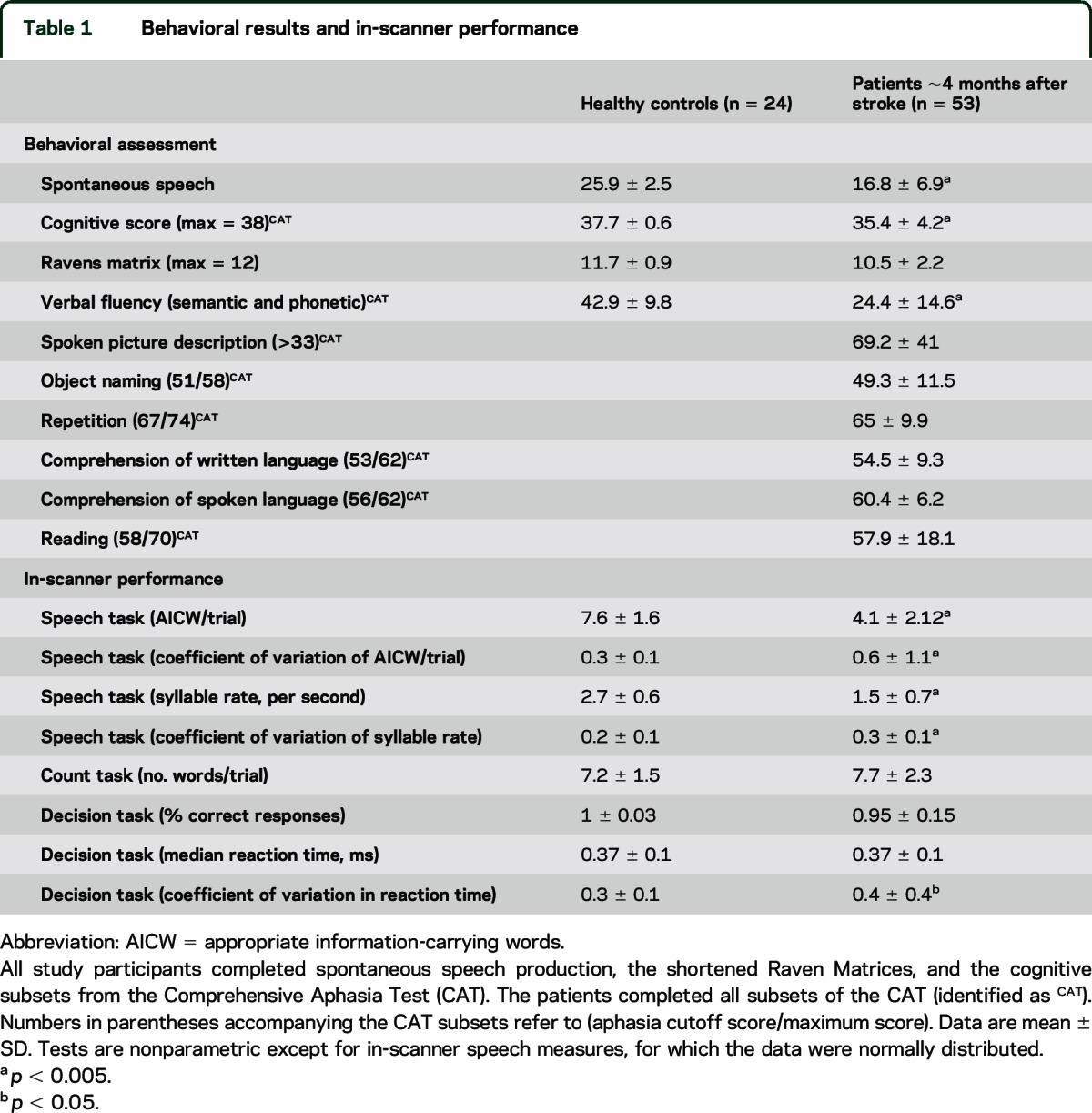
We tested patients at approximately 4 months (111 ± 27 days) poststroke using the Comprehensive Aphasia Test (CAT),27 modified detailed quantitative analysis of speech production (spontaneous speech),28 and shortened Raven Progressive Matrix Test.29 Controls underwent the same assessments, except only the cognitive sections of the CAT were used. In-scanner measures of task performance were assessed in all participants (table 1 and e-Methods). The in-scanner appropriate information-carrying words (AICW) measure was related to imaging measures, since principal component analysis showed that AICW related best to a dominant fluency/phonology factor that explained most variance for all assessments (e-Methods).
Table 1.
Behavioral results and in-scanner performance
fMRI procedure.
We acquired whole-brain fMRI data on a Siemens (Munich, Germany) Trio 3T scanner using gradient-echo sequences (e-Methods). A 1 mm3 T1-weighted image and field maps for registration purposes were also acquired approximately 4 months after ictus. We used sparse fMRI design to minimize artifacts associated with overt speech.6 Across each session, the participants performed 3 fMRI runs, each consisting of 4 conditions (20 speech, 16 count, 16 decision, and 15 rest), performed over 7-second epochs. During speech, the participants defined colored pictures of objects. During count, they counted up from 1 at a rate of 1/s. During the rest trials, they simply viewed a fixation cross. For the decision trials, participants were required to press a button upon seeing a target (a blue square), inhibiting their response for a distractor (an orange circle). See e-Methods for more detail.
Extraction of time courses from the reference networks.
For fMRI data preprocessing, we used FMRIB's Software Library (www.fmrib.ox.ac.uk/fsl) and previously published methods (see e-Methods).30 We then registered the preprocessed data to the Montreal Neurological Institute (152) standard space (e-Methods). We identified the reference networks of interest (LFTP, RFTP, CingOper, and DMN) using a temporal-concatenation group independent component analysis (ICA)31 from the controls' data. ICA is a multivariate data-driven approach that separates data into maximally independent spatiotemporal maps (or components), each explaining a unique level of variance in the data, each with a time course that may relate to a coherent neural signal associated with a specific task, artifact, or both. Using this technique, functional networks with opposing directions of activity can be extracted, even if there is spatial overlap, making it advantageous over subtractive univariate analysis.6,7,17,32
The ICA in this study was set to decompose the control data into 55 components that included the DMN, LFTP, RFTP, and CingOper (figure 1A) based on our previous study of ICA at different dimensionalities.6 In that study, ICA reliably extracted a consistent LFTP with a time course related to speech production at high dimensionalities (45, 50, 55, 60) but not at lower dimensionalities. In contrast, DMN, RFTP, and CingOper were consistently extracted at all dimensionalities. We extracted the time course from all the voxels in each of the 4 reference networks using regression analyses (for which each fMRI run was the dependent variable and the 55 spatial maps, including that of the 4 reference networks, was simultaneously entered as the independent variable).
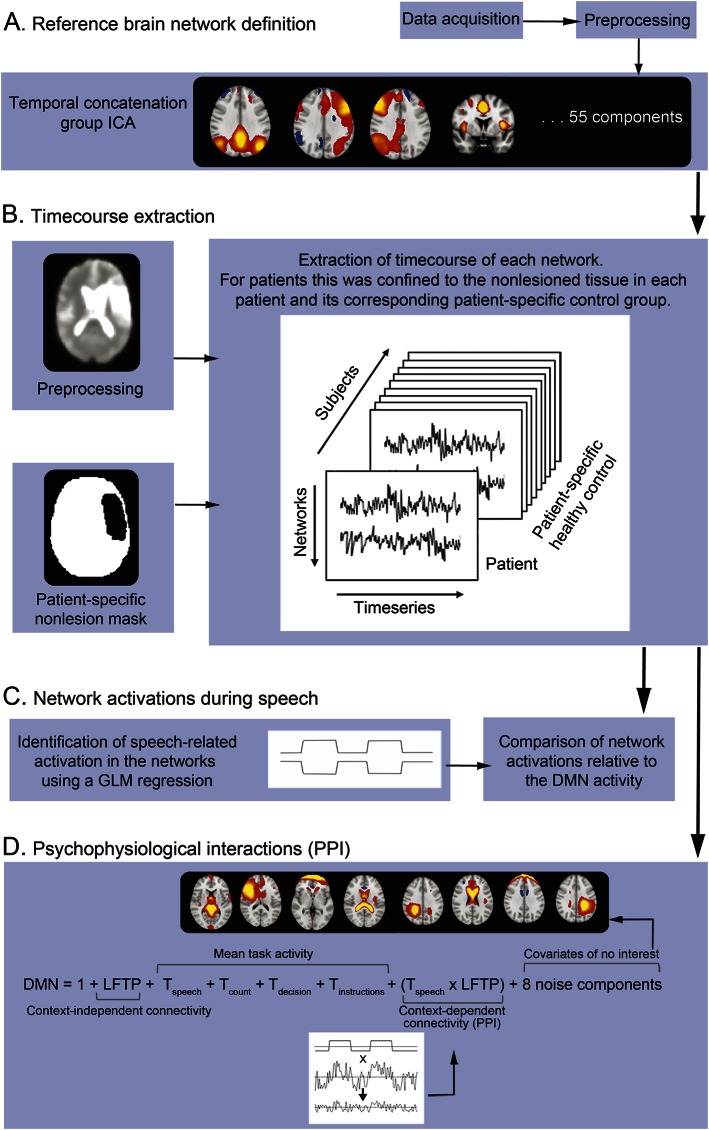
Figure 1. Methodology pipeline.
(A) We identified the reference brain networks using temporal concatenation group independent component analysis (ICA) that decomposed the control data into 55 components (published previously6). The 4 main networks of interest (left fronto-temporo-parietal network [LFTP] right fronto-temporo-parietal network, cingulo-opercular network, and default mode network [DMN]) are shown. (B) We extracted time courses for each component using regression analysis, in which the spatial maps of the networks were entered as the independent variable. For patients, we performed an additional step creating a patient-specific control group for each patient, extracting the network time courses only in the nonlesioned brain. Finally, we normalized the time course of each patient to the patient-specific controls. (C) We also performed further regression analysis, using the task design matrix as independent variable and the extracted time courses as dependent variable. We examined resulting activations during speech in 4 networks (referred to as activations in healthy controls and normalized activations in patients). We also investigated activity of these networks relative to the DMN (differential activations). (D) We also entered the time courses into PPI analyses for estimation of the speech task-specific functional connectivity between networks. The dependent variable was the time course for the DMN. Independent variables are shown.
For patients, we limited the time course extraction to nonlesioned tissue by including an individual-specific nonlesion mask in the regression step (figure 1B). For each patient, the same mask was applied to all fMRI runs from the controls. Thus, for each of the 53 patients a separate time course was derived for each control run, masking out the lesioned tissue allowing fMRI measures for each patient to be normalized (see below) against those of the control group. Using this method, we accounted for the heterogeneity of the lesions and effects on the estimation of network parameters when comparing to participants without lesions. The results were qualitatively similar when run without this normalization method.
Assessment of activity for each network during the speech task.
We performed further regression analyses to assess activity of the 4 networks during speech compared to each baseline task (figure 1C). The task design matrix (independent variable) was regressed against the run-specific time courses of each reference network derived from the previous step (dependent variable) to estimate the effect size (β). We refer to the resulting group averages as network activations in the controls. In the patients, we normalized these against the patient-specific controls (i.e., controls with the same nonlesioned tissue as that of the specific patient) by subtracting the mean activation of all of the patient-specific runs in the controls from the patient's mean network activation, referred to as normalized activations. A value of normalized activation close to zero indicated similar network activity in patients and controls. Values above zero suggested higher activity in the network in the patients compared to controls and vice versa. We entered data for patients and controls separately into repeated-measures analyses of variance with contrast (speech > rest, speech > count, speech > decision) and network activations as within-subjects factors, using Greenhouse-Geisser correction.
Network measures and residual speech production after stroke.
Since we hypothesized that the patients have altered relationships between networks, we investigated relative activation of the networks during speech by subtracting the normalized activations of the LFTP, RFTP, or CingOper from that of the DMN. We entered the resulting differential activations, as well as non-fMRI predictors (lesion volume, age, sex, and years of formal education) into a hierarchical multiple regression analysis to assess relation to speech performance (AICW).
Psychophysiological interactions (PPI).
PPI analyses are used to determine task-specific changes in the relationship between activity in different brain areas, a measure of functional connectivity.33 We performed PPI analyses to determine the speech task–specific functional connectivity between the DMN and the networks whose differential activations predicted speech performance (i.e., LFTP and RFTP) (figure 1D and e-Methods). A positive parameter estimate (PPI) indicated an increase in the strength of speech task–specific functional connectivity. For each patient, the PPI values were normalized against the patient-specific controls (normalized PPI). Normalized PPI that was close to zero indicated that speech task–specific functional connectivity was the same in patients and controls.
RESULTS
Behavioral.
The patients had reduced performance compared to controls on measures of spontaneous speech, verbal fluency, and in-scanner speech performance (table 1).
Network activity during speech.
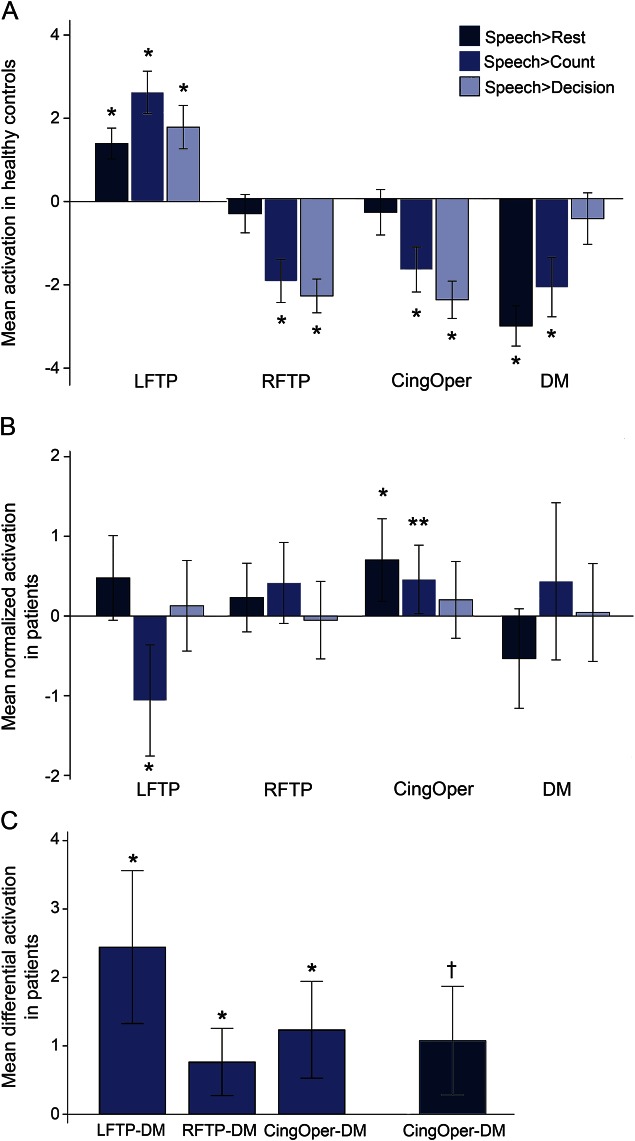
Figure 2A shows network activations in controls. There was a main effect for activation (F2.3,50.7 = 81.1, p < 0.001), no main effect for contrast (F1.3,30.5 = 2.3, p > 0.1), and an interaction (F4.6,105.3 = 74.1, p < 0.001). Post hoc comparisons indicated a larger mean activation of the LFTP during speech than at any baseline task. LFTP activation was higher than the other 3 networks, none of which was activated in any of the contrasts, and in many were significantly deactivated (p < 0.001). Figure 2B shows the network normalized activations in patients. There was no main effect for normalized activations (F33.5,1,379 = 1.3, p > 0.2) or contrast (F2,102 = 0.6, p > 0.6), but an interaction was present (F3.9,203.3 = 8.5, p < 0.001). Post hoc t tests indicated that patients had similar levels of activity compared to controls except greater activity in the CingOper during speech > rest (p < 0.01) and speech > count (p = 0.04), and reduced activity in the LFTP during speech > count (p < 0.01) (which, by extrapolation, was associated with greater LFTP activity in the contrast of count > rest in the patients relative to controls).
Figure 2. Network activations in controls.
Normalized activations in patients and differential activations in patients. (A) In the healthy state, activity in the left fronto-temporo-parietal network (LFTP) network was upregulated during speech compared to activity in the default mode network (DM), right fronto-temporo-parietal network (RFTP), and cingulo-opercular network (CingOper). (B) In general, patients showed similar levels of normalized activity compared to controls (normalized activation), evident from the 95% confidence intervals crossing the zero line. Exceptions were upregulation of activity in the CingOper network during speech > rest and for speech > count and reduced activity in the LFTP during speech > count. (C) Patient activations of the LFTP, RFTP, and CingOper relative to the DM (differential activations), normalized to that of controls. Error bars represent 95% confidence intervals. Zero on the Y axis represents the rest baseline in panel A, and mean values for the controls in panels B and C. **p < 0.05; †p < 0.01; *p < 0.005; results of post hoc 2-tailed t tests.
Altered differential activity between networks predicted residual speech production in a multiple regression model.
We investigated differences in activity of task-positive systems (LFTP, RFTP, and CingOper) and the DMN in patients by subtracting normalized activation of the DMN from each of the other networks, using the contrast speech > rest (figure 2C). In addition, we investigated differential activation of CingOper for decision trials, a task that the patients performed well. The differential activations of these networks were higher in the patients (p < 0.01, Bonferroni-corrected).
To investigate the relationship between these network measures and speech performance (AICW), we entered potential confounding variables (age, sex, lesion volume, and years of formal education) as predicting factors in the first step of the multiple regression model. The second step included the differential activations of the LFTP-DMN, RFTP-DMN, and CingOper-DMN (table 2). The first step accounted for 39% of the variance in AICW (adjusted R2 = 0.39, F4,48 = 9.6, p < 0.001). Addition of the 3 fMRI predictors accounted for an additional 17%, explaining 56% of variance (adjusted R2 = 0.56, F7,45 = 10.6, p < 0.001). Thus, increasing differential activation between the LFTP-DMN positively predicted AICW (p < 0.001), while increasing differential activation between the RFTP-DMN (p = 0.001) and lesion volume (p < 0.001) negatively predicted AICW. These predictors remained significant when using the out-of-scanner phonologic/fluency factor (derived from the principal component analysis, e-Methods) as the dependent variable, or when including subject-specific motion (average relative root mean square frame-wise displacement) as an extra predictor.
Table 2.
Multiple regression analysis with the in-scanner performance
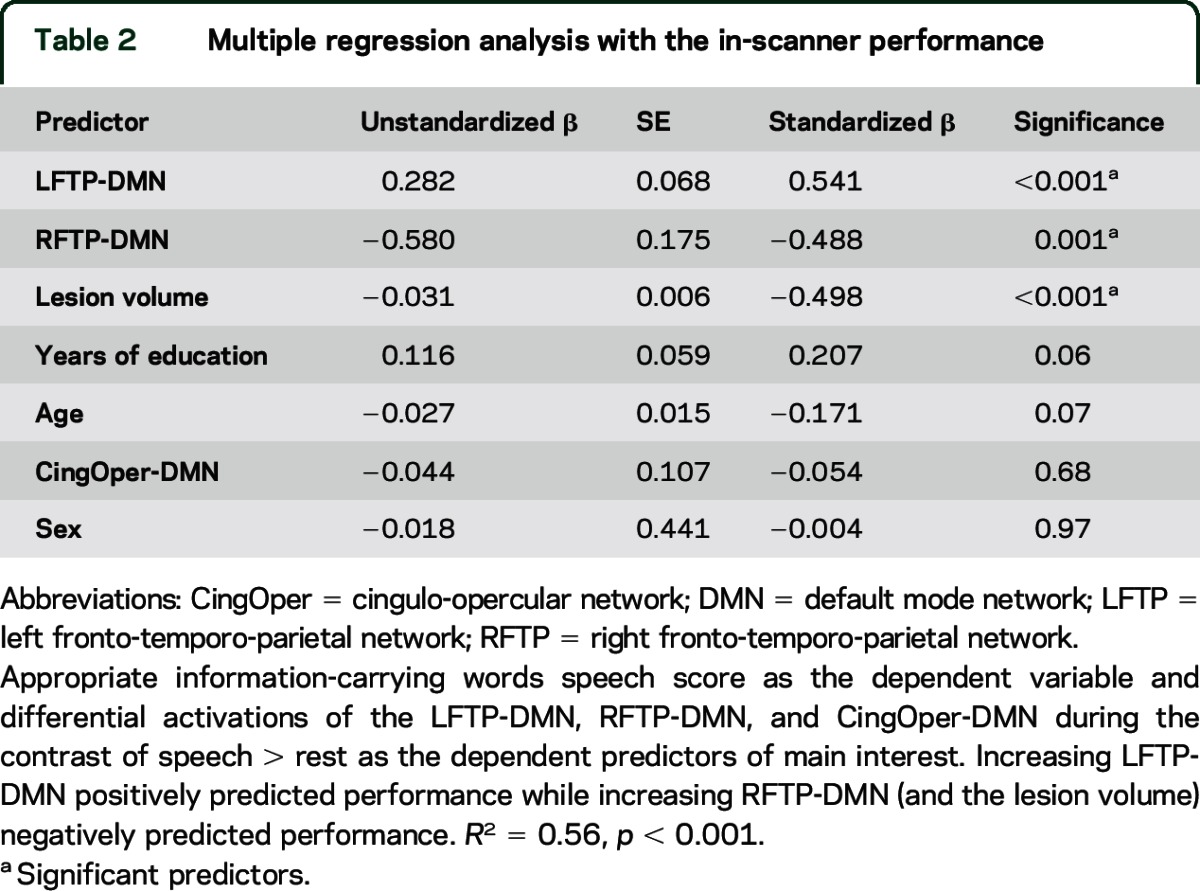
Although normalized activation of the LFTP and DMN did not correlate independently with speech performance (p > 0.5), differential activation between these networks (r = 0.3, p < 0.05, 2-tailed) correlated with speech performance. Differential activation between the CingOper-DMN during speech did not predict speech production ability, but its differential activation during decision trials (figure 2C) correlated negatively with reaction times on this task (r = −0.41, p < 0.01) even when controlled for lesion volume (r = −0.37, p < 0.01).
Altered functional connectivity between networks.
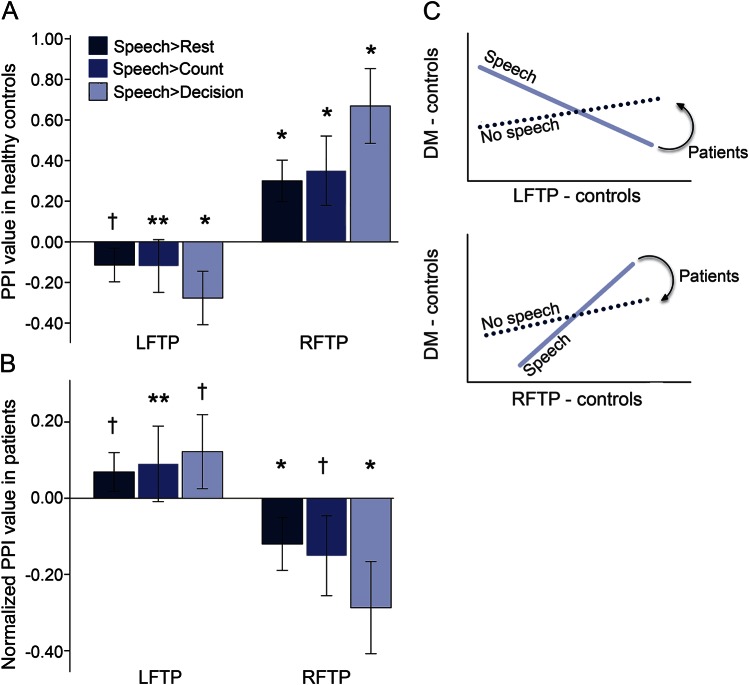
Differential activation among LFTP, RFTP, and DMN correlated with residual speech production after stroke. Using PPI analyses, we investigated pairwise speech-task-specific functional connectivity, a measure of trial-by-trial relationship between networks. In controls (figure 3A), there was a main effect for network PPI [F1,23 = 44.3, p < 0.001] and contrast [F1.4,32.7 = 4.9, p = 0.02] and interaction [F1.5,33.7 = 29.1, p < 0.001]. LFTP-DMN had a negative PPI (−0.17 ± 0.05, mean ± SE) and RFTP-DMN had a positive PPI (0.44 ± 0.06) (p < 0.001). In the controls there was increased trial-by-trial negative correlation between the LFTP and DMN during speech (figure 3C), such that, as activity in the LFTP increased, activity in the DMN decreased. The opposite occurred in RFTP, i.e., as RFTP became less active during speech, DMN also became less active. PPI was the same for the contrasts of speech > rest and speech > count (p = 0.5), suggesting that this interaction is the product of higher-level processes involved in speech rather than lower-level sensorimotor processes.
Figure 3. Speech-related psychophysiological interactions (PPIs) between the left fronto-temporo-parietal network (LFTP) and right fronto-temporo-parietal network (RFTP) with the default mode network (DMN).
Error bars represent 95% confidence intervals. The 3 shades of tone for the bars represent the PPI values contrasting speech against the different baselines. (A) Controls demonstrated a negative PPI for the LFTP network, reflecting an increase in trial-by-trial negative correlation between the LFTP and DMN during speech > baseline tasks. The opposite occurred with the RFTP network; speech > any baseline increased its functional connectivity with the DMN. (B) Patients demonstrated an increase in the normalized PPI for the LFTP (a positive normalized PPI) and a decrease for the RFTP (a negative normalized PPI) compared to controls. Zero on the Y axis represents the mean values for controls. (C) Illustration of the relationship between the LFTP and DMN (top) and RFTP and DMN (bottom). Shaded lines represent the task-independent connectivity between the 2 networks. Solid line represents the speech task-specific functional connectivity in the controls. Curved arrows represent the direction of significant change in the PPI in the patients. When the patients performed the speech task, the normal trial-by-trial negative correlation between the LFTP and DMN was significantly reversed. Likewise, the normal correlation between the RFTP and the DMN during speech was also significantly reversed. **p = 0.07; †p < 0.05; *p ≤ 0.001; post hoc 2-tailed t tests.
Figure 3B shows normalized PPI values in patients. There was a main effect for network normalized PPI [F1,52 = 17.8, p < 0.001], and an interaction [F1.4,76.0 = 9.0, p = 0.001], but no main effect for contrast [F1.3,68.8 = 2.0, p = 0.15]. The mean LFTP normalized PPI (0.94 ± 0.04) was greater than the RFTP normalized PPI (−0.19 ± 0.04) (p < 0.001). Post hoc t tests confirmed that the LFTP normalized PPI was greater (p = 0.009), while the RFTP normalized PPI was lower than in the controls (p = 0.001). When the patients were performing the speech task, the normal negative correlation between activity in the LFTP and DMN and the normal positive correlation between activity in the RFTP and the DMN was reduced (figure 3C).
Test of network specificity.
To test network specificity of these findings, we performed the above analyses on a network of noninterest (sensorimotor). The differential activation of this network in patients was similar to controls and did not predict speech performance in the multiple regression model. Similarly, there was no PPI difference in patients and controls for this network.
DISCUSSION
In this study, we emphasize the interactions between task-dependent activations and deactivations of brain networks, many remote from the site of infarction, for the production of propositional speech after left hemisphere stroke. This accords with the proposal that cognitive functions are the product of dynamic anticorrelated networks,2,34,35 and that language depends on interactions among language-specific and domain-general systems.15,16
The balance of activity between the LFTP and DMN positively predicted speech performance; that is, the combination of higher activity in the LFTP and lower activity in the DMN was associated with improved speech production. Conversely, differential activation between the RFTP and DMN negatively predicted speech production; that is, high RFTP activity relative to the DMN was detrimental to speech production. These results are in agreement with reports of beneficial language effects after noninvasive inhibition of the right inferior frontal gyrus.36,37
The degree of deactivation in the DMN correlates with task difficultly.9,11 The increase in differential activation between networks in patients may relate to increased activity in the fronto-temporo-parietal networks, or increased deactivation in the DMN as the patients found the task more difficult, or both. Although relative activity between these networks correlates with behavioral outcomes after stroke, activity within individual networks alone does not. This may be a consequence of combining 2 weak individual associations with speech production. Alternatively, it may be an effect mediated by functional connectivity of the many subregions within the DMN.7,38
Patients showed altered speech task-specific functional connectivity between the LFTP and DMN relative to controls, so that the normal negative correlation between these 2 networks during the speech task was reduced (figure 3). This reduction in speech task-specific functional connectivity is different from relative local changes in brain activity, as differential activity between these 2 networks was greater in patients compared to controls (figure 2C). Therefore, relatively reduced functional communication between brain regions occurred despite relative increased activity between the networks. Likewise, normal speech task-specific functional connectivity between the RFTP and the DMN that was seen in controls was reduced in patients. These changes in speech task-specific functional connectivity after left hemisphere stroke were observed in relation to higher-level processes associated with the speech task, and not influenced by the baseline task to which the speech task was related.
We cannot definitively ascertain the underlying cause for the altered relationship between the fronto-temporo-parietal and the DMN seen in patients during the speech task. Throughout the 7-second epochs, the patients produced much less speech than the controls. One possibility is that they were trying to produce words, thus attempting to suppress the DMN and upregulate the LFTP, but with variable success depending on the degree of damage to pathways mediating communication between the 2 networks. Alternatively, they may have briefly disengaged from the task at intervals, resulting in upregulation of activity in the DMN with no upregulation in LFTP.
Although there was a reduction of overall DMN activity during the speech task in this study and others,6,17 this does not imply that components of this network were not involved in the task: components of the DMN are involved in narrative language comprehension and production39,40 as well as semantic processing.8,9,14 The speech task-specific LFTP is functionally connected to the posterior cingulate cortex, a node within the DMN.7,17 Furthermore, we showed that the balance of activity between the DMN and the RFTP and LFTP predicts residual propositional speech production after stroke. Coordination between these networks may depend on common functional connectivity with components of the DMN in the posterior cingulate cortex7,17,38 or the medial frontal cortex6,38 or may be indirectly driven by another distant source (e.g., thalamic innervation). Other methods of analysis such as dynamic causal modeling (DCM) might elucidate if any causal relationship between these networks exists. However, application of DCM to data acquired by sparse sampling is methodologically suspect.
Only CingOper revealed increased activity during speech when comparing patients to controls. However, neither activity in this network nor its differential activation relative to the DMN during speech trials correlated with measures of speech production. Relative activity within CingOper compared to the DMN was also increased in patients compared to controls during decision trials, but for this task the relative activity correlated with performance on the decision trials in patients. This result indicates that CingOper is engaged in both communicative and noncommunicative tasks that require stimuli processing and response production; it also indicates that upregulation of its activity only contributes to performance when downstream domain-specific processes are intact, such as in the decision task.
Based on univariate analyses, increased activity in midline components of CingOper correlate with measures of language function after stroke.22,41,42 However, correlation with language behavior reported in some studies and not others is expected, given the heterogeneity of patients and task designs. Furthermore, the midline frontal components of the networks lie in close anatomical proximity to one another,6 therefore sampling this region with univariate analysis and assuming that it reflects the function of a single processing module32 may be misleading.
For this study, we only included patients with relatively mild stroke (table e-1), in order to obtain informed consent and cooperation with task performance. Location of the lesions within the left hemisphere and size was variable, reflecting consecutive recruitment of patients admitted in a clinical setting. As selection was not based on lesion location within the left hemisphere, inferences regarding the effect of lesion location on the networks could not be tested. Instead, the network measures of interest were normalized in each patient against a case-specific control group matched for the lesion.
Although the 2 groups were not matched for education and sex, we accounted for this in the multiple regression model. One further caveat is that the findings should be tested in a group of patients with a right hemisphere stroke, to confirm behavioral specificity.
Our results indicate that communication depends on functional interactions among domain-general and task-specific networks and that aphasia after a focal infarct is associated with widespread disruption of these interactions.15,16 Whether this is the cause or consequence of the reduction in speech production cannot be determined from our results. Nevertheless, the altered relationship between networks following left hemisphere stroke suggests that a much broader approach to the rehabilitation of aphasia is required. Recent evidence indicates that targeted modulation of distant nodes within the same dysfunctional brain network in a disease state may be effective for improving outcome.43 Therefore, stimulation within the LFTP network remote from the infarct and inhibition of the RFTP network may prove to be effective for modulating function of classic perisylvian language nodes or their mirror regions in the contralateral hemisphere.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and volunteers who took part in the study; the clinical staff at the stroke units at Charing Cross, Chelsea, and Westminster, Northwick Park and West Middlesex Hospitals in London, UK; and Claudia Cramer for analysis of the spontaneous speech recordings.
GLOSSARY
- AICW
appropriate information-carrying words
- CingOper
cingulo-opercular network
- CAT
Comprehensive Aphasia Test
- DCM
dynamic causal modeling
- DMN
default mode network
- ICA
independent component analysis
- LFTP
left fronto-temporo-parietal network
- RFTP
right fronto-temporo-parietal network
- PPI
psychophysiological interaction
Footnotes
Editorial, page 1277
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Geranmayeh is the awardee for the main funding for this study, contributed to study design, and led data collection and analysis and manuscript preparation. Dr. Leech contributed to study design, data analysis, and manuscript preparation. Prof. Wise was the study supervisor, and contributed to study, grant funding, and manuscript preparation.
STUDY FUNDING
F.G. was funded by the Welcome Trust (093957). R.J.W. was funded by the MRC (WMCR_P39389). The Article Processing Charge was paid by Wellcome Trust.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Kahn I, Snyder AZ, et al. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 2008;100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dosenbach NUF, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007;104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigneau M, Beaucousin V, Hervé PY, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 2006;30:1414–1432. [DOI] [PubMed] [Google Scholar]
- 6.Geranmayeh F, Wise RJS, Mehta A, et al. Overlapping networks engaged during spoken language production and its cognitive control. J Neurosci 2014;34:8728–8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci 2012;32:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphreys GF, Lambon Ralph MA. Fusion and fission of cognitive functions in the human parietal cortex. Cereb Cortex 2014;25:3547–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys GF, Hoffman P, Visser M, et al. Establishing task- and modality-dependent dissociations between the semantic and default mode networks. Proc Natl Acad Sci USA 2015;112:7857–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA 2013;110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage 2008;41:100–112. [DOI] [PubMed] [Google Scholar]
- 12.Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron 2013;80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regev M, Honey CJ, Simony E, et al. Selective and invariant neural responses to spoken and written narratives. J Neurosci 2013;33:15978–15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder JR, Desai RH, Graves WW, et al. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 2009;19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends Cogn Sci 2014;18:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geranmayeh F, Brownsett SLE, Wise RJS. Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain 2014;137:2632–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geranmayeh F, Brownsett SLE, Leech R, et al. The contribution of the inferior parietal cortex to spoken language production. Brain Lang 2012;121:47–57. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 2009;106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noonan KA, Jefferies E, Visser M, et al. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci 2013;25:1824–1850. [DOI] [PubMed] [Google Scholar]
- 20.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 21.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brownsett SLE, Warren JE, Geranmayeh F, et al. Cognitive control and its impact on recovery from aphasic stroke. Brain 2014;137:242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jilka SR, Scott G, Ham T, et al. Damage to the salience network and interactions with the default mode network. J Neurosci 2014;34:10798–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 2010;133:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp DJ, Turkheimer FE, Bose SK, et al. Increased frontoparietal integration after stroke and cognitive recovery. Ann Neurol 2010;68:753–756. [DOI] [PubMed] [Google Scholar]
- 26.Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage 2012;62:2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swinburn K, Porter G, Howard D. Comprehensive Aphasia Test, 1st ed Abingdon, UK: Psychology Press; 2005. [Google Scholar]
- 28.Rochon E, Saffran EM, Berndt RS, et al. Quantitative analysis of aphasic sentence production: further development and new data. Brain Lang 2000;72:193–218. [DOI] [PubMed] [Google Scholar]
- 29.Arthur W, Day DV. Development of a short form for the Raven Advanced Progressive Matrices test. Educ Psychol Meas 1994;54:394–403. [Google Scholar]
- 30.Geranmayeh F, Leech R, Wise RJS. Semantic retrieval during overt picture description: left anterior temporal or the parietal lobe? Neuropsychologia 2014;76:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 2005;360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Calhoun VD, Potenza MN. The absence of task-related increases in BOLD signal does not equate to absence of task-related brain activation. J Neurosci Methods 2014;240:125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Reilly JX, Woolrich MW, Behrens TEJ, et al. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci 2012;7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly AMC, Uddin LQ, Biswal BB, et al. Competition between functional brain networks mediates behavioral variability. NeuroImage 2008;39:527–537. [DOI] [PubMed] [Google Scholar]
- 35.Koyama MS, Di Martino A, Zuo XN, et al. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci 2011;31:8617–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang EK, Kim YK, Sohn HM, et al. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca's homologue area. Restor Neurol Neurosci 2011;29:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin PI, Naeser MA, Ho M, et al. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep 2009;9:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braga RM, Sharp DJ, Leeson C, et al. Echoes of the brain within default mode, association, and heteromodal cortices. J Neurosci 2013;33:14031–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awad M, Warren JE, Scott SK, et al. A common system for the comprehension and production of narrative speech. J Neurosci 2007;27:11455–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AbdulSabur NY, Xu Y, Liu S, et al. Neural correlates and network connectivity underlying narrative production and comprehension: a combined fMRI and PET study. Cortex 2014;57:107–127. [DOI] [PubMed] [Google Scholar]
- 41.Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology 2008;70:290–298. [DOI] [PubMed] [Google Scholar]
- 42.Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain 2006;129:1371–1384. [DOI] [PubMed] [Google Scholar]
- 43.Fox MD, Buckner RL, Liu H, et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA 2014;111:4367–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.