Abstract
Uric acid, once viewed as an inert metabolic end-product of purine metabolism, has been recently incriminated in a number of chronic disease states, including hypertension, metabolic syndrome, diabetes, non-alcoholic fatty liver disease, and chronic kidney disease. Several experimental and clinical studies support a role for uric acid as a contributory causal factor in these conditions. Here we discuss some of the major mechanisms linking uric acid to metabolic and cardiovascular diseases. At this time the key to understanding the importance of uric acid in these diseases will be the conduct of large clinical trials in which the effect of lowering uric acid on hard clinical outcomes is assessed. Elevated uric acid may turn out to be one of the more important remediable risk factors for metabolic and cardiovascular diseases.
Keywords: Uric acid, Metabolic syndrome, Hypertension, Diabetes mellitus, Kidney disease, Cardiovascular disease
1. Uric acid and metabolic syndrome
While the condition known as metabolic syndrome has been suggested to be a pathophysiological condition, studies in comparative physiology show that the syndrome, as well as many of its associated factors, is a simple consequence of excessive fat storage [1]. Indeed, most mammals and birds will store their excess fat not only in their adipose tissue, but also in their liver and serum (triglycerides), often in association with the development of insulin resistance and elevated blood pressure [1]. While the underlying mechanisms involved in fat storage involve multiple genetic and other factors, recent studies suggest a role for nucleic acid metabolism, in which stimulation of adenosine monophosphate (AMP) deaminase promotes fat storage and insulin resistance, whereas activation of AMP activated protein kinase stimulates fat degradation and decreases gluconeogenesis [2–4]. A key factor that appears to promote fat storage is the AMP deaminase product, uric acid [2,3,5,6]. Here we will briefly discuss the studies incriminating uric acid in these conditions.
2. Uric acid and hypertension
One of the earliest associations of hyperuricemia was with hypertension [7–9]. Asymptomatic hyperuricemia is both associated with [10,11], and predicts, the development of hypertension [11]. Studies in laboratory animals have been complicated by the fact that most mammals express uricase, which is an enzyme that breaks down uric acid. As a consequence, most mammals have uric acid levels of 1–3 mg/dl, whereas the great and lesser apes, and humans, have uric acid levels of 3 mg/dl or greater [12]. When rats are given a uricase inhibitor (oxonic acid), they develop mild hypertension [13]. Genetically raising uric acid by knocking down the enteric urate transporter (SLC2A9) also results in elevation in uric acid that responds to lowering of uric acid with allopurinol [14]. Animal models of metabolic syndrome also have mild hyperuricemia despite the presence of uricase, and lowering uric acid in these animals also lowers blood pressure [15,16]. Interestingly, studies suggest that over time elevated serum uric acid induces microvascular and inflammatory changes in the kidney; the latter results in enhanced sensitivity to the effects of salt. Enhanced salt sensitivity leads to salt-sensitive hypertension that occurs irrespective of serum uric acid levels [17]. This suggests that hyperuricemia is more likely playing a role in initiating hypertension, but over time microvascular alterations in the kidney maintain the hypertensive state.
Pilot studies also suggest that lowering uric acid may improve blood pressure, including in pre-hypertensive obese [18] and hypertensive adolescents [19], hypertensive children on an angiotensin converting enzyme inhibitor [20], and in adults with asymptomatic hyperuricemia [21,22], in older hypertensive adults [23,24], in subjects with gout [25], in obese prehypertensive adults [26], in some subjects with chronic kidney disease [27], and in hemodialysis patients [28]. However, not all studies have reported a lowering of blood pressure, especially in subjects with chronic kidney disease [29]. Nevertheless, the studies support the hypothesis that uric acid may be a remediable risk factor in subjects with hypertension.
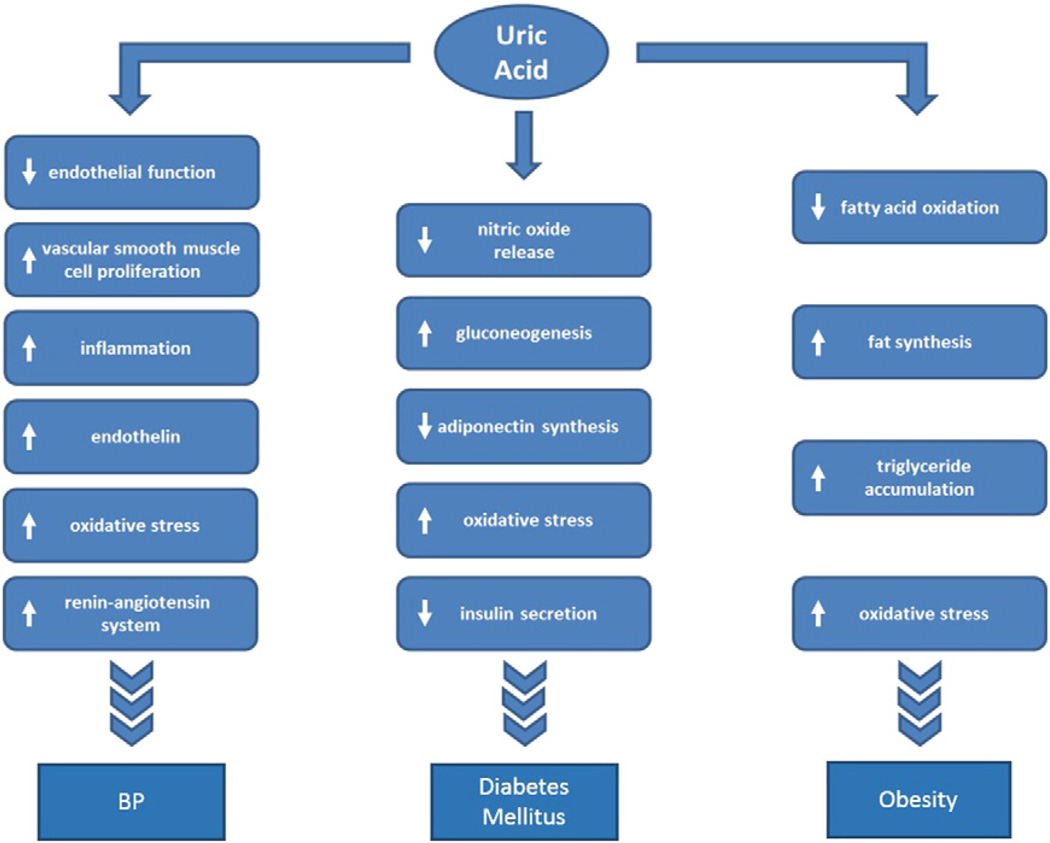
Experimental studies suggest that uric acid may raise blood pressure through several mechanisms, including impairing endothelial function [30–36], stimulating endothelin [37,38] and activating both the renal and intracellular renin angiotensin system [36,39,40] (Fig. 1). One of the more important pathogenic mechanisms by which uric acid raises blood pressure appears to be by stimulating intracellular oxidative stress by activation of NADPH oxidases both in the cytosol and mitochondria [5,40–43]. Indeed, blocking oxidative stress or improving endothelial function can lower blood pressure in hyperuricemic rats [35,44]. In addition, uric acid stimulates vascular smooth muscle cell proliferation and induces inflammatory changes in the kidney that may help perpetuate the hypertension [39,45,46].
Fig. 1.
Uric acid induced effects that may play a role in the pathogenesis of hypertension, diabetes, and obesity.
3. Uric acid and diabetes
Hyperuricemia has been linked with diabetes since the 1800s [47] and was associated with metabolic syndrome by the early 1920s [48]. Today there is overwhelming epidemiological evidence that shows that hyperuricemia is both present and predicts the development of insulin resistance and type 2 diabetes (reviewed in [49]). Historically, hyperuricemia was attributed as a secondary consequence to insulin resistance [50], but more recent studies suggest it may have a contributory causal role [49], especially since an elevated serum uric acid often precedes the development of insulin resistance [51]. A study of 5012 young adults found that baseline elevated serum uric acid predicted the onset of both diabetes (HR 1.87, CI 1.33–2.62) and insulin resistance (HR 1.36, CI 1.23–1.51). The elevation in baseline serum uric acid was not associated with plasma insulin concentration suggesting that serum uric acid is in fact an independent risk factor in the development of insulin resistance and subsequent diabetes [51]. Indeed, insulin resistance in models of metabolic syndrome can be improved by lowering serum uric acid [15,16], and uric acid has been shown to block AMP-activated protein kinase and to stimulate gluconeogenesis [3]. Uric acid also blocks insulin mediated endothelial nitric oxide release [43] that is critical for insulin action [52]. Furthermore, uric acid induces oxidative stress in adipocytes, leading to lower adiponectin synthesis [41]. Reducing uric acid can improve circulating adiponectin levels and insulin resistance in mice with metabolic syndrome [16]. Furthermore, uric acid has also been shown to induce oxidative stress in islet cells, and upregulation of urate transporters have been identified in islets of rats with sugar-induced diabetes [53]. Scott, et al. [54] also reported that serum insulin was decreased by 26% in rats in which uricase was inhibited after 4weeks in association with an increase in serum glucose by 24–38%. Finally, pancreatic islet cells from neonatal rats incubated with uric acid but not oxonate (the uricase inhibitor) reduced insulin secretion by 65%. Removing the uric acid from the medium rapidly restored insulin secretion suggesting uric acid could have a cytostatic or cytotoxic effect on β-cells in the pancreas.
The effect of lowering uric acid on insulin resistance in human studies is limited. However, insulin resistance (HOMA index) has been reported to be improved by benzbromarone [55] and allopurinol [56] in two small randomized trials. In addition, one study reported an improvement in hemoglobin A1C levels in normotensive diabetic subjects treated with allopurinol [57].
While the evidence that uric acid may have a causal role in type 2 diabetes is mounting, the primary argument against this relationship has been the use of Mendelian randomization studies in which genetic polymorphisms that predict an increase in uric acid can be used to predict the risk for gout but not diabetes [58,59] Again, the limitation of these studies is that they are evaluating serum (extracellular) uric acid as a risk factor when the metabolic mechanisms are mediated by intracellular uric acid, and by the fact that the polymorphisms involve urate transport and explain only 4–6% of the overall variance of serum uric acid levels [60].
4. Uric acid and fat storage (adipose and liver)
An elevated serum uric acid is also a potent predictor for the development of obesity [61] and fatty liver [62–67]. Experimentally uric acid has been shown to increase triglyceride accumulation in cultured liver cells [5,6] and hyperuricemia also increases triglyceride levels in the liver of rats [68]. The mechanism has been shown to be mediated by intracellular and mitochondrial oxidative stress [5,69]. The oxidative stress is associated with inhibition of aconitase in the Krebs cycle that leads to citrate accumulation and the stimulation of ATP citrate lyase resulting in increased fat synthesis, as well as an inhibition of enoyl CoA hydratase resulting in impaired beta fatty acid oxidation that is also potentiated by the inhibition of AMPK-activated protein kinase [2,5,70]. Lowering uric acid has been shown to reduce liver fat in several animal models of metabolic syndrome and also in alcohol-induced fatty liver [5,70,71]. To date we are unaware of any clinical trials to determine if lowering uric acid can reduce adiposity or hepatic steatosis in humans. However, one clinical trial found that allopurinol use resulted in less weight gain in adolescents compared to placebo treated controls [18]. More recently, another study reported that allopurinol treatment resulted in weight loss in obese, prehypertensive adults that was independent of energy intake [26].
5. Role of diet in uric acid mediated effects
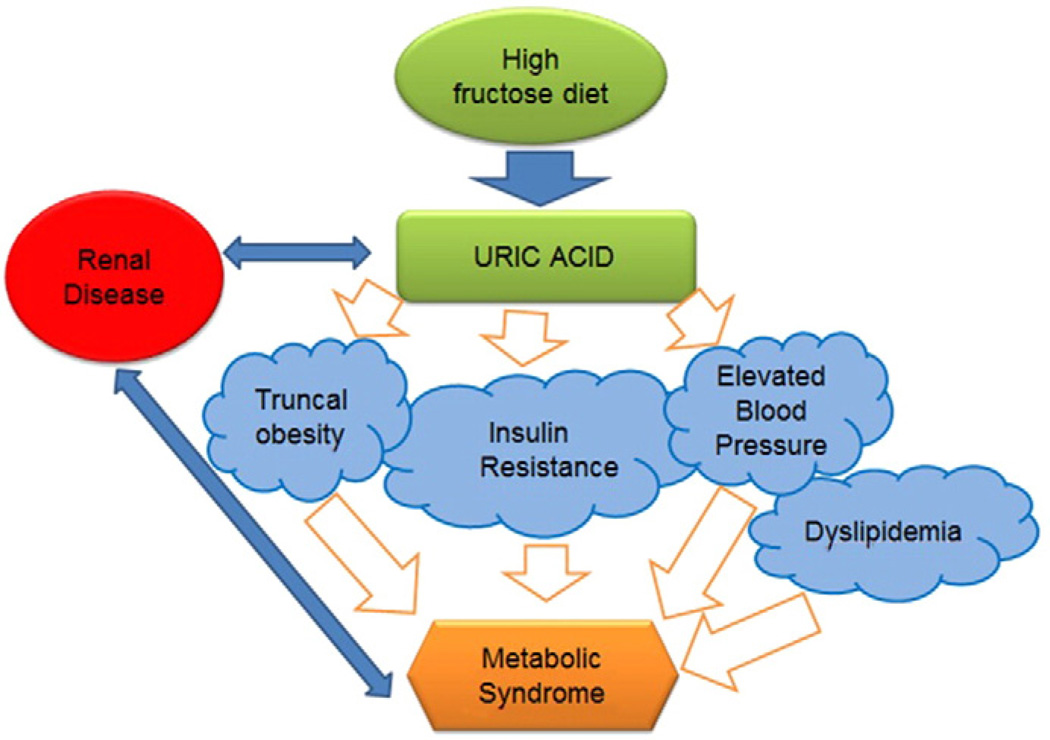
Uric acid can be increased in the circulation by high purine foods (such as beer) and by fructose. Classically the focus has been on high purine foods as risk factors for gout [72], of which most purine-rich foods fall in the umami-class of foods that are increasingly recognized as risk factors for metabolic syndrome [73]. High fat diets can also increase serum uric acid [74]. In western cultures, a major dietary source for increasing uric acid is fructose present in added sugars [75]. Indeed, there is remarkable evidence that sugary beverages play a major role in the epidemic of obesity and metabolic syndrome [76–78] and experimental studies show that it is likely the fructose component which is primarily responsible for uric acid elevation and subsequent development of metabolic syndrome[79–81] (Fig. 2). Experimental studies also suggest that a primary mechanism by which fructose induces metabolic syndrome is through its ability to increase intracellular uric acid [5,15,68,70,82]. Clinical studies also show that fructose can raise serum uric acid and induce features of metabolic syndrome [83–88]. In one study, high doses of fructose (200 g/day) were given to healthy adult males for two weeks with or without allopurinol [89]. In this study many of the features of metabolic syndrome were induced rapidly despite the short duration of the study. However, lowering uric acid was associated with prevention of the rise in blood pressure but no improvement in insulin resistance. Whether this is because of the high doses of fructose given, or whether this suggests the beneficial effects of lowering uric acid is unknown [89].
Fig. 2.
Relationship between high-fructose diet, generation of hyperuricemia and resulting metabolic syndrome. Renal disease is linked to both metabolic syndrome and hyperuricemia in a mutual way.
6. Uric acid and thrifty genes
Most mammals have low serum uric acid (1–2 mg/dl) due to the presence of uricase, an enzyme that degrades uric acid [90]. However, uricase activity was progressively attenuated (25 to 15 million years ago) and then silenced about 15 million years ago in apes and prehominids [91]. There is increasing evidence that the mutation may have provided survival advantages to the ancestral apes at the time, by increasing their ability to store fat in response to a decrease in food (fruit) availability that resulted from global cooling [92,93]. Indeed, studies of the resurrected ancestral uricase gene suggested that it was able to blunt the effect of uric acid to increase fat in response to fructose and to stimulate gluconeogenesis [3,91]. Other potential benefits of uric acid may include its ability to block oxidative stress (extracellularly) [94] and to stimulate foraging behavior [95].
Studies of modern apes and humans living on native diets suggest that the mutation of uricase only resulted in an increase of serum uric acid to the 3 to 4 mg/dl range [12]. However, with the introduction of western diet, serum uric acid has increased progressively over the last century [12]. The rise in western diet, coupled with the loss of uricase may account for why there is a world epidemic of obesity, diabetes and cardiovascular disease. Interestingly, Pacific Islanders have higher uric acid levels that appear to be genetic and which preceded the introduction of western diets [96]. We have postulated that the higher uric acid levels in this population may explain their higher frequency of obesity and diabetes compared to other peoples throughout the world [97].
7. Cardiovascular disease
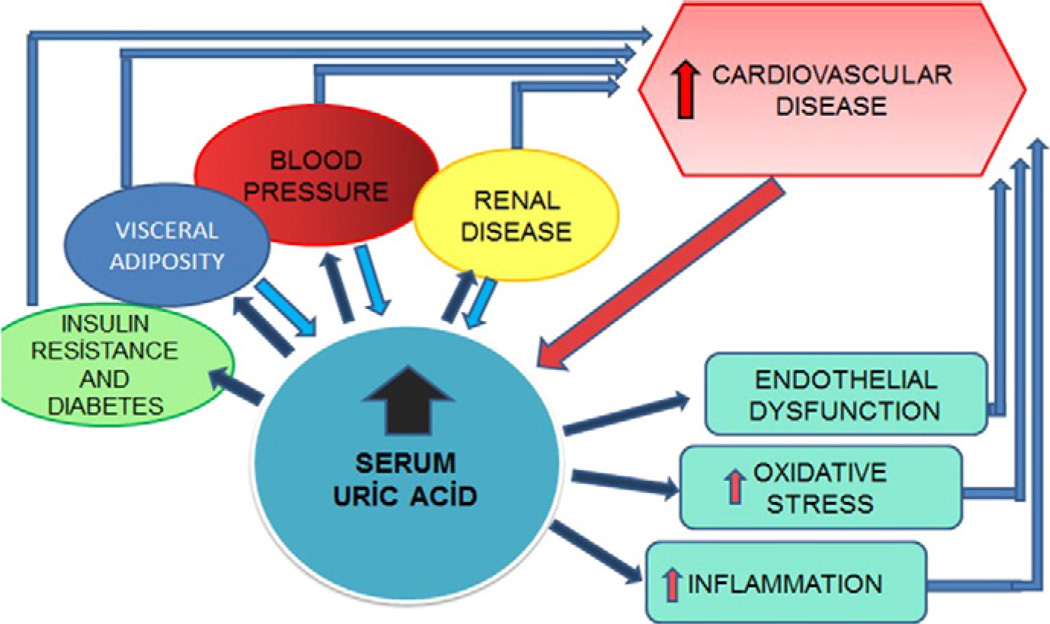
Uric acid has been associated with cardiovascular disease for decades [98]. For a long period, uric acid was thought to be purely secondary to obesity and hypertension, and was not considered a true cardiovascular risk factor [99,100]. A major problem with these early studies is that the assumption was that the relationship of uric acid with cardiovascular disease had to be direct, and the possibility that it increased cardiovascular disease as a consequence of causing hypertension, insulin resistance or kidney disease was not considered [101,102] (Fig. 3). The reawakening that uric acid might have a role in heart disease is now a hot topic, and can be best assessed by clinical trials. Early trials suggest benefits of lowering uric acid on carotid intimal thickness [23], angina [103], left ventricular hypertrophy [104], arterial stiffness [105] and cardiovascular events in subjects with and without chronic kidney disease [29,105–108].
Fig. 3.
Schematic diagram showing complex interaction of uric acid, components of metabolic syndrome and cardiovascular disease. Note that elevated uric acid can lead to development of individual components and these components in turn can lead to elevations in serum uric acid. Elevated serum and intracellular uric acid may lead to increased incidence of cardiovascular disease both directly through inflammation, oxidative stress and endothelial dysfunction and indirectly through developing other established cardiovascular risk factors such as hypertension, diabetes and visceral obesity.
8. Arguments against uric acid as a cardiovascular or metabolic risk factor
There are several arguments that suggest that uric acid may not be a true risk factor for metabolic or cardiovascular disease. First is the observation that acutely raising uric acid in the blood by infusing uric acid improves endothelial function [94,109]. The improvement in endothelial function is thought to be due to the ability of uric acid to function as an antioxidant [94]. However, uric acid is expected to be an antioxidant in the extracellular environment. However, numerous studies have shown that uric acid is a pro-oxidant in the intracellular environment [5,40–42,53,69]. Moreover, while uric acid inactivates peroxynitrite, it generates two urate-based radicals in the process [110,111], so the ffects of uric acid as an antioxidant are not without some radical generation.
Uric acid has also been proposed to be one reason that chlorthalidone was beneficial in the ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) study possibly due to its antioxidant properties [112]. However, this is unlikely, as in our experimental models thiazides lower blood pressure in metabolic syndrome, but the addition of allopurinol completely corrects the hypertension while at the same time improving endothelial function [113].
Some have also argued that the benefit of allopurinol on blood pressure may be via its effects to block oxidants generated during the reaction of xanthine with xanthine oxidase [114]; however, this fails to explain why probenecid (a uricosuric) lowered blood pressure in the trial by Soletsky et al [18]. In addition, the effect of xanthine oxidase inhibition to block fat accumulation in hepatocytes in response to fructose can be prevented by adding back uric acid to the culture media [5]. Finally some genetic studies have failed to link polymorphisms that raise uric acid with hypertension [26–28] whereas others have shown such a link [115,116]. However, the genetic studies have relied highly on polymorphisms in genes that alter urate transport, and hence may alter the normal relationship of serum with intracellular urate levels [60]. The complexity associated with assessing polymorphisms of urate transporters is best observed by noting that knocking down SLC2A9 in the liver results in hyperuricemia without hypertension, whereas blocking the same gene in the intestine results in hyperuricemia with hypertension that can be treated by lowering uric acid levels [14,117].
9. Summary
We have entered a new exciting period in the history of uric acid. While uric acid was once the lonely dinner conversation for those suffering from gout or kidney stones, it is now being evaluated as a potential master conductor in the worldwide symphony of obesity, diabetes, and cardiorenal disease. However, at this time, it is still premature to lower uric acid as a means for reducing metabolic and cardiovascular disease. Rather, it is time to recommend definitive, large-scale clinical trials to determine whether lowering uric acid can be beneficial in the prevention and treatment of hypertension, insulin resistance, obesity, fatty liver, and cardiovascular disease.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Financial disclosure
None.
References
- 1.Johnson RJ, Stenvinkel P, Martin SL, Jani A, Sanchez-Lozada LG, Hill JO, et al. Redefining metabolic syndrome as a fat storage condition based on studies of comparative physiology. Obesity. 2013;21(4):659–664. doi: 10.1002/oby.20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7(11):e48801. doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014;28(8):3339–3350. doi: 10.1096/fj.13-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanaspa MA, Epperson E, Li N, Cicerchi C, Garcia G, Roncal-Jimenez C, et al. Opposing activity changes in AMP deaminase and AMP-activated protein kinase in the hibernating ground squirrel. PLoS One. 2015;10(4):e0123509. doi: 10.1371/journal.pone.0123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287(48):40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94(10):1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 7.Mahomed FA. The etiology of Bright's disease and the prealbuminuric state. Med Chir Trans. 1874;39:197–228. doi: 10.1177/095952877405700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haig A. The connecting link between the high tension pulse and albuminuria. Br Med J. 1890;1:65–68. doi: 10.1136/bmj.1.1515.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis NC. The cardiovascular and renal relations and manifestations of gout. JAMA. 1897;29:261–262. [Google Scholar]
- 10.Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275(9):457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- 11.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25(1):3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 14.DeBosch BJ, Kluth O, Fujiwara H, Schurmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642. doi: 10.1038/ncomms5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Lozada LG, Nakagawa T, Kang DH, Feig DI, Franco M, Johnson RJ, et al. Hormonal and cytokine effects of uric acid. Curr Opin Nephrol Hypertens. 2006;15(1):30–33. doi: 10.1097/01.mnh.0000199010.33929.7f. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60(4):1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 18.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60(5):1148–1156. doi: 10.1161/HYPERTENSIONAHA.112.196980. [DOI] [PubMed] [Google Scholar]
- 19.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assadi F. Allopurinol enhances the blood pressure lowering effect of enalapril in children with hyperuricemic essential hypertension. J Nephrol. 2014;27(1):51–56. doi: 10.1007/s40620-013-0009-0. [DOI] [PubMed] [Google Scholar]
- 21.Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39(4):1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 23.Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees KR, et al. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart. 2014;100(14):1085–1092. doi: 10.1136/heartjnl-2014-305683. [DOI] [PubMed] [Google Scholar]
- 24.Beattie CJ, Fulton RL, Higgins P, Padmanabhan S, McCallum L, Walters MR, et al. Allopurinol initiation and change in blood pressure in older adults with hypertension. Hypertension. 2014;64(5):1102–1107. doi: 10.1161/HYPERTENSIONAHA.114.03953. [DOI] [PubMed] [Google Scholar]
- 25.Kim HA, Seo YI, Song YW. Four-week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J Korean Med Sci. 2014;29(8):1077–1081. doi: 10.3346/jkms.2014.29.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madero M, Rodriguez Castellanos FE, Jalal D, Villalobos-Martin M, Salazar J, Vazquez-Rangel A, et al. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: a randomized placebo controlled trial. J Am Soc Hypertens. 2015;9(11):837–844. doi: 10.1016/j.jash.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Pai BH, Swarnalatha G, Ram R, Dakshinamurty KV. Allopurinol for prevention of progression of kidney disease with hyperuricemia. Indian J Nephrol. 2013;23(4):280–286. doi: 10.4103/0971-4065.114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalalzadeh M, Nurcheshmeh Z, Mohammadi R, Mousavinasab N, Ghadiani MH. The effect of allopurinol on lowering blood pressure in hemodialysis patients with hyperuricemia. J Res Med Sci. 2012;17(11):1039–1046. [PMC free article] [PubMed] [Google Scholar]
- 29.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 31.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27(8):967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz IF, Grupper A, Chernichovski T, Hillel O, Engel A, Schwartz D. Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res. 2011;48(3):252–260. doi: 10.1159/000320356. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121(3–4):e71–e78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez-Lozada LG, Tapia E, Lopez-Molina R, Nepomuceno T, Soto V, Avila-Casado C, et al. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol. 2007;292(4):F1238–F1244. doi: 10.1152/ajprenal.00164.2006. [DOI] [PubMed] [Google Scholar]
- 36.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 37.Chao HH, Liu JC, Lin JW, Chen CH, Wu CH, Cheng TH. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008;29(11):1301–1312. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng TH, Lin JW, Chao HH, Chen YL, Chen CH, Chan P, et al. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. Int J Cardiol. 2010;139(1):42–49. doi: 10.1016/j.ijcard.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 40.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin–angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–1242. [PubMed] [Google Scholar]
- 41.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 42.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin–angiotensin system. J Hypertens. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Lozada LG, Rivard C, Lanaspa M, Roncal C, Franco M, Sautin Y, et al. Uric acid alters mitochondrial biogenesis in cultured human endothelial cells. Am Soc Nephrol Annual Meeting. 2009 (abstract) [Google Scholar]
- 44.Sanchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4):F1134–F1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266(13):8604–8608. [PubMed] [Google Scholar]
- 46.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 47.Duckworth D. A treatise on gout. London: C Griffin & Co; 1889. p. 476. [Google Scholar]
- 48.Kylin E. Studien uber das hypertonie–hyperglykamie–hyperurikamiesyndrome (Studies of the hypertension–hyperglycemia–hyperuricemia syndrome) Zbl inn Med. 1923;44:105–127. [Google Scholar]
- 49.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011. [PubMed] [Google Scholar]
- 51.Krishnan E, Pandya BJ, Chung L, Hariri A, Dabbous O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. 2012;176(2):108–116. doi: 10.1093/aje/kws002. [DOI] [PubMed] [Google Scholar]
- 52.Cook S, Hugli O, Egli M, Vollenweider P, Burcelin R, Nicod P, et al. Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly. 2003;133(25–26):360–363. doi: 10.4414/smw.2003.10239. [DOI] [PubMed] [Google Scholar]
- 53.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metab Clin Exp. 2011;60(9):1259–1270. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott FW, Trick KD, Stavric B, Braaten JT, Siddiqui Y. Uric acid-induced decrease in rat insulin secretion. Proc Soc Exp Biol Med. 1981;166(1):123–128. doi: 10.3181/00379727-166-41033. [DOI] [PubMed] [Google Scholar]
- 55.Ogino K, Kato M, Furuse Y, Kinugasa Y, Ishida K, Osaki S, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010;3(1):73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 56.Facchini C, Malfatto G, Giglio A, Facchini M, Parati G, Branzi G. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J Cardiovasc Med. 2015 doi: 10.2459/JCM.0000000000000226. (in press) [DOI] [PubMed] [Google Scholar]
- 57.Dogan A, Yarlioglues M, Kaya MG, Karadag Z, Dogan S, Ardic I, et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20(3):182–187. doi: 10.3109/08037051.2010.538977. [DOI] [PubMed] [Google Scholar]
- 58.Pfister R, Barnes D, Luben R, Forouhi NG, Bochud M, Khaw KT, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54(10):2561–2569. doi: 10.1007/s00125-011-2235-0. [DOI] [PubMed] [Google Scholar]
- 59.Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson RJ, Merriman T, Lanaspa MA. Causal or noncausal relationship of uric acid with diabetes. Diabetes. 2015;64(8):2720–2722. doi: 10.2337/db15-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42(4):474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 62.Sirota JC, McFann K, Targher G, Johnson RJ, Chonchol M, Jalal DI. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: liver ultrasound data from the National Health and Nutrition Examination Survey. Metab Clin Exp. 2013;62(3):392–399. doi: 10.1016/j.metabol.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JW, Cho YK, Ryan M, Kim H, Lee SW, Chang E, et al. Serum uric acid as a predictor for the development of nonalcoholic fatty liver disease in apparently healthy subjects: a 5-year retrospective cohort study. Gut Liver. 2010;4(3):378–383. doi: 10.5009/gnl.2010.4.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lonardo A, Loria P, Leonardi F, Borsatti A, Neri P, Pulvirenti M, et al. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig Liver Dis. 2002;34(3):204–211. doi: 10.1016/s1590-8658(02)80194-3. [DOI] [PubMed] [Google Scholar]
- 65.Petta S, Camma C, Cabibi D, Di Marco V, Craxi A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;34(7):757–766. doi: 10.1111/j.1365-2036.2011.04788.x. [DOI] [PubMed] [Google Scholar]
- 66.Ryu S, Chang Y, Kim SG, Cho J, Guallar E. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metab Clin Exp. 2011;60(6):860–866. doi: 10.1016/j.metabol.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Xu C, Yu C, Xu L, Miao M, Li Y. High serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study. PLoS One. 2010;5(7):e11578. doi: 10.1371/journal.pone.0011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tapia E, Cristobal M, Garcia-Arroyo FE, Soto V, Monroy-Sanchez F, Pacheco U, et al. Synergistic effect of uricase blockade plus physiological amounts of fructose–glucose on glomerular hypertension and oxidative stress in rats. Am J Physiol Renal Physiol. 2013;304(6):F727–F736. doi: 10.1152/ajprenal.00485.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Investig. 2014;94(10):1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 70.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7(10):e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kono H, Rusyn I, Bradford BU, Connor HD, Mason RP, Thurman RG. Allopurinol prevents early alcohol-induced liver injury in rats. J Pharmacol Exp Ther. 2000;293(1):296–303. [PubMed] [Google Scholar]
- 72.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 73.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Lanaspa MA, Tamura Y, Tanabe K, et al. Umami: the taste that drives purine intake. J Rheumatol. 2013;40(11):1794–1796. doi: 10.3899/jrheum.130531. [DOI] [PubMed] [Google Scholar]
- 74.Ogryzlo MA. Hyperuricemia induced by high fat diets and starvation. Arthritis Rheum. 1965;8(5):799–822. doi: 10.1002/art.1780080443. [DOI] [PubMed] [Google Scholar]
- 75.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86(4):899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 76.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basu S, Yoffe P, Hills N, Lustig RH. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One. 2013;8(2):e57873. doi: 10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2012;109(11):4320–4325. doi: 10.1073/pnas.1119908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013;58(5):1632–1643. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzicky P, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez-Lozada LG, Tapia E, Bautista-Garcia P, Soto V, Avila-Casado C, Vega-Campos IP, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2008;294(4):F710–F718. doi: 10.1152/ajprenal.00454.2007. [DOI] [PubMed] [Google Scholar]
- 83.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. 2012;66(2):201–208. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gammaglutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9(1):68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–E1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stanhope KL, Griffen SC, Keim NL, Ai M, Otokozawa S, Nakajima K, et al. Consumption of fructose-, but not glucose-sweetened beverages produces an atherogenic lipid profile in overweight/obese men and women. Diabetes. 2007;56(Suppl 1):A16. (abstr) [Google Scholar]
- 87.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, et al. Consumption of fructose-sweetened beverages for 10 weeks increases post-prandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008:1–6. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34(3):454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 90.Kahn K, Serfozo P, Tipton PA. Identification of the true product of the urate oxidase reaction. J Am Chem Soc. 1997;119(23):5435–5442. [Google Scholar]
- 91.Kratzer JT, Lanaspa MA, Murphy MN, Cicerchi C, Graves CL, Tipton PA, et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci U S A. 2014;111(10):3763–3768. doi: 10.1073/pnas.1320393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson RJ, Andrews P. Fructose, uricase, and the back-to-Africa hypothesis. Evol Anthropol. 2010;19:250–257. [Google Scholar]
- 93.Johnson RJ, Andrews P. The fat gene: a genetic mutation in prehistoric apes may underlie today's pandemic of obesity and diabetes. Sci Am. 2015;313:64–69. [Google Scholar]
- 94.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, et al. Impulsivity is associated with uric acid: evidence from humans and mice. Biol Psychiatry. 2014;75(1):31–37. doi: 10.1016/j.biopsych.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gosling AL, Matisoo-Smith E, Merriman TR. Gout in Maori. Rheumatology (Oxford) 2014;53(5):773–774. doi: 10.1093/rheumatology/ket299. [DOI] [PubMed] [Google Scholar]
- 97.Johnson RJ, Lanaspa MA, Sanchez-Lozada LG, Rivard CJ, Rodriguez-Iturbe B, Merriman TR, et al. Fat storage syndrome in Pacific peoples: a combination of environment and genetics? Public Health Dialog. 2014;20:11–16. [PubMed] [Google Scholar]
- 98.Gertler MM, Garn SM, Levine SA. Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann Intern Med. 1951;34(6):1421–1431. doi: 10.7326/0003-4819-34-6-1421. [DOI] [PubMed] [Google Scholar]
- 99.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 100.Vaccarino V, Krumholz HM. Risk factors for cardiovascular disease: one down, many more to evaluate. Ann Intern Med. 1999;131(1):62–63. doi: 10.7326/0003-4819-131-1-199907060-00012. [DOI] [PubMed] [Google Scholar]
- 101.Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB. Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis. 1999;33(2):225–234. doi: 10.1016/s0272-6386(99)70295-7. [DOI] [PubMed] [Google Scholar]
- 102.Johnson RJ, Tuttle KR. Much ado about nothing, or much to do about something? The continuing controversy over the role of uric acid in cardiovascular disease. Hypertension. 2000;35(3):E10. doi: 10.1161/01.hyp.35.3.e10. [DOI] [PubMed] [Google Scholar]
- 103.Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375(9732):2161–2167. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grimaldi-Bensouda L, Alperovitch A, Aubrun E, Danchin N, Rossignol M, Abenhaim L, et al. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. 2015;74(5):836–842. doi: 10.1136/annrheumdis-2012-202972. [DOI] [PubMed] [Google Scholar]
- 106.Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, et al. Allopurinol and progression of CKD and cardiovascular events: long-term Follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 107.Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, et al. The impact of serumuric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65(3):1041–1049. doi: 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 108.Terawaki H, Nakayama M, Miyazawa E, Murata Y, Nakayama K, Matsushima M, et al. Effect of allopurinol on cardiovascular incidence among hypertensive nephropathy patients: the Gonryo study. Clin Exp Nephrol. 2013;17(4):549–553. doi: 10.1007/s10157-012-0742-z. [DOI] [PubMed] [Google Scholar]
- 109.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci. 2003;105(4):425–430. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 110.Gersch C, Palii SP, Imaram W, Kim KM, Karumanchi SA, Angerhofer A, et al. Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids. 2009;28(2):118–149. doi: 10.1080/15257770902736400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Imaram W, Gersch C, Kim KM, Johnson RJ, Henderson GN, Angerhofer A. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic Biol Med. 2010;49(2):275–281. doi: 10.1016/j.freeradbiomed.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reyes AJ, Leary WP. The ALLHAT and the cardioprotection conferred by diuretics in hypertensive patients: a connection with uric acid? Cardiovasc Drugs Ther. 2002;16(6):485–487. doi: 10.1023/a:1023090823731. [DOI] [PubMed] [Google Scholar]
- 113.Reungjui S, Roncal CA, Mu W, Srinivas TR, Sirivongs D, Johnson RJ, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. 2007;18(10):2724–2731. doi: 10.1681/ASN.2007040416. [DOI] [PubMed] [Google Scholar]
- 114.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 115.Parsa A, Brown E, Weir MR, Fink JC, Shuldiner AR, Mitchell BD, et al. Genotype-based changes in serum uric acid affect blood pressure. Kidney Int. 2012;81(5):502–507. doi: 10.1038/ki.2011.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, et al. A polymorphism in the major gene regulating serum uric acid associates with clinic SBP and the white-coat effect in a family-based study. J Hypertens. 2014;32(8):1621–1628. doi: 10.1097/HJH.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 117.Preitner F, Pimentel A, Metref S, Berthonneche C, Sarre A, Moret C, et al. No development of hypertension in the hyperuricemic liver-Glut9 knockout mouse. Kidney Int. 2015;87(5):940–947. doi: 10.1038/ki.2014.385. [DOI] [PubMed] [Google Scholar]