Abstract
Clinical studies have reported associations between serum uric acid levels and the development of diabetic nephropathy, but the underlying mechanisms remain elusive. There is evidence from animal studies that blocking uric acid production protects the kidney from tubulointerstitial injury, which may suggest a causal role for uric acid in the development of diabetic tubular injury. In turn, when fructose, which is endogenously produced in diabetes via the polyol pathway, is metabolised, uric acid is generated from a side-chain reaction driven by ATP depletion and purine nucleotide turnover. For this reason, uric acid derived from endogenous fructose could cause tubulointerstitial injury in diabetes. Accordingly, our research group recently demonstrated that blocking fructose metabolism in a diabetic mouse model mitigated the development of tubulointerstitial injury by lowering tubular uric acid production. In this review we discuss the relationship between uric acid and fructose as a novel mechanism for the development of diabetic tubular injury.
Keywords: Diabetes, Diabetic nephropathy, Fructokinase, Fructose, Glucose, Kidney, Polyol pathway, Review, Tubular injury, Uric acid
Introduction
Diabetic nephropathy is the leading cause of end-stage renal disease and dialysis in the Western world [1–4]. New therapeutic strategies are urgently needed to improve the prognosis of this devastating complication of diabetes, which remains a significant public health burden. However, the development of new drugs to halt or prevent diabetic nephropathy and the translation of these therapies into clinical practice has been slow and disappointing. An economical approach to developing new drugs is to revisit old molecules.
Uric acid has both oxidant and antioxidant properties in the human body. While uric acid may also demonstrate antioxidant effects in the central nervous system [5], uric acid are associated with development of vascular complications in diabetes, in particular, diabetic nephropathy [6]. In this regard, targeting uric acid is advantageous, as uric acid production can be easily manipulated by either blocking xanthine oxidase or promoting urine uric acid (UUA) excretion. Moreover, methods to measure serum uric acid (SUA) and UUA are readily available and inexpensive.
One diabetes-related factor that could closely associate with uric acid is fructose. Fructose is endogenously produced as a consequence of activation of the polyol pathway under diabetic conditions. It is also a major component of added sugars and is distinct from other sugars in its ability to generate uric acid as a side product of its metabolism [7].
Our research group recently demonstrated that blocking fructose metabolism in a mouse model of diabetes mitigated the development of tubulointerstitial injury by lowering tubular uric acid production [8]. This discovery could provide new insights into the pathogenesis and therapeutic options for this intractable disease. Herein we review the literature and discuss future directions for research on the relationship between fructose, uric acid and diabetic nephropathy.
Clinical associations of uric acid and diabetic nephropathy
Several important clinical studies have demonstrated cross-sectional and longitudinal associations between SUA and diabetic nephropathy in patients with type 1 and 2 diabetes (Tables 1, 2 and 3).
Table 1.
The association of serum uric acid with diabetic nephropathy
Table 2.
Serum uric acid predicts the development of diabetic nephropathy
| Location | Number | Type of diabetes |
Follow- up period |
Outcome | Reference |
|---|---|---|---|---|---|
| Denmark | 263 | Type 1 | 18 years | Macroalbuminuria | [12] |
| USA | 443 | Type 1 | 6 years | Coronary atherosclerosis |
[85] |
| USA | 355 | Type 1 | 6 years | Early GFR loss | [9] |
| USA | 324 | Type 1 | 6 years | Albuminuria | [10] |
| USA | 652 | Type 1 | 6 years | Rapid GFR decline Microalbuminuria |
[86] |
| USA | 1,449 | Type 2 | 5 years | Incident CKD | [13] |
| UK | 270 | Type 2 | 8 years | eGFR decline | [14] |
| USA | 534 | Type 1 | 8 years | Early GFR decline | [87] |
Table 3.
Effects of lowering serum uric acid on diabetic nephropathy
Prospective data from The Second Joslin Study on the Natural History of Microalbuminuria in Type 1 Diabetes, a clinic-based study, identified a relationship between SUA levels at baseline and rapid decline in GFR (>3.3%/year) in adults with type 1 diabetes [9] (Table 2). Interestingly, they also reported a dose–response relationship between baseline SUA levels and future risk of early GFR loss. The unadjusted relative risk of developing increased GFR loss was 1.5 for each mg/dl (60 µmol/l) increase in SUA, which translated into a 2.4-fold increase in the risk of early GFR loss for SUA levels above the median (267.7 µmol/l) as compared with SUA levels below this value.
The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study also demonstrated strong relationships between SUA, incident albuminuria, rapid GFR decline, as well as the progression of subclinical atherosclerosis and diabetic retinopathy [10, 11] (Table 2). Similarly, a study based at the Steno Diabetes Center reported an association between SUA and the development of persistent macroalbuminuria. In an inception cohort study of 263 individuals with newly diagnosed type 1 diabetes, SUA measured shortly after the onset of type 1 diabetes was a significant independent predictor of macroalbuminuria 18 years later (HR 2.37, 95% CI 1.04– 5.37, p=0.04, for every 101.1 µmol/l increase in SUA) [12] (Table 2).
The role of SUA as a predisposing factor for diabetic nephropathy does not appear to be limited to type 1 diabetes. In an Italian cohort of type 2 diabetic patients with normal kidney function and without overt proteinuria, the risk of chronic kidney disease (CKD) during a 5 year follow-up was significantly higher in participants with hyperuricaemia compared with those without [13] (Table 2). In a study with adults with type 2 diabetes and chronic kidney disease, SUA was found to predict progression of established nephropathy [14] (Table 2). Furthermore, a post hoc analysis of the Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) Trial found that lowering SUA levels with losartan treatment (which reduces SUA levels by facilitating urinary uric acid excretion) accounted for 20% of the renoprotective benefit of this medication [15] (Table 3). A study on the effect of allopurinol on type 2 diabetic patients with macroalbuminuria reported that lowering SUA levels significantly reduced urinary proteinuria [16] (Table 3). In a recent randomized parallel-controlled study, allopurinol was also shown to improve renal function in patients with type 2 diabetes [17] (Table 3).
While the majority of studies have found positive associations between SUA and incident CKD and several have demonstrated relationships between SUA and CKD progression [18, 19], not all findings have been positive [20, 21]. Furthermore, the identification of SUA as a risk factor for the development of both early and late phenotypes of diabetic nephropathy does not necessarily imply causation; however, increasing evidence implicates SUA in the pathogenesis of nephropathy and the deterioration of kidney function observed in type 1 and 2 diabetes.
Females have lower SUA levels than males, irrespective of diabetes status, and the difference between sexes is thought to stem from the uricosuric effect of oestrogen on the urate transporter URAT1 [22, 23]. Interestingly, studies have demonstrated that women carry a greater risk for CKD than men for the same uric acid level [24].
The Preventing Early Renal Function Loss in Diabetes (PERL) allopurinol study is an ongoing multi-centre double-blind randomised clinical trial with allopurinol to lower SUA in individuals with type 1 diabetes in an attempt to prevent early diabetic nephropathy [6]. If PERL produces promising results, similar studies could be conducted with other micro-vascular and macrovascular complications as endpoints. The recent discovery that fructose-mediated generation of uric acid may contribute to vascular complications provides an additional opportunity for important diet and lifestyle modifications [7].
Diabetic tubulopathy
Diabetic nephropathy is characterised not only by glomerular disease but also by tubulointerstitial injury. The tubular changes associated with diabetic nephropathy include basement membrane thickening, tubular hypertrophy, epithelial–mesenchymal transition, glycogen accumulation and interstitial inflammation. Although glomerular changes have received significantly more attention than tubulointerstitial changes in diabetes from researchers and clinicians, the tubular injury is more closely associated with renal function than glomerular injury [25]. In fact, tubular proteinuria may precede microalbuminuria in children with type 1 diabetes [26], suggesting that tubular damage may be induced earlier than glomerular injury in the course of diabetic nephropathy.
In a rat model of diabetes, there is a 67% increase in the weight of the kidney within 50 days after induction of diabetes, and such changes are thought to be attributable to tubular hypertrophy [27]. In the hypertrophic response, tubular cells likely undergo both proliferation and cellular hypertrophy under diabetic conditions, resulting in an elongation of tubular length, an increase in cellular volume and tubular dilation in both proximal and distal tubular cells [28]. Such changes are likely to be evident in the cortex and the outer stripe of the outer medulla, but not in collecting ducts [27]. The tubular dilation can be induced by other mechanisms, including tubular cell apoptosis and tubular epithelial mesenchymal transition [29].
Uric acid targets tubular epithelial cells in the mouse model of diabetes
Exactly how uric acid contributes to the development of diabetic nephropathy remains elusive. However, a series of murine studies has shed light on some possible underlying mechanisms. In the first study, the db/db mouse model was used to examine the effects of lowering SUA levels in the kidney [30]. This mouse model is known to develop renal injury accompanied with albuminuria, mesangial matrix expansion and mild tubulointerstitial disease. Of importance, this model also exhibits mild hyperuricaemia. We found that lowering SUA levels with allopurinol significantly reduced albuminuria and ameliorated the tubulointerstitial injury [30]. In the same study, we also demonstrated that uric acid directly stimulates cultured human proximal tubular epithelial cells to induce the expression of intercellular adhesion molecule 1 (ICAM-1), an important inflammatory cytokine [30]. Similarly, the KK-Ay/Ta mouse, another mouse model of type 2 diabetes, was found to develop diabetic tubular injury that was attenuated by allopurinol [31]. This attenuation is likely to be attributable to the ability of allopurinol to inhibit the fibrogenic effect by activation of the TGF-β–Smad pathway [31]. Rats with streptozotocin-induced diabetes were found to exhibit hyperuricaemia, which likely activated the NLR protein 3 (NLRP3) inflammasome, and allopurinol administration was able to block this activation [32]. Taken together, these findings suggest that uric acid might directly act on the proximal tubular epithelial cells to cause inflammation in diabetic nephropathy. Consistent with this notion, Verzola et al recently demonstrated that uric acid increases the permissiveness of human proximal tubular cells to apoptosis by triggering a pathway involving NADPH oxidase signalling and URAT1 transport [33].
Renal handling of uric acid in patients with diabetes
Renal uric acid handling in humans may be altered in people with diabetes and may also be different according to diabetes type. This notion could account for the fact that SUA concentrations are often lower in patients with type 1 diabetes compared with their non-diabetic peers [10, 11], although among people with type 1 diabetes, the risk for renal disease increases with SUA levels [11]. Golembiewska et al demonstrated that poor glycaemic control was associated with hypouricaemia as a result of an increase in the fractional excretion of uric acid (FeUA) in patients with type 1 diabetes [34]. Although the precise mechanisms involved remain unknown, it is likely that the glycosuria-driven osmotic gradient across the apical membrane may prevent reabsorption of UUA. Since the threshold for glucose reabsorption at the proximal tubules is approximately 10 mmol/l, glycaemia exceeding this threshold results in glycosuria and increased osmolarity [35]. An alternative mechanism, based on the finding that glucose is three times more potent than mannitol in facilitating UUA excretion [36], is that glucose exerts an effect in addition to osmotic stress on the excretion of uric acid into urine.
In contrast, type 2 diabetes and the metabolic syndrome are often associated with higher SUA concentrations [37, 38], although some studies have reported the presence of hypouricaemia in type 2 diabetes [39, 40]. The increase might be accounted for by the presence of insulin resistance and hyperinsulinaemia. Several studies have documented that insulin sensitivity could be maintained in renal tubules even in the systemic insulin-resistant state [38, 41]. If this is the case, a higher concentration in serum insulin could stimulate the renal tubular cells to reabsorb sodium coupling with uric acid. Interestingly, adolescents and adults with type 1 diabetes are also recognised as being significantly more insulin resistant than their non-diabetic counterparts, but in contrast to type 2 diabetes, the associations between SUA and insulin sensitivity in type 1 diabetes are weak [11]. The precise mechanisms as to how either glucose or insulin affect the transport of urate in the proximal tubular cells remain unclear.
Urinary glucose reabsorption and uric acid in patients with diabetes
Under physiological conditions in humans serum glucose is filtered at the glomerulus and reabsorbed in the proximal tubular cells. A major mediator for glucose reabsorption is thought to be the sodium–glucose co-transporter 2 (SGLT2). This transporter is expressed in the apical membrane of the proximal convoluted tubular cells, accounts for 90% of glucose reabsorption and is dependent on sodium to co-transport glucose and sodium [42]. In type 1 and 2 diabetes, the activated sodium–potassium ATPase in the basolateral membrane creates the inward sodium gradient across the apical membrane, which facilitates SGLT2 transport activity [42]. Hyperglycaemia enhances SGLT2 expression, resulting in increased tubular reabsorption of urinary glucose [43]. In turn, the accumulation of intracellular glucose activates the polyol pathway.
SGLT2 inhibitors reduce proximal tubular sodium reabsorption, thereby increasing distal sodium delivery to the macula densa, causing afferent vasoconstriction and decreased hyperfiltration via tubuloglomerular feedback [44]. Treatment with SGLT2 inhibitors for 8 weeks was reported to attenuate renal hyperfiltration (≥135 ml min−1 1.73 m−2) in adults with type 1 diabetes [44]. SGLT2 inhibitors are primarily used in clinical medicine as an adjunctive therapy to improve hyperglycaemia. While SLGT2 inhibitors are capable of reducing serum glucose concentrations in diabetic patients, this compound has another interesting effect, which is to lower SUA by facilitating UUA excretion [45, 46]. Recently, Lytvyn et al demonstrated that an induction of glycosuria with SGLT2 inhibition during euglycaemia leads to a decrease in SUA and an increase in FeUA in adults with type 1 diabetes [47]. However, the molecular mechanisms underlying the uricosuric effect of glucose remain unclear.
SUA concentration relies on both the exogenous pool of uric acid, which depends on dietary intake and the endogenous reservoir, which is mainly regulated by hepatic synthesis, intestinal secretion and renal secretion. Approximately 100% of SUA is excreted into urine, but is almost immediately reabsorbed, followed by the secretion of about half of the original filtered load, and a post-secretory reabsorption at the proximal convoluted tubule. Consequently, 90% filtered SUA is reabsorbed, and approximately 10% of the originally filtered SUA is excreted. UA reabsorption occurs via several urate transporters on the apical membrane; mainly URAT1, the more recently discovered GLUT9b and the organic anion transporters OAT4 and OAT10. Human GLUT9 has two splice variants with different expression patterns; GLUT9a is located on basolateral membrane whereas GLUT9b is expressed in the apical membrane of the polarised cells [48].
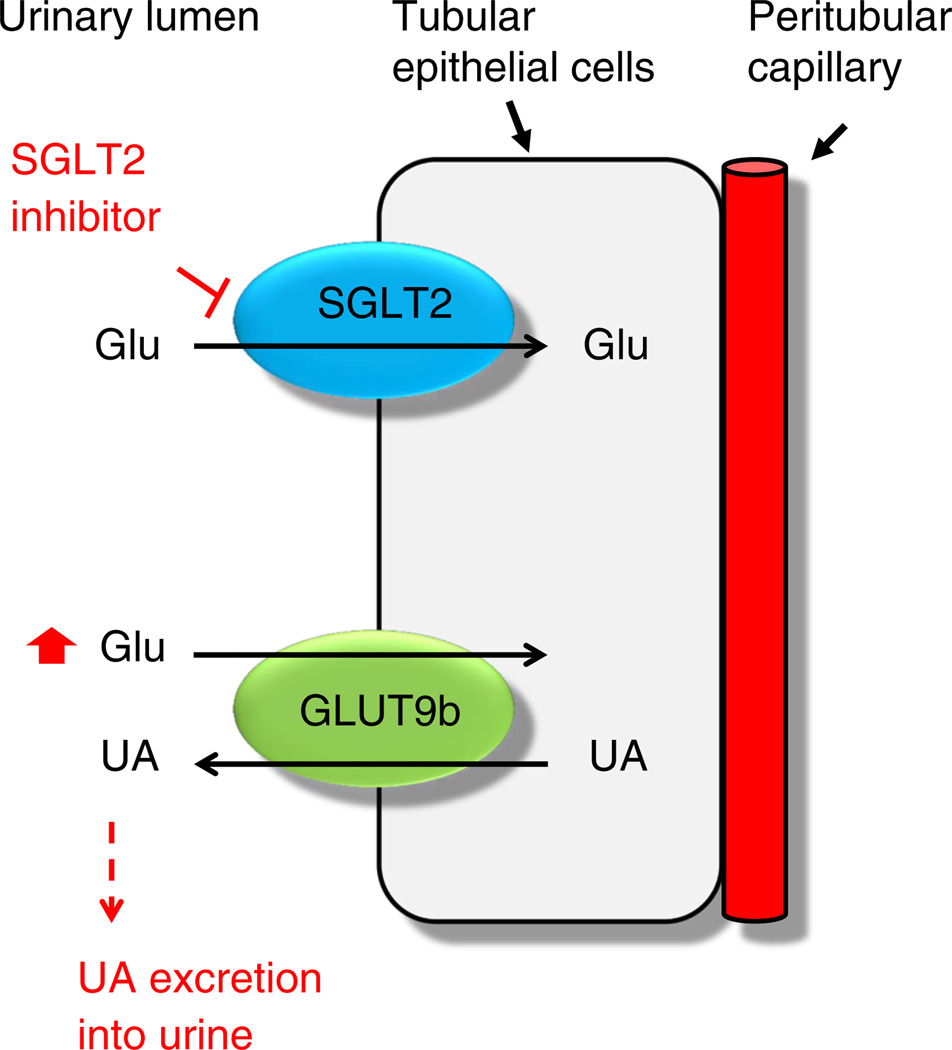
A recent study has shown that glucose is capable of trans-stimulating GLUT9b for the efflux of uric acid in Xenopus oocytes [49]. Given that urinary glucose is elevated in diabetes and is further increased by SGLT2 inhibitors, GLUT9b could be stimulated by high concentrations of glucose in the apical membrane, leading to uricosuria. However, this is unlikely since this isoform has been localised to the collecting duct rather than the proximal tubules [50]. It thus remains unclear how GLUT9b in the collecting duct is involved in diabetes. As both uric acid transporter and SGLTs are found in the proximal tubular cells, interaction between these transporters by an unknown mechanism is also highly plausible and might result in blocking uric acid reabsorption (Fig. 1).
Fig. 1.
A proposed mechanism by which a high concentration of urinary glucose my stimulate urate excretion. Glu, glucose; SGLT, sodium-dependent glucose transporter; UA, uric acid
Endogenous fructose is produced in diabetes
Animals
In diabetes, glucose is reduced to sorbitol in the polyol pathway by aldose reductase, and then sorbitol is oxidised by sorbitol dehydrogenase to produce fructose. In the kidney, aldose reductase is predominantly expressed in the inner stripe of the outer medulla, inner medulla and papillary tip and is also expressed in the proximal tubular cells and podocytes [51]. Aldose reductase activity has also been observed in the renal cortex and outer medulla of non-diabetic rats [52]. In diabetes, the enzyme can be upregulated by hyperglycaemia-induced hyperosmolarity, as well as by glucose per se [7, 53]. Ghahary et al demonstrated an elevation in both aldose reductase expression and activity in the kidney of streptozotocin-induced diabetic rat [54] and the diabetes-prone BioBreeding/Worcester (BB/Wor) rat [55]. Interestingly, a transgenic mouse line carrying human aldose reductase 2 (ALR2) cDNA was shown to develop thrombosis in renal vessels and deposits in Bowman’s capsule, which partially resemble the histological changes associated with human diabetic nephropathy [56].
Activation of the polyol pathway in diabetic rats increases fructose levels in several organs, including the retina, endoneurium and lens, compared with those in non-diabetic rats [57]. We recently demonstrated an elevation in fructose content in the kidney of the streptozotocin-induced diabetic mouse [8]. Importantly, blockade of fructose production through inhibition of sorbitol dehydrogenase has been shown to ameliorate vascular and neuronal dysfunction in diabetic animals [57], suggesting that fructose accumulation could be involved in the development of diabetic complications.
Humans
Diabetic patients exhibit higher fructose levels than non-diabetic individuals in serum and urine [58]. Interestingly, the degree of fructose accumulation in the proximal tubular cells is likely to vary between individuals. In one study, fructose levels were increased in 75% of human proximal tubular epithelial cells isolated from 21 non-diabetic individuals following exposure to 27.5 mmol/l glucose in vitro, but were not increased in the remaining 25% [59]. This variation in fructose variation may explain why some patients with diabetes develop diabetic nephropathy, whereas others do not. Furthermore, inhibition of aldose reductase has been tested in diabetic murine models. For example, a pyridazinone recently discovered to be an aldose reductase inhibitor was shown to improve survival and inhibit cataract development and normalise retinal sorbitol and fructose in rat model of diabetes [60], but clinical trials in humans with aldose reductase inhibitors have been less conclusive. The Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group [61] reported the prevention of progression of diabetic nephropathy with epalrestat, but other studies have failed to show a delay in diabetic nephropathy [62, 63].
Fructose and uric acid
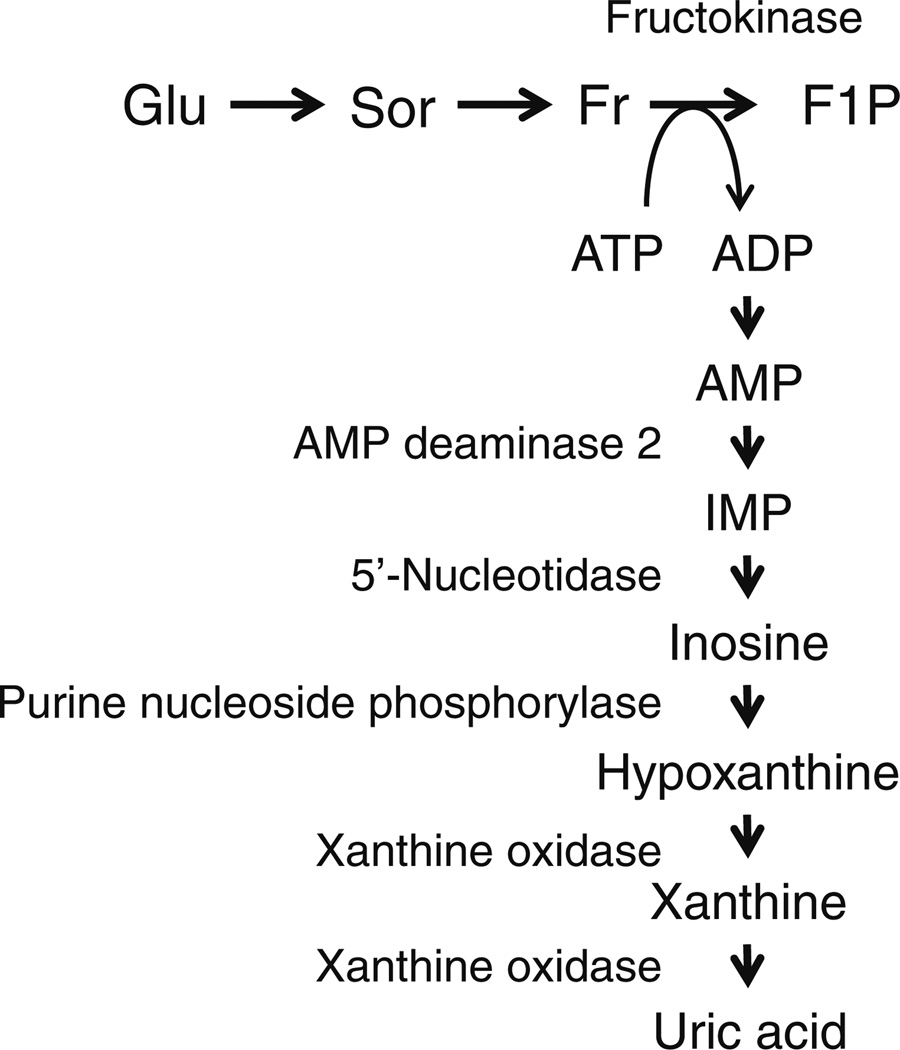
Intracellular fructose produced as a result of activation of the polyol pathway can be further metabolised, generating uric acid as a side product by a sequence of multiple enzymatic activations (Fig. 2). Fructokinase (also known as ketohexokinase), an enzyme predominantly expressed in the liver, phosphorylates fructose to produce fructose 1-phos-phate. ATP is the phosphate donor in this reaction and is converted into ADP. While the reaction with fructokinase is rapid, it is not associated with negative feedback, which leads to a reduction in intracellular phosphate and ATP when fructose levels are high. A reduction in intracellular phosphate subsequently stimulates AMP deaminase 2, which in turn stimulates uric acid production (Fig. 2) [53, 64]. Uric acid production is increased not only through degradation of adenine nucleotides but also by increased de novo purine biosynthesis [65, 66]. Emerging data support the notion that fructose metabolism elevates SUA levels in animals and humans [67, 68].
Fig. 2.
From polyol pathway to uric acid production. F1P, fructose-1-phosphate; Fr, fructose; Glu, glucose; IMP, inosine monophosphate; Sor, sorbitol
Animals
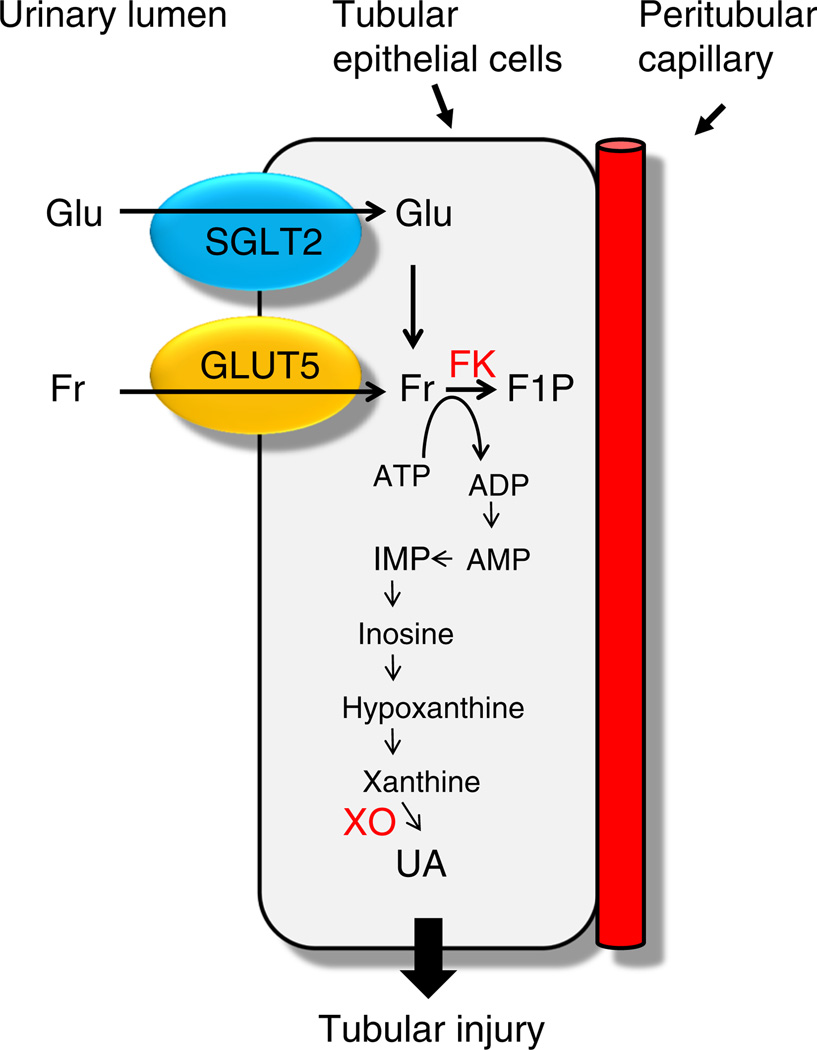
In the kidney, urinary glucose is absorbed by the SGLTs into the proximal tubular epithelial cells, where fructokinase is predominantly expressed [69]. It is conceivable that reabsorbed glucose in the proximal tubules activates the polyol pathway to produce endogenous fructose, which is then further metabolised by fructokinase [8, 53]. Furthermore, the activity of xanthine oxidase, which is required for uric acid production, is highest in the proximal tubular cells [70]. These findings indicate that it is likely that uric acid is produced in the proximal tubular cells (Fig. 3).
Fig. 3.
A proposed mechanism for tubular injury in diabetes. F1P, fructose-1-phosphate; FK, fructokinase; Fr, fructose; Glu, glucose; UA, uric acid; XO, xanthine oxidase
The proximal tubular epithelial cells, particularly those in the S3 segment, have another way of increasing their fructose levels, through the fructose transporter GLUT5, which is expressed in their apical membrane [69]. It is therefore likely that there are at least two mechanisms involved in the accumulation of fructose in the proximal tubular cells in diabetes (Fig. 3).
Humans
A dose of fructose of 1 g/kg body weight has been reported to rapidly increase SUA levels in humans by 59– 118 µmol/l in 2 h [71]. Similarly, in a clinical study, consumption of fructose-containing beverages representing 25% of energy requirements for 10 weeks significantly raised SUA concentrations compared with the isoenergetic consumption of glucose-containing beverages [72]. In the National Health and Nutrition Examination Survey (NHANES), the consumption of sugar-sweetened soft drinks was positively associated with SUA concentrations in adults, suggesting that the fructose in soft drinks could increase uric acid levels [73].
Fructose causes tubular injury in animal models
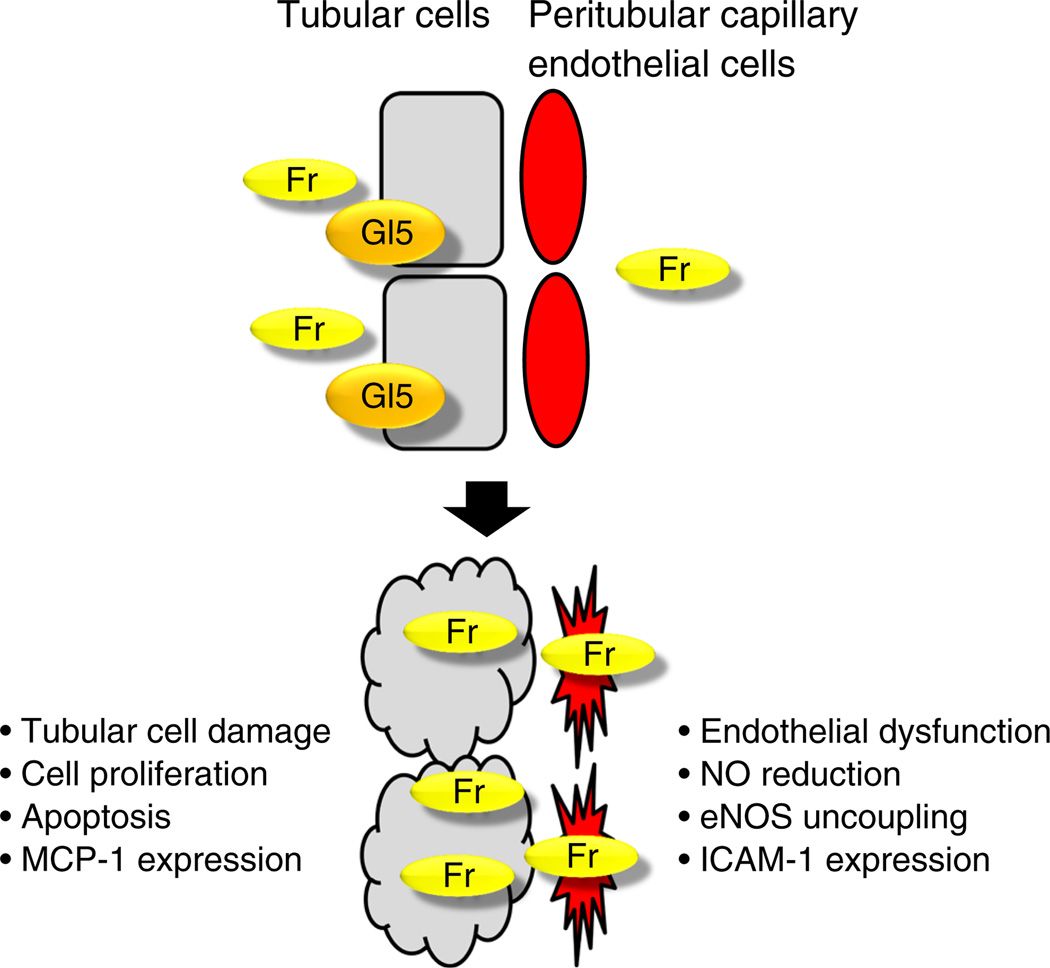
A recent murine study examining the renal effects of dietary fructose provided evidence that fructose induces mild tubulointerstitial injury in normal rats [69] (Fig. 4). More specifically, some proximal tubular epithelial cells were shown to express vimentin and/or exhibit a cellular proliferative response [69]. An increase in type III collagen deposition was also detected around the impaired tubular cells [69]. A similar study on mice also reported that dietary fructose could initiate the development of tubulointerstitial injury [74].
Fig. 4.
Fructose impairs renal tubular epithelial cells and endothelial cells. Fr, fructose; Gl5, GLUT5
To investigate how fructose is implicated in the progression of renal disease we examined the effects of dietary fructose in the rat remnant kidney model of chronic kidney disease [75]. Dietary fructose dramatically accelerated the progression of renal injury, including glomerular and tubulointerstitial disease [75]. Given that fructose is freely filtered by the glomerulus, and urinary fructose is absorbed in the proximal tubular epithelial cells where the fructose transporter GLUT5 is expressed, in theory, the tubular cells could be directly stimulated by fructose (Fig. 3). Consistent with this notion, monocyte chemoattractant protein 1 (MCP-1) expression and proliferative responses were found to be induced in cultured human proximal tubular epithelial cells in response to fructose [75]. Importantly, these responses were significantly decreased by a xanthine oxidase inhibitor, suggesting that fructose-induced MCP-1 expression can be partly mediated by uric acid [76].
Another mechanism of fructose-induced renal disease may involve endothelial dysfunction [77]. In fructose-fed rats, the glomerular and peritubular capillary endothelial cells exhibit ICAM-1 induction accompanied by an increase in serum ICAM-1 concentration [77]. Fructose-induced ICAM-1 expression was also confirmed in the cultured endothelial cells, suggesting that fructose directly acts on endothelial cells. A likely mechanism for endothelial dysfunction involves a reduction in the availability of NO [77]. The active form of the enzyme endothelial nitric oxide synthase (eNOS) is a dimer. Fructose prevents the dimerisation of eNOS monomers, resulting in inhibition of NO production and an increase in ROS [78]. Consistent with this notion, fructose-induced ICAM-1 expression was mitigated in the presence of an NO donor [77]. Moreover, uric acid has been shown to block insulin stimulation of NO in endothelial cells [79]
Blocking fructose metabolism mitigates the progression of diabetic tubular injury in animal models
Our animal study demonstrated that uric acid could be a mediator of the development of diabetic tubular injury [30]. As discussed above, we hypothesised that endogenous fructose, a metabolite of the polyol pathway, is a source of uric acid in diabetic tubular epithelial cells. We confirmed that the diabetic kidney has a higher concentration of uric acid than the non-diabetic kidney. Importantly, elevated intrarenal fructose content is also accompanied by an increase in fructokinase activity in diabetic kidney disease [8]. In streptozotocin-induced diabetic mice lacking the fructokinase gene, intrarenal uric acid levels and urinary albumin excretion were reduced compared with those in the wild-type streptozotocin-induced diabetic mice, and tubular injury was ameliorated [8]. Interestingly, glomerular injury, as evidenced by mesangial expansion and an increase in type IV collagen deposition, was also significantly mitigated in diabetic fructokinase-deficient mice, although the precise mechanism involved remains unclear. These data suggest that fructose and uric acid could be risk factors for diabetic tubular injury
From bench to bed
Data from animal studies indicate that uric acid may induce tubular injury in diabetic nephropathy, and that endogenous fructose, a metabolite of the polyol pathway in diabetic tubular epithelial cells, is a potential source of uric acid. These findings suggest that the fructose–uric acid axis may partially account for some of the clinical phenotypes of diabetic nephropathy in humans, including GFR decline and tubular albuminuria. Uric acid reduction could be a promising therapeutic option for diabetic nephropathy, and important clinical trials are underway.
Acknowledgments
Funding This work was supported by a fund from the Gout Research Foundation.
Abbreviations
- CKD
Chronic kidney disease
- eNOS
Endothelial nitric oxide synthase
- FeUA
Fractionated excretion of uric acid
- GFR
Glomerular filtration rate
- ICAM-1
Intercellular adhesion molecule 1
- MCP-1
Monocyte chemotactic protein 1
- NLRP3
NOD-like receptor pyrin domain containing 3
- OAT
Organic anion transporter
- PERL
Preventing early renal loss in diabetes study
- ROS
Reactive oxygen species
- SGLT2
Sodium–glucose co-transporter 2
- SUA
Serum uric acid
- URAT1
Urate transporter 1
- UUA
Urine uric acid
Footnotes
Duality of interest PB, ML, Ishimoto, TK, SK, DJ, DMM, and JKSB have no conflict of interest to disclose. RJJ and TNN are listed as inventors of a couple of patent applications that propose lowering serum uric acid as a treatment for various metabolic and renal disorders. RJJ is listed as an inventor on a patent application from the University of Colorado related to developing isoform-specific fructokinase inhibitors in the treatment of obesity and insulin resistance and diabetic nephropathy. RJJ and TN have equity with XORT Therapeutics that is interested in pursuing xanthine oxidase inhibitors for the treatment of diabetic nephropathy. RJJ also discloses that he has consulted for Danone, is on the scientific board of Amway and that he has received grants from the NIH and from Amway, Cardero, Danone and Questcor.
Contribution statement PB researched, wrote, contributed to discussion, and reviewed/edited the manuscript; ML, TI, TK, DJ obtained original supportive data, contributed to discussion and reviewed/edited the manuscript; SK, DMM, JKSB, RJJ wrote, contributed to discussion, and reviewed/edited the manuscript; and TN obtained original supportive data, wrote, contributed to discussion, and reviewed/edited the manuscript as the senior author. All authors approved the final version of the manuscript.
References
- 1.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus-progress made, more to be done. J Clin Endocrinol Metab. 2006;91:3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 2.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–2319. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7, e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014;21:279–286. doi: 10.1097/MED.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maahs DM, Caramori L, Cherney DZ, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanaspa MA, Ishimoto T, Cicerchi C, et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol. 2014;25:2526–2538. doi: 10.1681/ASN.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33:1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25:1865–1869. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornstad P, Snell-Bergeon JK, McFann K, et al. Serum uric acid and insulin sensitivity in adolescents and adults with and without type 1 diabetes. J Diabetes Complicat. 2014;28:298–304. doi: 10.1016/j.jdiacomp.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58:1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35:99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD) Nephrol Dial Transplant. 2012;27:1847–1854. doi: 10.1093/ndt/gfr561. [DOI] [PubMed] [Google Scholar]
- 15.Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 16.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4:128–132. [PubMed] [Google Scholar]
- 17.Liu P, Chen Y, Wang B, Zhang F, Wang D, Wang Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol. 2014 doi: 10.1111/cen.12673. [DOI] [PubMed] [Google Scholar]
- 18.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650. [PubMed] [Google Scholar]
- 19.Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43:347–352. doi: 10.1016/j.exger.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langford HG, Blaufox MD, Borhani NO, et al. Is thiazide-produced uric acid elevation harmful? Analysis of data from the Hypertension Detection and Follow-up Program. Arch Intern Med. 1987;147:645–649. doi: 10.1001/archinte.147.4.645. [DOI] [PubMed] [Google Scholar]
- 22.Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol (Copenh) 1977;85:198–208. doi: 10.1530/acta.0.0850198. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi A, Kamatani N. Control of renal uric acid excretion and gout. Curr Opin Rheumatol. 2008;20:192–197. doi: 10.1097/BOR.0b013e3282f33f87. [DOI] [PubMed] [Google Scholar]
- 24.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–697. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 26.Ginevri F, Piccotti E, Alinovi R, et al. Reversible tubular proteinuria precedes microalbuminuria and correlates with the metabolic status in diabetic children. Pediatr Nephrol. 1993;7:23–26. doi: 10.1007/BF00861555. [DOI] [PubMed] [Google Scholar]
- 27.Rasch R. Tubular lesions in streptozotocin-diabetic rats. Diabetologia. 1984;27:32–37. doi: 10.1007/BF00253498. [DOI] [PubMed] [Google Scholar]
- 28.Rasch R, Dorup J. Quantitative morphology of the rat kidney during diabetes mellitus and insulin treatment. Diabetologia. 1997;40:802–809. doi: 10.1007/s001250050752. [DOI] [PubMed] [Google Scholar]
- 29.Verzola D, Bertolotto MB, Villaggio B, et al. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol. 2004;15(Suppl 1):S85–S87. doi: 10.1097/01.asn.0000093370.20008.bc. [DOI] [PubMed] [Google Scholar]
- 30.Kosugi T, Nakayama T, Heinig M, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Ren Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SM, Choi YW, Seok HY, et al. Reducing serum uric acid attenuates TGF-beta1-induced profibrogenic progression in type 2 diabetic nephropathy. Nephron Exp Nephrol. 2012;121:e109–e121. doi: 10.1159/000343567. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE. 2012;7:e38285. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verzola D, Ratto E, Villaggio B, et al. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One. 2014;9:e115210. doi: 10.1371/journal.pone.0115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golembiewska E, Ciechanowski K, Safranow K, Kedzierska K, Kabat-Koperska J. Renal handling of uric acid in patients with type 1 diabetes in relation to glycemic control. Arch Med Res. 2005;36:32–35. doi: 10.1016/j.arcmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Cook DG, Shaper AG, Thelle DS, Whitehead TP. Serumuric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986;62:1001–1006. doi: 10.1136/pgmj.62.733.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skeith MD, Healey LA, Cutler RE. Urate excretion during mannitol and glucose diuresis. J Lab Clin Med. 1967;70:213–220. [PubMed] [Google Scholar]
- 37.Vuorinen-Markkola H, Yki-Jarvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab. 1994;78:25–29. doi: 10.1210/jcem.78.1.8288709. [DOI] [PubMed] [Google Scholar]
- 38.Muscelli E, Natali A, Bianchi S, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9:746–752. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 39.Shichiri M, Iwamoto H, Marumo F. Diabetic hypouricemia as an indicator of clinical nephropathy. Am J Nephrol. 1990;10:115–122. doi: 10.1159/000168065. [DOI] [PubMed] [Google Scholar]
- 40.Golik A, Weissgarten J, Cotariu D, et al. Renal uric acid handling in non-insulin-dependent diabetic patients with elevated glomerular filtration rates. Clin Sci (Lond) 1993;85:713–716. doi: 10.1042/cs0850713. [DOI] [PubMed] [Google Scholar]
- 41.Quinones Galvan A, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268:E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 42.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011;79(Suppl 120):S20–S27. doi: 10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]
- 43.Freitas HS, Anhe GF, Melo KF, et al. Na+-glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1α expression and activity. Endocrinology. 2008;149:717–724. doi: 10.1210/en.2007-1088. [DOI] [PubMed] [Google Scholar]
- 44.Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86:1057–1058. doi: 10.1038/ki.2014.246. [DOI] [PubMed] [Google Scholar]
- 45.Skeith MD, Healey LA, Cutler RE. Effect of phloridzin on uric acid excretion in man. Am J Physiol. 1970;219:1080–1082. doi: 10.1152/ajplegacy.1970.219.4.1080. [DOI] [PubMed] [Google Scholar]
- 46.Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012;44:375–393. doi: 10.3109/07853890.2011.560181. [DOI] [PubMed] [Google Scholar]
- 47.Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Ren Physiol. 2015;308:F77–F83. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]
- 48.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279:16229–16236. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 49.Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35:391–404. doi: 10.1002/bdd.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T, Takahashi M, Yan K, Sakurai H. Expression of SLC2A9 isoforms in the kidney and their localization in polarized epithelial cells. PLoS ONE. 2014;9:e84996. doi: 10.1371/journal.pone.0084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludvigson MA, Sorenson RL. Immunohistochemical localization of aldose reductase. II. Rat eye and kidney. Diabetes. 1980;29:450–459. doi: 10.2337/diab.29.6.450. [DOI] [PubMed] [Google Scholar]
- 52.Terubayashi H, Sato S, Nishimura C, Kador PF, Kinoshita JH. Localization of aldose and aldehyde reductase in the kidney. Kidney Int. 1989;36:843–851. doi: 10.1038/ki.1989.270. [DOI] [PubMed] [Google Scholar]
- 53.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghahary A, Luo JM, Gong YW, Chakrabarti S, Sima AA, Murphy LJ. Increased renal aldose reductase activity, immunoreactivity, and mRNA in streptozocin-induced diabetic rats. Diabetes. 1989;38:1067–1071. doi: 10.2337/diab.38.8.1067. [DOI] [PubMed] [Google Scholar]
- 55.Ghahary A, Chakrabarti S, Sima AA, Murphy LJ. Effect of insulin and statil on aldose reductase expression in diabetic rats. Diabetes. 1991;40:1391–1396. doi: 10.2337/diab.40.11.1391. [DOI] [PubMed] [Google Scholar]
- 56.Yamaoka T, Nishimura C, Yamashita K, et al. Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia. 1995;38:255–261. doi: 10.1007/BF00400627. [DOI] [PubMed] [Google Scholar]
- 57.Tilton RG, Chang K, Nyengaard JR, Van den Enden M, Ido Y, Williamson JR. Inhibition of sorbitol dehydrogenase. Effects on vascular and neural dysfunction in streptozocin-induced diabetic rats. Diabetes. 1995;44:234–242. doi: 10.2337/diab.44.2.234. [DOI] [PubMed] [Google Scholar]
- 58.Kawasaki T, Akanuma H, Yamanouchi T. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care. 2002;25:353–357. doi: 10.2337/diacare.25.2.353. [DOI] [PubMed] [Google Scholar]
- 59.Flath MC, Bylander JE, Sens DA. Variation in sorbitol accumulation and polyol-pathway activity in cultured human proximal tubule cells. Diabetes. 1992;41:1050–1055. doi: 10.2337/diab.41.9.1050. [DOI] [PubMed] [Google Scholar]
- 60.Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi M. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes. 2006;55:2757–2762. doi: 10.2337/db06-0138. [DOI] [PubMed] [Google Scholar]
- 61.Hotta N, Kawamori R, Fukuda M, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29:1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohmura C, Watada H, Azuma K, et al. Aldose reductase inhibitor, epalrestat, reduces lipid hydroperoxides in type 2 diabetes. Endocr J. 2009;56:149–156. doi: 10.1507/endocrj.k08e-237. [DOI] [PubMed] [Google Scholar]
- 63.Ramana KV. Aldose reductase: new insights for an old enzyme. Biomol Concepts. 2011;2:103–114. doi: 10.1515/BMC.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 65.Raivio KO, Becker A, Meyer LJ, Greene ML, Nuki G, Seegmiller JE. Stimulation of human purine synthesis de novo by fructose infusion. Metab Clin Exp. 1975;24:861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- 66.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33:276–280. doi: 10.1136/ard.33.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Ren Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 68.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes. 2010;34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 69.Nakayama T, Kosugi T, Gersch MS, et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Ren Physiol. 2010;298:F712–F720. doi: 10.1152/ajprenal.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doctor RB, Mandel LJ. Minimal role of xanthine oxidase and oxygen free radicals in rat renal tubular reoxygenation injury. J Am Soc Nephrol. 1991;1:959–969. doi: 10.1681/ASN.V17959. [DOI] [PubMed] [Google Scholar]
- 71.Stirpe F, Della Corte E, Bonetti E, Abbondanza A, Abbati A, De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1311. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- 72.Cox CL, Stanhope KL, Schwarz JM, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/ obese humans. Nutr Metab (Lond) 2012;9:68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serumuric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 74.Aoyama M, Isshiki K, Kume S, et al. Fructose induces tubulointerstitial injury in the kidney of mice. Biochem Biophys Res Commun. 2012;419:244–249. doi: 10.1016/j.bbrc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Gersch MS, Mu W, Cirillo P, et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Ren Physiol. 2007;293:F1256–F1261. doi: 10.1152/ajprenal.00181.2007. [DOI] [PubMed] [Google Scholar]
- 76.Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glushakova O, Kosugi T, Roncal C, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008;19:1712–1720. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shinozaki K, Kashiwagi A, Nishio Y, et al. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2 imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 79.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71–e78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tseng CH. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 2005;68:796–801. doi: 10.1111/j.1523-1755.2005.00459.x. [DOI] [PubMed] [Google Scholar]
- 81.Fukui M, Tanaka M, Shiraishi E, et al. Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism. 2008;57:625–629. doi: 10.1016/j.metabol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Behradmanesh S, Horestani MK, Baradaran A, Nasri H. Association of serum uric acid with proteinuria in type 2 diabetic patients. J Res Med Sci. 2013;18:44–46. [PMC free article] [PubMed] [Google Scholar]
- 84.Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J Diabetes Complicat. 2014;28:124–129. doi: 10.1016/j.jdiacomp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Rodrigues TC, Maahs DM, Johnson RJ, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33:2471–2473. doi: 10.2337/dc10-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bjornstad P, Maahs DM, Rivard CJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014;51:783–791. doi: 10.1007/s00592-014-0611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krolewski AS, Niewczas MA, Skupien J, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37:226–234. doi: 10.2337/dc13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]