Summary
Anxiety helps us anticipate and assess potential danger in ambiguous situations [1, 2, 3]; however, the anxiety disorders are the most prevalent class of psychiatric illness [4, 5, 6]. Emotional states are shared between humans and other animals [7], as observed by behavioral manifestations [8], physiological responses [9], and gene conservation [10]. Anxiety research makes wide use of three rodent behavioral assays—elevated plus maze, open field, and light/dark box—that present a choice between sheltered and exposed regions [11]. Exposure avoidance in anxiety-related defense behaviors was confirmed to be a correlate of rodent anxiety by treatment with known anxiety-altering agents [12, 13, 14] and is now used to characterize anxiety systems. Modeling anxiety with a small neurogenetic animal would further aid the elucidation of its neuronal and molecular bases. Drosophila neurogenetics research has elucidated the mechanisms of fundamental behaviors and implicated genes that are often orthologous across species. In an enclosed arena, flies stay close to the walls during spontaneous locomotion [15, 16], a behavior proposed to be related to anxiety [17]. We tested this hypothesis with manipulations of the GABA receptor, serotonin signaling, and stress. The effects of these interventions were strikingly concordant with rodent anxiety, verifying that these behaviors report on an anxiety-like state. Application of this method was able to identify several new fly anxiety genes. The presence of conserved neurogenetic pathways in the insect brain identifies Drosophila as an attractive genetic model for the study of anxiety and anxiety-related disorders, complementing existing rodent systems.
Graphical Abstract
Highlights
-
•
Drosophila orthologs of anxiety genes affect fly wall following
-
•
Conserved anxiety genes influence fly defense behaviors similarly to mouse anxiety
-
•
New candidate anxiety genes are identified from fly defense behavior screen
-
•
Drosophila identified as a new neurogenetic tool for anxiety research
Mohammad et al. show that orthologs of mammalian anxiety factors govern defense behaviors in the fly and use these behaviors to identify new conserved candidate anxiety genes. Thus, rodent anxiety research may be complemented by Drosophila neurogenetic models.
Results
Flies Follow the Walls of an Enclosed Chamber
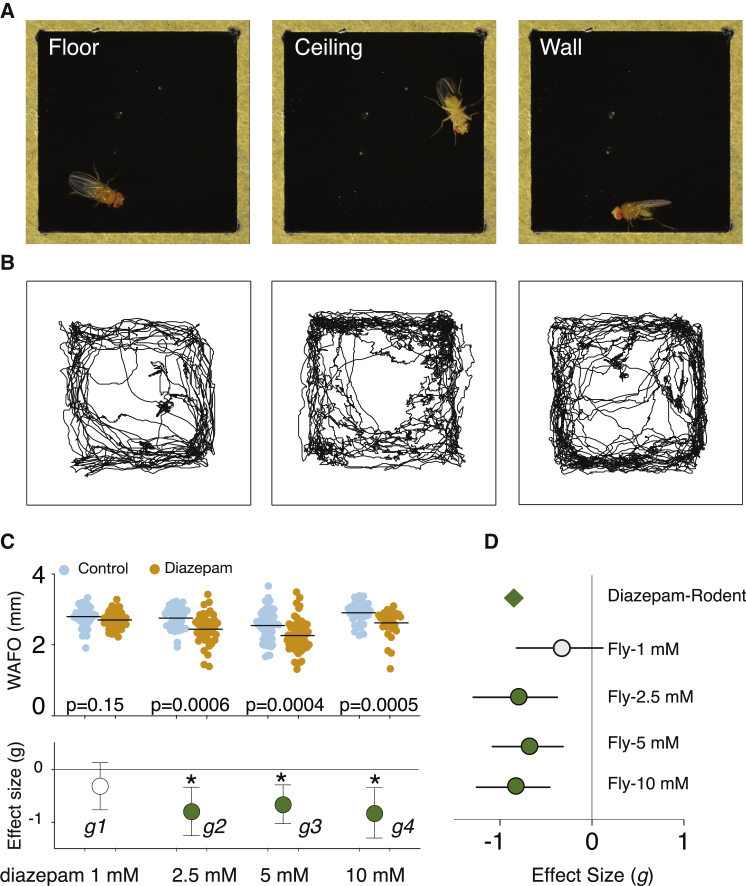
Flies in enclosed chambers spent a large proportion of time near the walls (Figures 1 and S1) [18, 19]. While flies were able to crawl on all surfaces—floor, walls, and ceiling (Figure 1A)—cumulative locomotion traces were strikingly similar to rodent thigmotaxis data from open fields (Figure 1B) [14]. Flies on all surfaces were close to the wall, often 3–4 mm away from the center of a 5-mm chamber (Figure S1C). This behavioral feature, but not locomotion itself, was persistent (Figures S1D–S1F). We termed this behavior “wall following” (WAFO).
Figure 1.
Drosophila Wall Following Behavior Is Reduced by Diazepam
(A) Flies in a glass-topped arena walk on all interior surfaces.
(B) Tracking data from a 10-min experiment reveal that flies mainly walk in the perimeter of the arena.
(C) Flies fed with diazepam had decreased WAFO compared with controls (g1 = −0.32, g2 = −8.0, g3 = −0.67, g4 = −0.83, n = 40, 40). Fly WAFO was measured as mean distance from center in millimeters. Dots indicate the mean distance from center for individual flies; horizontal line indicates the mean distance from center (mm). p values determined by Mann-Whitney U. The lower axis represents the effect size in Hedges’ g with 95% CI. Green circles and asterisk (∗) mark a statistically significant (p < 0.05) decrease in behavior.
(D) Standardized mean effect sizes of diazepam effects on rodent anxiety (−0.85 g [95 CI −0.74, −0.96]) and fly WAFO (−0.83 g [95 CI −0.42, −0.91]) have comparable magnitudes.
See also Figure S1.
Diazepam Reduces Fly Wall Following
Benzodiazepines reduce anxiety by modulating GABAA receptors [20], and their binding site is evolutionarily conserved [21]. Diazepam reduces anxiety in three important rodent defense behavior assays: the open field (OF), the elevated plus maze (EPM), and the light/dark box [11]. In flies, diazepam had a pronounced effect on fly WAFO at three doses (Figure 1C). Raw behavioral metrics may have an indirect relationship to internal state and are not comparable across diverse experimental systems, for example, between different assays in distinct species. To contextualize the diazepam result, we calculated a standardized effect size (Hedges’ g) from the diazepam-induced WAFO change (Figure 1C, lower panel) and compared it with a meta-analytic rodent anxiety diazepam effect size calculated from 382 published rodent experiments (http://dx.doi.org/10.1101/020701). Diazepam effect sizes in both systems were comparable (Figure 1D).
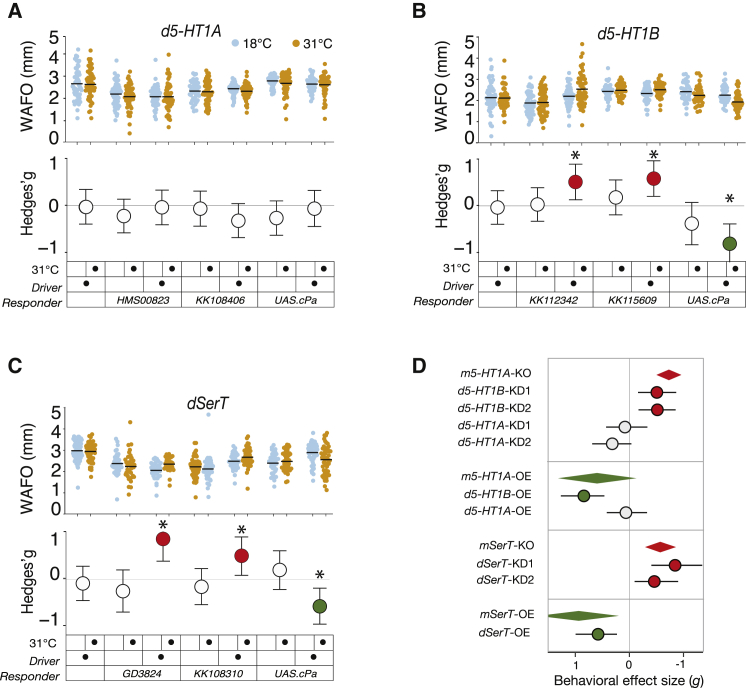
Altering d5-HT1B Function Has WAFO Effects that Are Concordant with Mouse Anxiety
Genetic experiments in mouse previously demonstrated that deleting and overexpressing the gene for the mammalian 5-HT1A receptor (m5-HT1A) produced moderate effects on rodent anxiety (http://dx.doi.org/10.1101/020701). Drosophila has two serotonin class 1 receptor genes with similarity to m5-HT1A: d5-HT1A and d5-HT1B. The function of these genes was knocked down in adult flies with lines expressing RNAi under the control of a warm-induced pan-neuronal driver, nSyb-Gal4, tub-Gal80ts [22, 23]. Alterations of d5-HT1A expression with two RNAi lines and one cDNA responder produced only minor changes in WAFO (Figure 2A). However, the use of RNAi and overexpression to alter levels of d5-HT1B produced pronounced effects on WAFO (Figure 2B). These d5-HT1B effect sizes were of a comparable magnitude to the mouse anxiety effects from m5-HT1A lesions (Figure 2D) (http://dx.doi.org/10.1101/020701). Control experiments with warm treatment of control flies had trivial WAFO effects (Figures 2A and 2B). We conclude that manipulating d5-HT1B function influences fly WAFO in ways that parallel the effects that altering m5-HT1A expression has on mouse defense behaviors.
Figure 2.
Anxiety-Concordant Effects of Serotonin Gene Lesions on Fly WAFO
(A) Genetic lesions of d5-HT1A produced only minor effects in WAFO. Blue dots are untreated flies; orange dots are pre-warmed to 31°C as for GAL80ts derepression. The lower axes show Hedges’ g; responder alleles are named in the boxes. The driver is nSyb-Gal4, Tub-Gal80ts.
(B) Genetic lesions of d5-HT1B had moderate and statistically significant effects on WAFO: knockdown caused increases (d5-HT1BKK112342g = 0.51, p = 9 × 10−3; d5-HT1BKK115609g = 0.58, p = 2 × 10−3), while overexpression elicited a decrease (g = −0.82, p = 7.4 × 10−5, n = 53, 54). Red and green circles indicate a statistically significant WAFO change.
(C) Knockdowns of mSerT with two RNAi lines produced consistent WAFO increases (SerTGD3824g = 0.63, p = 8.2 × 10−4, n = 60, 55; SerTKK108310g2 = 0.48, p = 0.2 × 10−2, n = 60, 40), and overexpression decreased WAFO (dSerTScer∖UAS·cPag = −0.53, p = 1.8 × 10−3, n = 73, 75). Warm-treated controls for d5-HT1A, d5-HT1B, and mSerT UAS transgenes underwent modest, non-statistically significant changes.
(D) A comparison of mouse anxiety gene effect sizes and fly ortholog WAFO effect sizes indicates they are concordant in direction and magnitude, except for d5-HT1A knockdowns. Diamonds indicate averaged meta-analytic values; circles indicate fly WAFO effect; lateral vertices and error lines are 95% CI.
See also Figure S2.
Concordant SERT Effects on Fly WAFO and Mouse Anxiety
Deletion of mSert produces an increase in mouse anxiety (http://dx.doi.org/10.1101/020701). In flies, reducing dSerT mRNA levels with either of two RNAi alleles increased WAFO (Figure 2C). Flies expressing transgenic dSerT at 12× elevated levels (Figure S2P) had lowered WAFO (g = −0.53; Figure 2E), echoing the low anxiety observed in mice expressing elevated mSert (http://dx.doi.org/10.1101/020701). Control, warm-treated flies underwent no WAFO change (Figure 2C).
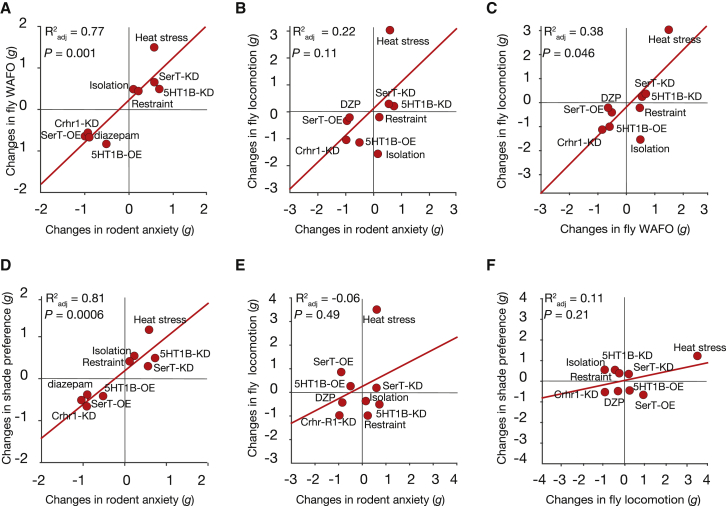
Concordant Stress Effects on Fly WAFO and Mouse Anxiety
Environmental stress drives anxiety [24]. Subjecting flies to heat shock stress elicited a large WAFO increase (Figure 3A), concordant with the effect of acute pain on rodent anxiety (Figure S3D) ([25]; http://dx.doi.org/10.1101/020701). Diazepam reduced WAFO in heat-stressed flies, much as it did for flies at 25°C (Figures S2Q and S2R). Physically restraining flies produced a WAFO increase that was concordant with the anxiogenic effect of restraint in rodents (Figure S2E) (http://dx.doi.org/10.1101/020701). Ten days of social isolation stress increased fly WAFO (Figure S2F), an outcome that is concordant with isolation’s effect on rodent anxiety (http://dx.doi.org/10.1101/020701). The corticotropin-releasing hormone receptor 1 (CRHR1) is associated with mammalian stress, and knockout mice have lower anxiety; the fly homolog is the diuretic hormone 44 receptor 1 (DH44-R1) [26, 27]. Reducing Dh44-R1 expression (Figure S2O) reduced WAFO (Figure S2K), consistent with mouse data (http://dx.doi.org/10.1101/020701). Interestingly, Dh44-R1 mRNA levels were dramatically altered by all three stressors (Figure S4D).
Figure 3.
Fly Defense Behavior Outcomes Are Concordant with Anxiety Outcomes
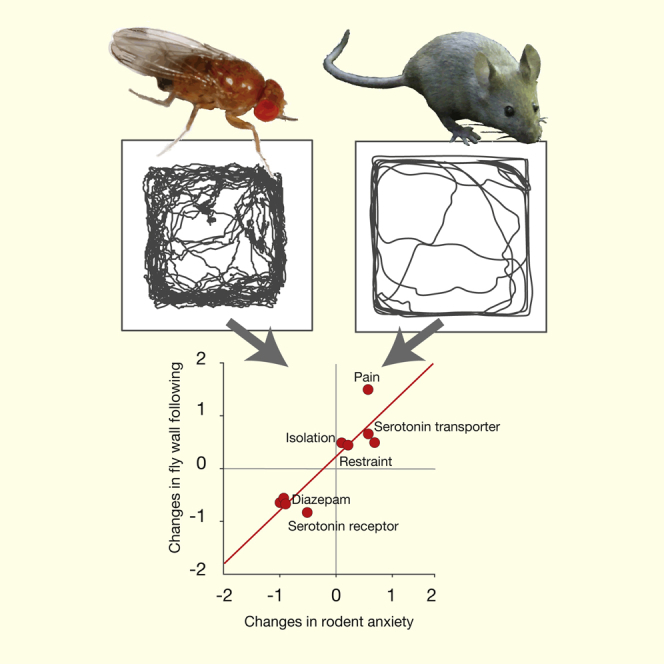
(A) A strong correlation between rodent anxiety and fly WAFO data for nine comparable manipulations (R2adj = 0.77 [95 CI 0.58, 0.83]). The horizontal axis shows rodent meta-analytic g values; the vertical axis displays fly WAFO g values. The red line is the least-squares fit; p is for the F statistic of the model.
(B) Walking speed changes in the square arena are weakly correlated with rodent anxiety outcomes (R2adj = 0.22 [95 CI 0.0, 0.30]).
(C) WAFO is moderately related to locomotion in the square arena (R2adj = 0.38 [95 CI 0.06, 0.49]).
(D) Light/dark choice outcomes are strongly correlated with rodent effect sizes (R2adj = 0.81 [95 CI 0.64, 0.86]).
(E) Changes in locomotion in the light/dark arena are weakly correlated with rodent anxiety outcomes (R2adj = 0.06 [95 CI 0.0, 0.09]).
(F) Light/dark choice outcomes are poorly correlated with locomotion (R2adj = 0.11 [95 CI 0.0, 0.14]).
See also Figure S3.
Anxiotropic Manipulations Influence Drosophila Light/Dark Choice
A second fly shelter/exposure assay with anxiety concordance would verify that exposure avoidance correlates with fly anxiety. The rodent light/dark choice assay examines light avoidance [12]. We used a simple chamber (Figure S3A) to measure changes in fly light/dark choice in response to anxiety manipulations. Of nine interventions, six (diazepam, d5-HT1B loss of function, dSerT overexpression, heat, restraint, and social isolation) had substantial statistical effects that were concordant with rodent anxiety data (Figure S3H). The other three were also directionally concordant but had modest, non-statistically significant effects on light/dark choice (d5-HT1B overexpression, dSerT knockdown, and Dh44-R1 knockdown). These data largely verify the hypothesis that exposure avoidance measures a fly anxiety state.
Effects in Drosophila WAFO and Light/Dark Choice Are Predictive of Rodent Anxiety Effects
Fly and rodent effect sizes for all interventions were subjected to cross-species linear regression. The regression models indicated that fly ΔWAFO data are largely predictive of rodent anxiety changes (R2adj = 0.77 95% confidence interval [95 CI 0.47, 0.75]), as are the fly Δlight/dark choice outcomes (R2adj = 0.81 [95 CI 0.58, 0.82]; Figures 3A and 3D). These results are compatible with the hypothesis that fly WAFO and fly light/dark choice, like rodent anxiety assays, test an anxiety-related brain state.
Fly Defense Behaviors Are Distinct from Motor Activity
Motor activity and anxiety behavior are related phenotypes. Tranquilizers like diazepam also have sedative effects, and such overlap might also apply to neurogenetic systems. If WAFO and/or light/dark choice changes were purely a result of speed changes, this would erode confidence in their specificity to anxiety. However, this was not the case. Walking speed was altered in ways that were dissociated from WAFO (Figures S1F, S2A–S2C, and S2H–S2K). Individual flies’ WAFO metrics were poorly correlated with “raw” walking speed (WAFO-locomotion R2adj = 0.18 [95 CI 0.17, 0.19], p = 1.0 × 10−91, n = 2,046), as were their light/dark preferences (shade preference-locomotion R2adj = 0.05 [95 CI 0.04, 0.06], p = 1.0 × 10−13, n = 1,138). Additional regression analyses of fly walking speed changes (Δspeed) indicated that these could explain less than four-tenths of WAFO change variance (ΔWAFO; Figure 3C) and only a tenth of Δlight/dark variance (Figure 3F). Cross-species analyses indicated that fly speed changes were weakly predictive of rodent anxiety: only a fifth (WAFO; Figure 3B) and 6% (light/dark; Figure 3E) of variance was explained. Thus, while locomotor changes contribute to ΔWAFO and Δlight/dark choice, they are not the main driver.
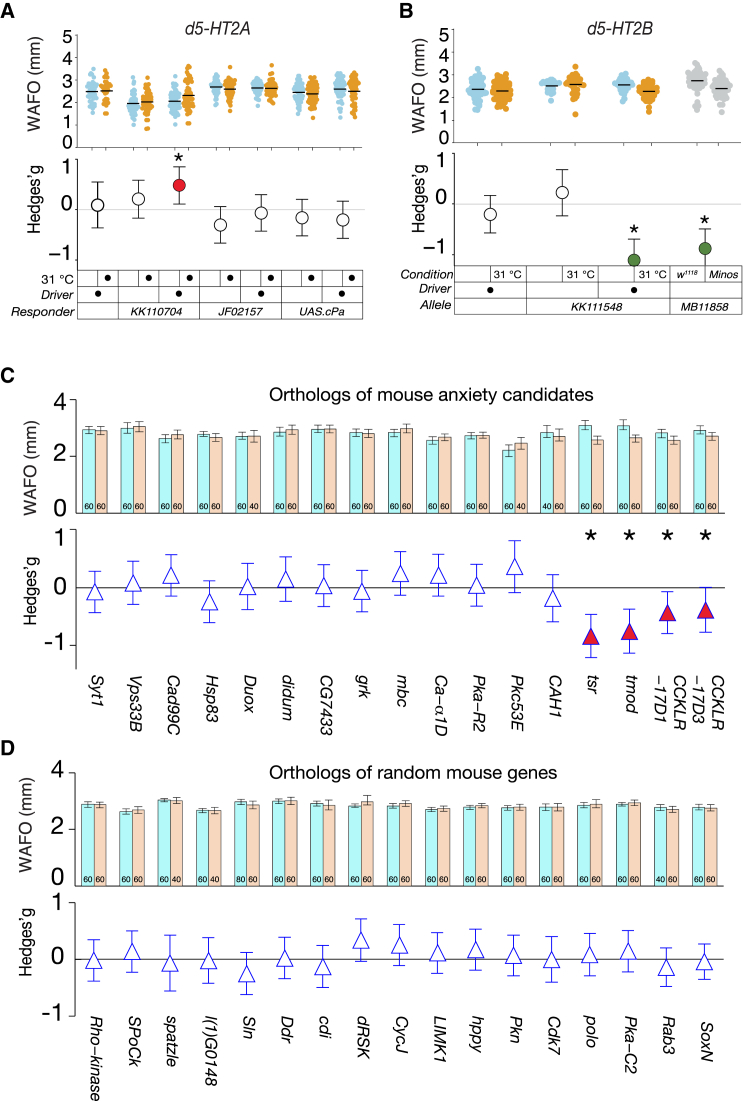
Identification of 5-HT2B, tsr, tmod, CCKLR-17D1, and CCKLR-17D3 as Fly Anxiety Factors
Wall following assays were used to identify fly anxiety gene candidates. Systematic review found that serotonin class 2 receptor knockouts have not been tested for their mouse anxiety role (http://dx.doi.org/10.1101/020701). Functional alterations of the two fly class 2 receptor genes, d5-HT2A and d5-HT2B, found that only the latter had consistent, substantial effects on WAFO (Figure 4B). Fly orthologs of candidate anxiety genes found at quantitative trait loci (QTLs) identified from a mouse genetic experiment were screened [28]. Of 17 genes, four showed WAFO alterations: twinstar (tsr), two Cholecystokinin-like receptor genes (CCKLR-17D3 and CCKLR-17D1), and tropomodulin (tmod) (Figure 4), which are homologs of mouse cofilin 1 (Cfl1), cholecystokinin B receptor (Cckbr), and Tropomodulin-2 (Tmod2), respectively. Control tests of 17 randomly selected orthologs found none produced WAFO effects (Figure 4D). Interestingly, two mouse orthologs of the four fly anxiety candidate genes are known to anxiety research. Cofilin-1 is a mouse anxiety gene with a knockout having a concordant outcome to the fly WAFO result [28]. Mouse Cckbr codes for cholecystokinin receptor, and its deletion has an effect concordant with knockdown effects of fly WAFO [29].
Figure 4.
Identification of Candidate Fly Anxiety Genes
(A) RNAi knockdown with d5-HT2AKK110704 increased WAFO (g = 0.48, p = 1 × 10−2), but this effect was not confirmed by a second RNAi allele (d5-HT2AJF02157g = −0.07, p = 6.9 × 10−1) or overexpression (d5-HT2AScer∖UAS.cPag = −0.21, p = 0.28). Warm-treated controls underwent non-statistical WAFO alterations.
(B) Knockdown of d5-HT2B with d5-HT2BKK111548 produced a decrease in WAFO (g = −1.1, p = 6.8 × 10−08) as did a Minos transposon insertion into the gene: d-HT2BMB11858 (g = −0.88, p = 4.1 × 10−06).
(C) Orthologs of candidate mouse anxiety genes were knocked down in the adult fly and tested for WAFO changes. Four knockdowns produced statistically significant reductions in WAFO: tsrKK108706 (g = −0.89, p = 5.0 × 10−6); tmodKK108701 (g = −0.81, p = 1.8 × 10−5); CCKLR−17D1KK108482 (g = −0.45, p = 3.5 × 10−4); and CCKLR−17D3KK110484 (g = −0.40, p = 1.2 × 10−2). Sample sizes are indicated at the base of the bars.
(D) Seventeen randomly selected orthologs’ knockdowns had trivial effects on WAFO.
See also Figure S4.
Discussion
The results verify the hypothesis that exposure avoidance behaviors of Drosophila share underlying neurogenetic pathways with mammalian anxiety. A GABA-modulating drug, serotonin receptor and transporter alterations, a stress peptide receptor, and environmental stressors produced effects that were concordant with comparable manipulations in mammalian anxiety-related behaviors. A regression comparison of fly behavior data and rodent anxiety data indicated that the two are similar. The high coefficients of determination observed in the interspecies comparisons are remarkable in that they would not be expected to account for sources of variance that include sampling error, within- and between-lab heterogeneity, publication bias, >600 million years of evolutionary divergence, or the difference between semi-acute knockdowns and lifelong knockouts.
A candidate survey newly implicated d5-HT2B, tsr, tmod, CCKLR-17D3, and CCKLR-17D1 in fly anxiety. The anxiolytic effect of tsr supports the hypothesis that actin microfilament stability is connected to anxiety [28], consistent with ideas that actin polymerization influences anxiety via aversive memory formation and stability [30] and/or related processes [31]. Similarly, that CCK-like receptor knockdowns reduce fly anxiety supports the hypothesis that CCK receptors are involved in anxiety and fear [32, 33], with a role proposed specifically for the mammalian cholecystokinin B receptor (CCKBR) [29]. In flies, the putative ligand for the CCKLR receptors is DROSULFAKININ (DSK); intriguingly, CCKLR-17D1 and dsk mutants have deficits in a larval stress-induced escape behavior [34]. The implication of CCK-like receptors in fly defense behaviors suggests that this is an anxiety-related signaling system, like GABA, serotonin, and Dh44-R1/CRHR1. Most of the orthologous gene knockdowns produced no WAFO effect, suggesting that the QTL hits include false positives and that WAFO genes are relatively rare.
Anxiety research has struggled to find new therapeutics [11]. Bringing the neurogenetic tools and larger sample sizes of Drosophila to bear on anxiety promises to complement rodent model analysis of anxiety and anxiety disorders.
Author Contributions
Conceptualization, F.M. and A.C.-C.; Methodology, F.M. and A.C.-C.; Software, F.M., S.A., J.H., J.C.S., and A.C.-C.; Investigation, F.M., T.L.T., N.A.N., and A.E.; Writing, F.M. and A.C.-C.; Visualization, F.M.; Supervision, A.C.-C.; Project Administration, A.C.-C.; Funding Acquisition, A.C.-C.
Acknowledgments
We thank Jonathan Flint, Gero Miesenböck, Justin Blau, Ajay Mathuru, and members of the A.C.-C. lab for their comments. We thank Helen Whitley, Martin Goodson, and Shankar Chandrasekhar Iyer for technical assistance. The authors were supported by Biomedical Research Council block grants to the Neuroscience Research Partnership and the Institute of Molecular and Cell Biology. F.M. and A.C.-C. received support from Duke-NUS Graduate Medical School. A.C.-C. received support from a Nuffield Department of Medicine Fellowship, a Wellcome Trust block grant to the University of Oxford, and NARSAD Young Investigator Award 17741. J.H., S.A., and A.E. were supported by awards from the A∗STAR Graduate Academy.
Published: March 24, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.02.031.
Supplemental Information
References
- 1.Blanchard D.C., Griebel G., Blanchard R.J. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 2.McNaughton N., Corr P.J. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Belzung C., Philippot P. Anxiety from a phylogenetic perspective: is there a qualitative difference between human and animal anxiety? Neural Plast. 2007;2007:59676. doi: 10.1155/2007/59676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler R.C., Heeringa S., Lakoma M.D., Petukhova M., Rupp A.E., Schoenbaum M., Wang P.S., Zaslavsky A.M. Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. Am. J. Psychiatry. 2008;165:703–711. doi: 10.1176/appi.ajp.2008.08010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiLuca M., Olesen J. The cost of brain diseases: a burden or a challenge? Neuron. 2014;82:1205–1208. doi: 10.1016/j.neuron.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin D.S., Anderson I.M., Nutt D.J., Allgulander C., Bandelow B., den Boer J.A., Christmas D.M., Davies S., Fineberg N., Lidbetter N. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. (Oxford) 2014;28:403–439. doi: 10.1177/0269881114525674. [DOI] [PubMed] [Google Scholar]
- 7.Anderson D.J., Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darwin C. Project Gutenberg; 1998. The Expression of the Emotions in Man and Animals. [Google Scholar]
- 9.Cannon W.B. Internet Archive; 2005. Bodily Changes in Pain, Hunger, Fear and Rage. [Google Scholar]
- 10.Saudou F., Boschert U., Amlaiky N., Plassat J.L., Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griebel G., Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawley J., Goodwin F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 13.Pellow S., Chopin P., File S.E., Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 14.Simon P., Dupuis R., Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 15.Besson M., Martin J.-R. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J. Neurobiol. 2005;62:386–396. doi: 10.1002/neu.20111. [DOI] [PubMed] [Google Scholar]
- 16.Lima S.Q., Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Iliadi K.G. The genetic basis of emotional behavior: has the time come for a Drosophila model? J. Neurogenet. 2009;23:136–146. doi: 10.1080/01677060802471650. [DOI] [PubMed] [Google Scholar]
- 18.Martin J.R., Ernst R., Heisenberg M. Temporal pattern of locomotor activity in Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1999;184:73–84. doi: 10.1007/s003590050307. [DOI] [PubMed] [Google Scholar]
- 19.Soibam B., Mann M., Liu L., Tran J., Lobaina M., Kang Y.Y., Gunaratne G.H., Pletcher S., Roman G. Open-field arena boundary is a primary object of exploration for Drosophila. Brain Behav. 2012;2:97–108. doi: 10.1002/brb3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigel E., Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J. Neurosci. 1988;8:289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson T., MacAllan D., Lunt G., Battersby M. gamma-Aminobutyric acid receptor complex of insect CNS: characterization of a benzodiazepine binding site. J. Neurochem. 1986;47:1955–1962. doi: 10.1111/j.1471-4159.1986.tb13114.x. [DOI] [PubMed] [Google Scholar]
- 22.Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 23.McGuire S.E., Le P.T., Osborn A.J., Matsumoto K., Davis R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 24.van Praag H.M. Can stress cause depression? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Marsch R., Foeller E., Rammes G., Bunck M., Kössl M., Holsboer F., Zieglgänsberger W., Landgraf R., Lutz B., Wotjak C.T. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J. Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson E.C., Bohn L.M., Taghert P.H. Drosophila CG8422 encodes a functional diuretic hormone receptor. J. Exp. Biol. 2004;207:743–748. doi: 10.1242/jeb.00818. [DOI] [PubMed] [Google Scholar]
- 27.Chang C.L., Hsu S.Y.T. Ancient evolution of stress-regulating peptides in vertebrates. Peptides. 2004;25:1681–1688. doi: 10.1016/j.peptides.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Goodson M., Rust M.B., Witke W., Bannerman D., Mott R., Ponting C.P., Flint J. Cofilin-1: a modulator of anxiety in mice. PLoS Genet. 2012;8:e1002970. doi: 10.1371/journal.pgen.1002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwanzger P., Domschke K., Bradwejn J. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress. Anxiety. 2012;29:762–774. doi: 10.1002/da.21919. [DOI] [PubMed] [Google Scholar]
- 30.Lamprecht R. The roles of the actin cytoskeleton in fear memory formation. Front. Behav. Neurosci. 2011;5:39. doi: 10.3389/fnbeh.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soetanto A., Wilson R.S., Talbot K., Un A., Schneider J.A., Sobiesk M., Kelly J., Leurgans S., Bennett D.A., Arnold S.E. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch. Gen. Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch. Gen. Psychiatry. 1989;46:511–517. doi: 10.1001/archpsyc.1989.01810060031006. [DOI] [PubMed] [Google Scholar]
- 33.Bradwejn J., Koszycki D., Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch. Gen. Psychiatry. 1991;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- 34.Chen X., Peterson J., Nachman R.J., Ganetzky B. Drosulfakinin activates CCKLR-17D1 and promotes larval locomotion and escape response in Drosophila. Fly (Austin) 2012;6:290–297. doi: 10.4161/fly.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.