The start-up syndrome associated with initiation of truvada as pre-exposure prophylaxis peaks within the first month of use and resolves by the third month. It is experienced by 16% of users and has a modest effect on adherence.
Keywords: HIV prevention, pre-exposure prophylaxis, tenofovir/emtricitabine, PrEP
Abstract
Background. Blinded clinical trials have reported a modest and transient “start-up syndrome” with initiation of tenofovir-based pre-exposure prophylaxis (PrEP). We evaluate this phenomenon and its effect on adherence in an open-label PrEP study.
Methods. In the iPrEx open-label extension (OLE) study, an 18-month open-label, multi-site PrEP cohort taking daily oral co-formulated tenofovir/emtricitabine, we examined the prevalence and duration of PrEP-associated symptoms and their effect on adherence, assessed by drug levels in dried blood spots tested monthly for the first 3 months.
Results. Symptom reports peaked within the first month, with 39% reporting potentially PrEP-related symptoms compared to 22% at baseline. Symptoms largely resolved to pre-PrEP levels by 3 months.
Symptoms varied substantially in frequency by study site (range in 1-month symptoms: 11% to 70%). Nongastrointestinal (GI) symptoms were not associated with adherence (odds ratio [OR] = 1.2, 95% confidence interval [CI], .4–3.7); however, GI-associated symptoms in the first 4 weeks were inversely associated with adherence at 4 weeks (OR = 0.47, 95% CI, .23–.96). Reports of GI symptoms were associated with 7% (95% CI, 4%–11%) of suboptimal adherence in this cohort.
Conclusions. PrEP-associated symptoms in the open-label setting occur in a minority of users and largely resolve within 3 months. GI symptoms are associated with a modest reduction in PrEP adherence, but good adherence is possible even in the presence of frequent symptom reports.
Clinical Trials Registration. Clinicaltrials.gov NCT00458393.
Clinical trials have demonstrated that pre-exposure prophylaxis (PrEP) with oral tenofovir disoproxil fumarate (TDF) or coformulated TDF with emtricitabine (FTC/TDF, Truvada) prevents human immunodeficiency virus (HIV) acquisition [1–7], with a few safety signals that include subclinical effects on bone [8–10] and kidney [11–13]. Although well tolerated overall, PrEP users have described a “start-up syndrome” associated with TDF and FTC/TDF of gastrointestinal (GI) and non-GI symptoms. In clinical trials, those assigned to FTC/TDF have reported nausea in 5%–19% [1, 3–5, 7], abdominal pain in 5%–13% [1–3, 7], flatulence in 8% [2], and vomiting in 4%–8% [3–5], as well as non-GI symptoms including dizziness in 15% [4] and fatigue in 11% [2]. These symptoms tend to peak in frequency within the first month after PrEP initiation and resolve to baseline levels by 3 months.
This may not reflect routine clinical experience, as placebo-controlled blinded trials may over- or underestimate symptom frequency depending on participant perception of treatment arm. In addition, the decline in symptom reports after 1 month could be due, in part, to the discontinuation of a drug from symptoms ascribed to medication. Adverse effects and poor tolerability have been associated with lower adherence in clinical settings in HIV treatment [14] and HIV post-exposure prophylaxis [15] and may also influence PrEP adherence.
We investigated the frequency of symptom reports and their potential effect on adherence using data from the iPrEx open-label extension (OLE) study [16]: a research study examining uptake of and adherence to oral FTC/TDF PrEP among a cohort of HIV-negative participants who were all assigned male sex at birth—1085 cis men who have sex with men (MSM) and 140 transgender women (TW). Participants enrolled from 11 sites in 6 countries (Brazil, Ecuador, Peru, South Africa, Thailand, United States of America). This study previously reported that: [1] concern about possible side-effects of FTC/TDF was the leading reason that participants in iPrEx OLE elected not to receive PrEP in OLE, [2] that adherence declined over the observation period, and [3] that adherence in the first month was highly associated with adherence during the study.
METHODS
Study Design
iPrEx OLE (OLE) was an open-label research study that enrolled HIV-negative former participants of 3 randomized PrEP trials [1, 17, 18] and offered them to receive FTC/TDF for up to 18 months or be followed without taking FTC/TDF. Follow-up visits were scheduled 1, 2, and 3 months after PrEP initiation and then quarterly for up to 18 months. The population and procedures have been reported previously [16]. Study drug was ended for iPrEx participants in August 2010, and enrollment in iPrEx OLE occurred between June 2011 and June 2012. The median gap in participation was 16 months (interquartile range: 12 months to 18 months).
Symptom and Adherence Assessment
At each visit, all participants completed a structured interview by study staff that recorded the presence of non-GI symptoms (arthralgia, fatigue, headache) and Gl symptoms (abdominal pain, diarrhea, flatulence, nausea, vomiting) during the previous month. Participants were also asked about the duration of symptoms, whether they attributed them to FTC/TDF, if symptoms had resolved or were ongoing, but they were not asked to rate symptom severity.
Of 1225 participants taking PrEP, 1108 (90%), had participated in the randomized phase of the iPrEx study. Because symptoms were ascertained in a similar way, it was possible to link symptom reports in the first months of OLE to the subset randomized to FTC/TDF during the iPrEx trial (n = 558). This allowed us to compare symptom reports within the same participant under the 2 different study conditions.
To estimate adherence with pharmacology, dried blood spots (DBS) were collected at every visit after PrEP was initiated. The DBS were assayed for intracellular levels of tenofovir diphosphate (TFV-DP) in red blood cells [19]. TFV-DP levels were categorized into TFV-DP levels of <350, ≥350 to <700, ≥700 fmol/punch which mapped to average adherence of <2 pills, 2–3 pills, and ≥4 pills per week (respectively) during the previous month [19]. A TFV-DP level <700 fmol/punch was considered suboptimal adherence, and levels ≥700 fmol/punch were associated with substantial protection [16]. DBS specimens were selected for TFV-DP testing using a case-cohort design [20]. This design tested all collected DBS specimens for every HIV-seroconverter (“cases”), and all collected DBS specimens in a randomly (site-stratified) selected 27% of participants who did not seroconvert (“cohort”). Interruption of FTC/TDF dispensation and reasons for drug interruption were recorded on case report forms.
Statistical Analysis
Ordinal logistic regression [21] with weights to reflect the case-cohort design and a robust variance for within-person clustering was used to determine the association between symptoms and TFV-DP dosing categories (<350, ≥350 to <700, ≥700 fmol/punch) after adjusting for potential confounders: a pre-specified set of variables identified in previous analyses [16], including site, baseline report condomless anal intercourse (insertive and/or receptive), baseline sexual partners (number and HIV status), age, education, baseline report of alcohol or drug use, and baseline creatinine. We adjusted proportions to clarify the direct influence of symptoms on adherence. Adjusted proportions were obtained by margining over potential confounders (by the “margins” command in Stata). Logistic regression with robust variance (clustered by participant) was used to evaluate potential correlates and predictors of symptoms over time. Paired proportions (eg, symptom reports of a participant between visits within OLE or across iPrEx randomized and OLE) were compared using McNemar's test [21]. All analyses were performed using Stata 14.
RESULTS
Symptoms and Enrollment in OLE
As previously reported [16], 1603 participants (58% of which had been participants in the iPrEx trial) enrolled and, of those, 1225 participants elected to take PrEP (44% of which were iPrEx participants). To assess whether previous experience with FTC/TDF or symptoms during the iPrEx randomized phase influenced the choice to return and take PrEP in OLE, we compared the PrEP opt-in rates by iPrEx randomized assignment and symptom experience. The choice to return for PrEP in OLE was not influenced by randomized assignment in the iPrEx trial, with odds ratio (OR) = 1.0 with 95% confidence interval (CI), .9–1.2 (P = .79) for assignment to FTC/TDF vs placebo. There was similar likelihood of enrollment and choosing PrEP among those who experienced any symptoms vs none and GI symptoms vs not in the first 3 months of the iPrEx randomized trial (any symptoms, OR = 1.1, 95% CI, .9–1.3, P = .51; GI symptoms, OR = 1.1, 95% CI, .9–1.3, P = .32) after adjustment for study site.
Symptoms During OLE
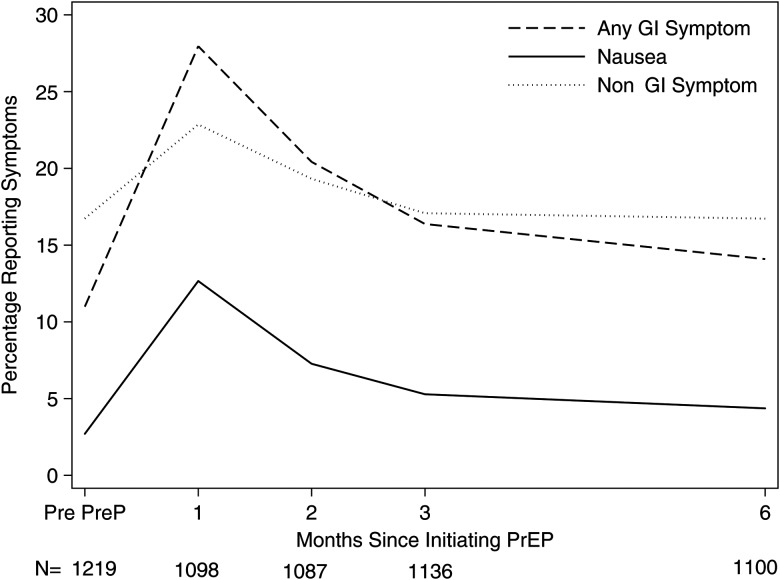
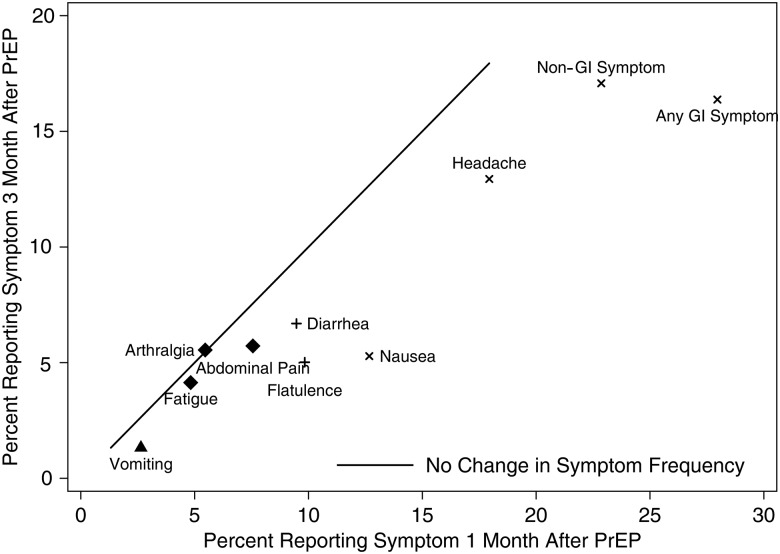
To assess potential transience of symptoms considered part of the “start-up syndrome,” we compared symptom reports between month 1 and subsequent visits. Symptom reports peaked within 1 month after starting PrEP and tended to return to baseline levels by 3 months (Figure 1). At least one non-GI symptom was reported by 23% at 1 month vs 17% at 3 months (P < .0001). One GI symptom was reported in 17% vs 11% (P < .0001) and 11% vs 5% for more than 1 GI symptom (P < .0001) at 1 month vs 3 months, respectively (Figure 2). For specific symptoms, 18% vs 13% (P < .0001) reported headache at 1 month vs 3 months, 13% vs 5% (P < .0001) for nausea, 10% vs 5% (P < .0001) for flatulence, 10% vs 7% (P = .008) for diarrhea, 8% vs 6% (P = .054) for abdominal pain, and 3% vs 1% (P = .03) for vomiting (Figure 2).
Figure 1.
Percentage of participants reporting any of the targeted symptoms prior and in the 6 months following initiation of emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) as pre-exposure prophylaxis (PrEP). Data below the axis gives the number of participants with available symptom interviews at each visit. Abbreviation: GI, gastrointestinal.
Figure 2.
Percentage of participants reporting symptoms at 1 and 3 months following initiation of pre-exposure prophylaxis (PrEP) by specific symptom and all gastrointestinal (GI) symptoms (any report of abdominal pain, diarrhea, flatulence, nausea, or vomiting) and non-GI symptoms (any report of arthralgia, fatigue, headache). Calculated among 1092 participants with symptom data at both visits. The solid line represents equal reports at month 1 and month 3. Values below the line are less frequent at month 3 compared to month 1. Symbol legend: x: P < .0001, +: P < .01, triangle P < .05, diamond: P > .05.
Of the 307 (28%) of participants reporting GI symptoms at 1 month, 170 attributed at least one of their GI symptoms to FTC/TDF. This represented 14% (170/1225) of those taking PrEP. Nausea was the only single symptom reported in the first month that was attributed to FTC/TDF in more than half of those who reported it (65%).
To assess if the open-label setting influenced the likelihood of symptom reports (compared to a blinded randomized trial), we examined the group of participants randomized to FTC/TDF in the iPrEx trial and who went on to choose to take PrEP in OLE (n = 558). We compared the frequency of symptom reports during OLE vs during the iPrEx trial period. Symptom frequency in the first month of PrEP use was comparable for nausea (iPrEx: 10%, OLE: 10%, P = .81), GI symptoms (iPrEx: 24%, OLE: 24%, P = .93), and any symptom (iPrEx: 36%, OLE: 33%, P = .18). Headache was less common in OLE (iPrEx: 19%, OLE: 13% P = .004).
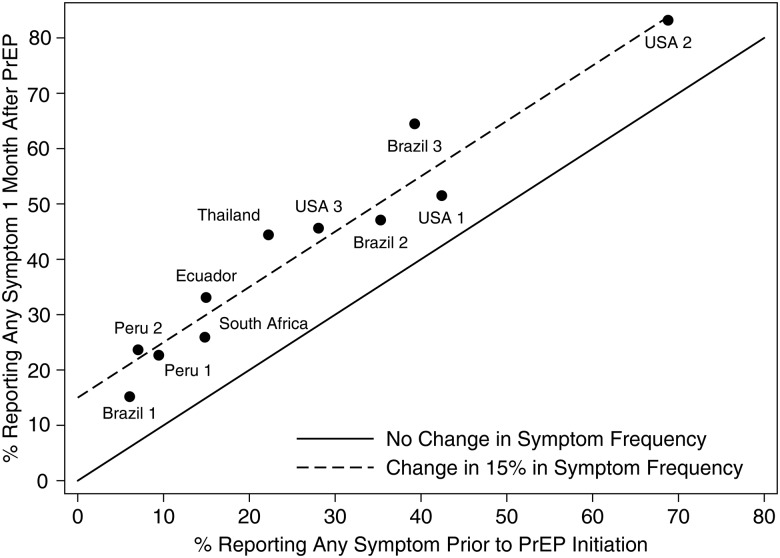
Symptom frequency in the first month of OLE varied greatly by site of enrollment; the median frequency was 28% (range 11% to 70%) for GI symptoms and 24% (range 3% to 59%) for non-GI symptoms. This variation in symptom reports one month after PrEP tracked tightly with the variation of symptom reports at the baseline visit, prior to participants starting PrEP (Figure 3). The average difference in reports of any symptom after 1 month on PrEP compared to enrollment was 15% (range of average by site: 9%–25%) showing substantially less variation by site. After adjustment for study site, symptom report in the first month was not associated with age, sexual practices (number of partners, condomless anal intercourse), gender identity (MSM vs TW), alcohol or drug use, body mass index, or creatinine clearance. Only post-secondary education was significantly associated with a higher frequency of GI symptoms (OR = 1.7; 95% CI, 1.1–2.7, P = .02) but was not associated with non-GI symptoms.
Figure 3.
Proportion of participants reporting any symptom prior to pre-exposure prophylaxis (PrEP) initiation and 1 month following PrEP initiation by enrolling site. There are 11 sites in 6 countries (Peru: 2 sites, Brazil: 3 sites, United States of America: 3 sites; Ecuador, Thailand, and South Africa: 1 site each). Reference lines of equal reports and an absolute increase of 15% from pre-PrEP to 1 month after are plotted.
Drug Interruptions for Adverse Effects
PrEP dispensation was interrupted during OLE in 56 (5%) participants for “adverse effects.” The participant or the study medical staff could initiate interruptions. The discontinuation was temporary for 22 participants; the combined duration of these temporary interruptions totaled 10 person-years, a 0.6% (10 person-years of a possible 1784 person-years) reduction in total possible person years in OLE. Discontinuation was permanent for 34 participants and decreased total possible use of PrEP by 35 person years, 2.9% of the possible use in the OLE cohort. The most common adverse effects cited for non-dispensation were nausea/abdominal pain (0.9% of possible use), diarrhea (0.4%), and skin problems/itching, headache, and flatulence (0.3% each).
Symptoms and Drug Levels
Reports of non-GI symptoms were not associated with drug levels by DBS stratum at week 4 (OR = 1.2, 95% CI, .40–3.7, P = .73) after adjustment for prespecified confounders. GI symptom reports were inversely associated with odds of higher drug levels at week 4 (OR = 0.48, 95% CI, .25–.91, P = .03), with similar results after adjustment for the set of pre-specified confounders (OR = 0.47, 95% CI, .23–.96, P = .04). This effect was similar across all GI symptoms except vomiting and flatulence (Figure 4). The effect was slightly more pronounced when the participant attributed the symptom(s) to FTC/TDF and clearly more pronounced in symptoms that had resolved by the 1-month visit (Figure 4). There was no greater effect with multiple GI symptom reports.
Figure 4.
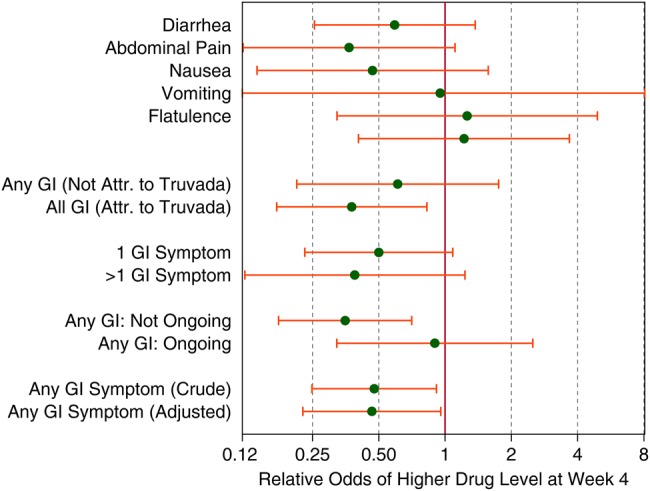
Relative odds (and 95% confidence intervals) of higher tenofovir diphosphate levels (<350, ≥350 to <700, ≥700 fmol/punch) at week 4 in dried blood spots (DBS) from an ordinal logistic regression model by type of symptom at week 4 adjusted for site, condomless anal intercourse (insertive and/or receptive), sexual partners (number and human immunodeficiency virus [HIV] status), age, education, alcohol or drug use, and creatinine and weighted by case-cohort testing of DBS samples. The “crude” odds ratio is adjusted only for study site. Abbreviation: GI, gastrointestinal.
An estimated 42% of those with GI symptoms and 54% without GI symptoms had DBS levels of TFV-DP ≥700 fmol /punch (consistent with ≥4 pills per week) at 1 month—compared to 52% of the overall cohort. By the population attributable fraction [22], approximately 7% (95% CI, 4%–11%) of use at <4 pills per week (TFV-DP <700) can be associated with reports of GI symptoms.
We examined if the effects of GI symptoms in the first month on adherence (DBS levels of TFV-DP ≥700 fmol/punch at the 1-month visit) varied by age (<30 vs ≥30), baseline report of non-condom receptive anal intercourse (ncRAI), or site. There was an indication that GI symptoms were more strongly inversely associated with adherence among younger participants. Among participants under 30 the proportion with TFV-DP ≥700 fmol/punch was 23% among those with GI symptoms and 47% among those without GI symptoms For those aged ≥30 the proportions were 57% vs 64%, respectively (P for interaction = .09). There was also a nonstatistically significant trend toward less effect of symptoms on adherence among those reporting ncRAI. Among those reporting ncRAI: 48% (GI symptoms) vs 59% (no GI symptoms) with TFV-DP ≥700 fmol/punch compared to 25% vs 48% who did not report ncRAI (P for interaction = .14). Table 1 compares the association between adherence and GI symptoms across study sites (P for interaction = .25). It is notable that at 1 month, the site with the highest adherence by DBS had the highest GI symptom reports (70%), and the 2 sites with the lowest adherence had among the lowest GI symptom reports (10% and 11%), suggesting that there are contexts where frequent symptom reports and high adherence can coexist.
Table 1.
Percentage of Participants With Drug Levels Consistent With ≥4 Pill per Week (tenofovir diphosphate [TFV-DP] ≥700 fmol/punch) at the 1 Month Visit by Report of Gastrointestinal Symptoms During the First Month Stratified by Site of Enrollment
| Study Site | No GI Symptoms Reported |
GI Symptoms Reported |
||
|---|---|---|---|---|
| N | % ≥700 fmol TFV-DP | N | % ≥700 fmol TFV-DP | |
| Brazil 1 | 18 | 67 | 2 | 50 |
| Brazil 2 | 11 | 91 | 5 | 40 |
| Brazil 3 | 13 | 62 | 17 | 59 |
| Ecuador | 24 | 54 | 8 | 50 |
| Peru 1 | 63 | 37 | 7 | 29 |
| Peru 2 | 42 | 45 | 5 | 20 |
| South Africa | 14 | 57 | 1 | 0 |
| Thailand | 13 | 54 | 5 | 20 |
| USA 1 | 10 | 60 | 6 | 17 |
| USA 2 | 11 | 82 | 16 | 94 |
| USA 3 | 9 | 67 | 4 | 50 |
Abbreviations: GI, gastrointestinal; TFV-DP, tenofovir diphosphate.
Reports of GI symptoms at 1 month were also inversely associated with odds of higher drug levels at month 3 (OR = 0.47, 95% CI, .25–.92, P = .03) but not at month 2 (OR = 0.85, 95% CI, .38–1.86, P = .69) . However, there was no inverse association between 2-month or 3-month GI symptoms with drug levels at 2 (OR = 1.3, 95% CI, .60 to 2.7, P = .54) or 3 months (OR = 1.7, 95% CI, .82–3.4, P = .16), respectively.
DISCUSSION
We observed a “start-up syndrome” that peaked within 1 month and symptoms that resolved to pre-PrEP levels within 3 months. The syndrome includes several symptoms such as nausea, flatulence, diarrhea, headache, and abdominal pain. These symptoms are relatively common even prior to PrEP initiation, but frequency of report varies considerably by site. The symptom reports increase by an absolute 16% (95% CI, 13% to 19%) from baseline to 1 month post PrEP—a number that was quite consistent across study sites (Figure 3). Most people with a symptom did not attribute it to their use of PrEP. Previous PrEP trials have reported early symptoms associated with truvada initiation, but our article is the first we are aware of to consider adverse effects in detail.
The exact timing of onset of the start-up syndrome cannot be discerned from our data; the peak may have occurred as early as within a day or weeks after starting PrEP. We focused on a set of symptoms shown to be elevated in PrEP trials but may not have captured all the physiologic aspects of PrEP initiation. Symptom reports were not more frequent in an open-label compared to a blinded setting.
Post-secondary education was the only participant-level factor associated with symptom reports. Enrolling study site showed substantial variation in the rate of symptom reports; a tendency seen prior to and during the initiation of PrEP. The variation in symptom reporting across study sites was large despite the use of the same structured survey at all OLE sites. Although different translations could explain differences between Brazil (in Portuguese), Peru and Ecuador (in Spanish), and the United States (in English), we also observed large variation within regions using the same language (eg, Brazil and the United States). Background gastrointestinal syndromes (unrelated to PrEP) will vary according to human genetics, exposure to intestinal parasites, dietary habits, water quality, and alcohol use. The higher frequency of symptom reports among people with higher education may reflect a greater understanding regarding the importance of reporting all adverse experiences, however mild, and regardless of whether treatment for the symptom was desired. These same participants may also have a greater commitment to use PrEP as part of this research study. As PrEP use transitions from research settings to clinical practice, the determinants of PrEP use may reflect more individualized weighing of risks and benefits, including protection from HIV and adverse effects. The finding that symptoms undermine PrEP use less among those with more compelling indications for PrEP (ncRAI) suggests that such individualized weighing of costs and benefits may be successful and could sustain much of PrEP's impact on HIV transmission even while some PrEP users stop due to adverse effects.
We saw an association between reports of GI symptoms, though not non-GI symptoms, with lower adherence. Our data lack measurements prior to 1 month, which might help us determine whether the symptoms preceded low adherence or vice versa. The association with GI symptoms report in the first month appeared to largely persist with adherence beyond the first month. Symptoms reports 2 and 3 months after PrEP initiation were not inversely associated with adherence at months 2 and 3. There were indications that symptoms may decrease adherence in specific population groups (eg, younger people, those not reporting ncRAI), but these associations were not statistically significant. The overall magnitude of nonadherence ascribed to symptoms associated with FTC/TDF use is low, accounting for about 7% of suboptimal drug concentrations at month 1 and 2.5% of potential person time lost due to medication interruptions and/or discontinuations.
Fifty percent of all participants who chose not to take PrEP in iPrEx OLE stated that their reason for making this decision was their concern regarding adverse effects [16]. This proportion seems incongruent with the relatively small proportion of persons who actually developed symptoms and their typically brief duration. It is intriguing that one study site with the largest proportion of reported symptoms also had very high adherence; there is considerable variability in the manner in which symptoms and adherence coexist. Taken together, the findings of this analysis suggest that education and counseling should focus on the transient nature of a “start-up syndrome” and advise PrEP users that the vast majority of those who do experience a “start-up syndrome” successfully “overcome” it within the first few months of PrEP use.
Notes
Acknowledgments. The authors gratefully acknowledge the participants of the iPrEx OLE study, Lane Bushman, Suwat Chariyalertsak, Linda-Gail Bekker, Martin Casapia, Orlando Montoya, Esper Kallas, Valdilea Veloso, and Javier Lama.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases. (U01 AI106499, UM1 AI068619, U01 AI064002, R03 AI120819, R03 AI122908). Study medication was donated by Gilead Sciences. The iPrEx studies were sponsored by the US National Institutes of Health with cofunding from the Bill and Melinda Gates Foundation.
Potential conflicts of interest. Study medication was donated by Gilead Sciences, which also supported travel expenses for non-US investigators to attend study meetings. R. M. G. and D. V. G. have received fees from and R. M. G. has received a research grant from ViiV, a manufacturer of an investigational compound being investigated for use as PrEP. P. L. A. receives study drug and contract work from and S. G. H. received unrestricted educational grant funds from Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme L, Corneli A, Ahmed K et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Richardson BA et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina J-M, Capitant C, Charreau I et al. On demand PrEP with oral TDF-FTC in MSM results of the ANRS Ipergay trial [abstract 23LB]. In: Program and abstracts of 22nd Congress of Retroviruses and Opportunistic Infections Seattle, WA, 23–26 February 2015. [Google Scholar]
- 8.Mulligan K, Glidden DV, Anderson PL et al. Effects of Emtricitabine/Tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial: DXA results from iPrEx. Clin Infect Dis 2015; 61:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michael K, Niska RW, Rose C et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One 2014; 9:e90111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu AY, Vittinghoff E, Sellmeyer DE et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One 2011; 6:e23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon MM, Lama JR, Glidden DV et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS 2014; 28:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M, Vanichseni S, Suntharasamai P et al. Renal function of participants in the Bangkok tenofovir study—Thailand, 2005–2012. Clin Infect Dis 2014; 59:716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mugwanya KK, Wyatt C, Celum C et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine–tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med 2015; 175:246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammassari A, Murri R, Pezzotti P et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr 2001; 28:445–9. [DOI] [PubMed] [Google Scholar]
- 15.Mayer KH, Mimiaga MJ, Cohen D et al. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston Community Health Center. J Acquir Immune Defic Syndr 2008; 47:494–9. [DOI] [PubMed] [Google Scholar]
- 16.Grant RM, Anderson PL, McMahan V et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grohskopf L, Chillag KL, Gvetadze R et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr 2013; 64:79–86. [DOI] [PubMed] [Google Scholar]
- 18.Hosek S, Siberry G, Bell M et al. Project PrEPare (ATN082): The acceptability and feasibility of an HIV pre-exposure prophylaxis (PrEP) trial with young men who have sex with men (YMSM). J Acquir Immune Defic Syndr 2013; 62:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo-Mancilla JR, Zheng JH, Rower JE et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986; 73:1–11. [Google Scholar]
- 21.Agresti A. Analysis of Ordinal Categorical Data. Hoboken, NJ: Wiley, 2010. [Google Scholar]
- 22.Fleiss JL. Inference about the population attributable risk from cross-sectional studies. Am J Epidemiol 1979; 110:103–4. [DOI] [PubMed] [Google Scholar]