In the US, Xpert demonstrated excellent sensitivity in individuals with smear-positive tuberculosis and identified the majority with smear-negative tuberculosis. One Xpert predicted the absence of smear positive tuberculosis in 99.7% of specimens, indicating a role in respiratory isolation algorithms.
Keywords: Xpert MTB/RIF, tuberculosis diagnosis, respiratory isolation, nontuberculous mycobacteria, HIV/tuberculosis coinfection
Abstract
Background. The Xpert MTB/RIF (Xpert) assay is a rapid nucleic acid amplification test widely used in settings of high tuberculosis prevalence to detect tuberculosis as well as rpoB mutations associated with rifampin resistance. Data are needed on the diagnostic performance of Xpert in lower-prevalence settings to inform appropriate use for both tuberculosis detection and the need for respiratory isolation.
Methods. Xpert was compared to 2 sputum samples, each evaluated with acid-fast bacilli (AFB) smear and mycobacterial culture using liquid and solid culture media, from participants with suspected pulmonary tuberculosis from the United States, Brazil, and South Africa.
Results. Of 992 participants enrolled with evaluable results, 22% had culture-confirmed tuberculosis. In 638 (64%) US participants, 1 Xpert result demonstrated sensitivity of 85.2% (96.7% in participants with AFB smear-positive [AFB+] sputum, 59.3% with AFB smear-negative [AFB–] sputum), specificity of 99.2%, negative predictive value (NPV) of 97.6%, and positive predictive value of 94.9%. Results did not differ between higher- and low-prevalence settings. A second Xpert assay increased overall sensitivity to 91.1% (100% if AFB+, 71.4% if AFB–), with specificity of 98.9%. In US participants, a single negative Xpert result predicted the absence of AFB+/culture-positive tuberculosis with an NPV of 99.7%; NPV of 2 Xpert assays was 100%, suggesting a role in removing patients from airborne infection isolation. Xpert detected tuberculosis DNA and mutations associated with rifampin resistance in 5 of 7 participants with rifampin-resistant, culture-positive tuberculosis. Specificity for rifampin resistance was 99.5% and NPV was 98.9%.
Conclusions. In the United States, Xpert testing performed comparably to 2 higher-tuberculosis-prevalence settings. These data support the use of Xpert in the initial evaluation of tuberculosis suspects and in algorithms assessing need for respiratory isolation.
(See the Editorial Commentaries by DiNardo, Lange, and Mandalakas on pages 1089–91.)
The Xpert MTB/RIF (Xpert) assay (Cepheid, Sunnyvale, California) is an automated nucleic acid amplification test that can detect both Mycobacterium tuberculosis and mutations associated with rifampin resistance in <2 hours with minimal hands-on time and technical expertise. In previous studies, Xpert demonstrated a pooled specificity of 99% and sensitivity of 98% in acid-fast bacilli smear-positive (AFB+) and 67% in AFB smear-negative (AFB–) sputum specimens in mostly high-tuberculosis-prevalence settings [1]. Xpert testing has been widely implemented in resource-limited settings with high tuberculosis prevalence and as a frontline tuberculosis diagnostic in patients infected with human immunodeficiency virus (HIV) [2].
Xpert was authorized by the US Food and Drug Administration (FDA) in 2013 for detection of M. tuberculosis and reporting of rifampin resistance directly from sputum samples [3] and as an aid in decisions regarding respiratory isolation in 2015 [4]. However, published data for Xpert use in settings of low tuberculosis prevalence such as the United States are limited. Lower tuberculosis prevalence may impact positive and negative predictive values, and data for Xpert performance in clinical settings where nontuberculous mycobacteria (NTM) are common are also limited.
We conducted a longitudinal multicenter study to evaluate Xpert performance in the United States in comparison to 2 higher-tuberculosis-prevalence settings: Rio de Janeiro, Brazil (local annual tuberculosis incidence of 96.9/100 000 [5]), and Johannesburg, South Africa (annual tuberculosis incidence of 466/100 000 [6]).
METHODS
Participants
Individuals aged ≥18 years undergoing evaluation for pulmonary tuberculosis (defined as individuals with cough, fever, night sweats, or weight loss who had sputum tested by AFB smear and culture) were enrolled from 21 sites in the United States and a site from Rio de Janeiro, Brazil, and Johannesburg, South Africa. Participants received <48 hours of tuberculosis treatment in the 6 months prior to sputum collection for Xpert testing. All were tested for HIV infection.
Specimen Collection and Processing
All participants provided 2 sputum specimens for AFB smear with fluorescent staining and mycobacterial culture (using both liquid and solid media), conducted according to the local standard of care. Results from additional AFB smears and cultures were recorded. Mycobacterial species identification was by FDA-approved methods, and participants with M. tuberculosis growth had 1 culture tested for rifampin susceptibility using proportions method on Middlebrook agar [7] as well as rpoB gene sequencing [8]. Xpert testing was conducted on either residual sputum after processing for AFB smear/cultures or on additional sputum specimens collected within 7 days of the 2 required AFB smears and cultures. Xpert testing was conducted according to the manufacturer's recommendations [9] within 7 days of specimen collection at 2 US laboratories and locally at laboratories in Brazil and South Africa. If initial Xpert results were invalid, testing was repeated using the same specimen when the sample volume was adequate. Sputum processing (concentrated vs unconcentrated) and method of collection (induced vs expectorated) were determined by specimen availability and the local standard of care. See Supplementary Appendix for additional details.
Statistical Methods
The primary objectives of this study were to estimate sensitivity of 1 Xpert assay in AFB+ participants and in AFB– participants and specificity among US participants compared with the results of mycobacterial culture. Sample size for this study was determined within each subgroup, based on the point estimates and 95% confidence intervals (CIs) reported in a prior study [10]. This study was not powered for the comparisons made in the secondary objectives; no adjustments were made for multiple comparisons. Unless otherwise specified, all secondary analyses were planned. AFB+ status was defined as at least 1 of the 2 sputum specimens having ≥1 positive AFB smear. Culture-confirmed tuberculosis was defined as at least 1 of the 4 cultures (2 sputum specimens, each cultured on liquid and solid media) with M. tuberculosis growth. The reference comparator for tuberculosis detection by Xpert assay was culture-confirmed M. tuberculosis and, for rifampin resistance, culture-based drug susceptibility testing. The 95% CIs were calculated using the Wilson score binomial method. Comparisons of sensitivities and specificities between independent and overlapped subgroups of interest were assessed using Fisher and McNemar exact tests, respectively; other statistical comparisons were done using 2-sided Wilcoxon, Fisher exact, and χ2 tests. All analyses were done using SAS software version 9.2, with results considered significant at a level of P < .05.
Human Subjects Review
The protocol was approved by institutional review board/ethics committee at each site and the Centers for Disease Control and Prevention.
RESULTS
Study Population
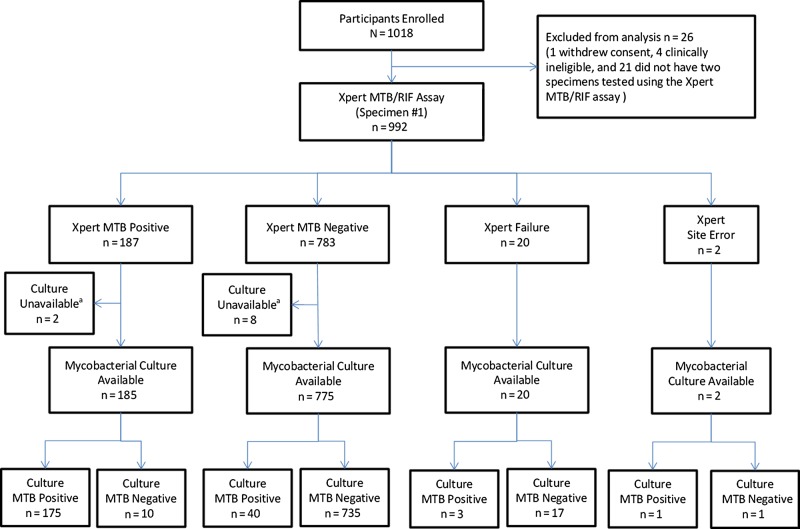
Between May 2012 and November 2013, 1018 participants undergoing evaluation for pulmonary tuberculosis were enrolled, and 992 were evaluable (Figure 1) . Sixty-four percent (n = 638) were enrolled from US sites, 20% (n = 199) were from South Africa, and 16% (n = 155) were from Brazil (Table 1). Thirteen percent had a history of prior tuberculosis and 19% had initiated tuberculosis treatment (<48 hours at time of sputum collection). Seventy-eight percent of US and 21% of non-US participants were inpatients at the time of evaluation.
Figure 1.
Flow diagram of diagnostic evaluation of the Xpert MTB/RIF assay in comparison to mycobacterial culture (reported according to Standards for the Reporting of Diagnostic Accuracy [11]). aMycobacterial culture results were not available for 10 subjects due to contamination (n = 3) or site error (n = 7). Abbreviation: MTB, Mycobacterium tuberculosis.
Table 1.
Demographics and Baseline Results
| Characteristic | Overall (N = 992) | ≥1 Culture TB Positive (n = 219) | Results of First Xpert Assay (n = 970)a |
Results of 2 Xpert Assays (n = 990)b |
||||
|---|---|---|---|---|---|---|---|---|
| Positive (n = 187) | Negative (n = 783) | P Valuec | Positive (n = 210) | Negative (n = 780) | P Valuec | |||
| Age at entry, median (IQR) | 46 (35–54) | 40 (30–51) | 38 (28–51) | 47 (37–54) | <.001 | 39 (28–51) | 47 (38–55) | <.001 |
| Male sex | 617 (62.2) | 129 (58.9) | 116 (19.1) | 490 (80.9) | .889 | 130 (21.1) | 485 (78.9) | .942 |
| Site of enrollment | <.001 | <.001 | ||||||
| Brazil | 155 (15.6) | 37 (16.9) | 32 (20.6) | 123 (79.4) | 37 (23.9) | 118 (76.1) | ||
| South Africa | 199 (20.1) | 90 (41.1) | 76 (39.2) | 118 (60.8) | 84 (42.2) | 115 (57.8) | ||
| United States | 638 (64.3) | 92 (42.0) | 79 (12.7) | 542 (87.3) | 89 (14.0) | 547 (86.0) | ||
| HIV infection status | ||||||||
| HIV infected | 446 (45.0) | 89 (40.6) | 71 (16.2) | 367 (83.8) | .028 | 85 (19.1) | 361 (80.9) | .133 |
| On ART at enrollment (HIV infected only) | ||||||||
| Yes | 155 (34.8) | 12 (13.5) | 12 (8.1) | 137 (91.9) | <.001 | 15 (9.7) | 140 (90.3) | <.001 |
| No | 291 (65.2) | 77 (86.5) | 59 (20.4) | 230 (79.6) | 70 (24.1) | 221 (75.9) | ||
| Presenting symptomsd | ||||||||
| Cough | 923 (93.0) | 205 (93.6) | 177 (19.6) | 725 (80.4) | .322 | 199 (21.6) | 722 (78.4) | .267 |
| Fevere | 526 (53.0) | 122 (55.7) | 108 (21.0) | 406 (79.0) | .118 | 119 (22.7) | 406 (77.3) | .052 |
| Weight lossf | 608 (61.3) | 171 (78.1) | 150 (25.2) | 446 (74.8) | <.001 | 165 (27.2) | 442 (72.8) | <.001 |
| Night sweatsg | 545 (54.9) | 125 (57.1) | 106 (20.1) | 422 (79.9) | .644 | 123 (22.6) | 421 (77.4) | .354 |
| Any of the above | 982 (99.0) | 217 (99.1) | 186 (19.4) | 774 (80.6) | .455 | 208 (21.2) | 772 (78.8) | .925 |
| CXR result | <.001 | <.001 | ||||||
| Abnormal | 776 (78.2) | 197 (90.0) | 172 (22.6) | 588 (77.4) | 190 (24.5) | 585 (75.5) | ||
| CXR findingsh (abnormal only) | ||||||||
| Infiltrate | 380 (49.0) | 139 (70.6) | 124 (33.3) | 248 (66.7) | <.001 | 134 (35.4) | 245 (64.6) | <.001 |
| Cavitation | 125 (16.1) | 73 (37.1) | 74 (59.7) | 50 (40.3) | <.001 | 77 (62.1) | 47 (37.9) | <.001 |
| Pleural effusion | 115 (14.8) | 25 (12.7) | 20 (18.2) | 90 (81.8) | .228 | 21 (18.3) | 94 (81.7) | .091 |
| Other | 419 (54.0) | 79 (40.1) | 70 (16.9) | 343 (83.1) | <.001 | 76 (18.2) | 342 (81.8) | <.001 |
| Location of evaluation | ||||||||
| Inpatient | 572 (57.7) | 98 (44.7) | 84 (15.1) | 471 (84.9) | <.001 | 92 (16.1) | 478 (83.9) | <.001 |
| Outpatient | 420 (42.3) | 121 (55.3) | 103 (24.8) | 312 (75.2) | 118 (28.1) | 302 (71.9) | ||
| TB treatment historyi | <.001 | <.001 | ||||||
| Current TB treatment | 189 (19.1) | 111 (50.7) | 105 (57.4) | 78 (42.6) | 112 (59.6) | 76 (40.4) | ||
| Prior TB treatment | 129 (13.0) | 20 (9.1) | 19 (14.8) | 109 (85.2) | 21 (16.3) | 108 (83.7) | ||
| No history of TB treatment | 666 (67.1) | 88 (40.2) | 63 (9.7) | 588 (90.3) | 77 (11.6) | 588 (88.4) | ||
| AFB smear statusj | ||||||||
| ≥1 AFB+ | 152 (15.3) | 133 (60.7) | 131 (87.3) | 19 (12.7) | <.001 | 135 (88.8) | 17 (11.2) | <.001 |
| 2 AFB– | 838 (84.5) | 86 (39.3) | 56 (6.8) | 762 (93.2) | 75 (9.0) | 761 (91.0) | ||
| Final TB determination | <.001 | <.001 | ||||||
| AFB+/TB culture positive | 133 (13.4) | 133 (60.7) | 129 (98.5) | 2 (1.5) | 133 (100.0) | 0 (0.0) | ||
| AFB–/TB culture positive | 86 (8.7) | 86 (39.3) | 46 (54.8) | 38 (45.2) | 59 (69.4) | 26 (30.6) | ||
| Clinical TB | 57 (5.7) | 0 (0.0) | 2 (3.7) | 52 (96.3) | 4 (7.0) | 53 (93.0) | ||
| Extrapulmonary TB | 2 (0.2) | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | ||
| No TB | 702 (70.8) | 0 (0.0) | 7 (1.0) | 680 (99.0) | 10 (1.4) | 691 (98.6) | ||
| Indeterminate | 12 (1.2) | 0 (0.0) | 3 (25.0) | 9 (75.0) | 4 (33.3) | 8 (66.7) | ||
Data are presented as No. (%) unless otherwise specified, with column percentages under the Overall and ≥1 Culture TB Positive results and row percentages under the Xpert results.
Abbreviations: AFB, acid-fast bacilli; AFB–, AFB smear negative; AFB+, AFB smear positive; ART, antiretroviral therapy; CXR, chest radiograph; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis; Xpert, Xpert MTB/RIF assay.
a Xpert results on sputum sample #1. Invalid results (n = 20) and missing results due to site or laboratory errors (n = 2) were excluded.
b Per-participant Xpert results based on 2 sputum samples (positive = 1 or 2 Xpert-positive results; negative = 2 Xpert-negative or 1 negative plus 1 invalid/missing result). Invalid results (n = 2) were excluded.
c χ2 test, except weight loss, night sweats, AFB status, and final TB determination (Fisher exact test), and age (Wilcoxon test).
d Participants may report >1 symptom.
e There were 21, 6, 6, 14, 8, and 13, respectively, participants whose presence of fever was unknown.
f There were 37, 4, 3, 33, 4, and 33, respectively, participants whose presence of weight loss was unknown.
g There were 25, 2, 3, 21, 3, and 22, respectively, participants whose presence of night sweats was unknown.
h Participants may have >1 abnormal CXR finding.
i There were 8, 0, 0, 8, 0, and 8, respectively, participants whose tuberculosis treatment history was unknown.
j There were 2, 2, 0, 2, 0, and 2, respectively, participants whose AFB status was unknown due to site/laboratory errors.
AFB Smear Status and Microbiologic Diagnoses
Twenty-two percent of participants had M. tuberculosis recovered from 1 or more sputum cultures; 61% had ≥1 AFB+ sputum results. Culture-confirmed tuberculosis was diagnosed in 14% (92/638) of US participants (68% with AFB+ sputum) and in 36% (127/354) of participants from non-US sites (55% with AFB+ sputum). Six percent (57/992) of participants' cultures failed to grow M. tuberculosis but were judged to have clinical tuberculosis on basis of response to empiric treatment. Twelve percent of US participants had cultures that grew NTM. Fifteen percent (152/992) of participants had 1 or more sputum specimens that were AFB+, 88% (133/152) of whom had M. tuberculosis recovered by culture.
Xpert Assay Performance
Sensitivity of the first Xpert result was 81.4% (Table 2). Sensitivity was 98.5% in AFB+ and 54.8% in AFB– participants. The first Xpert result had specificity of 98.7% in participants without culture-confirmed tuberculosis. In participants from whom NTM were cultured, the specificity of the first Xpert remained high at 92% (87/95) and correctly excluded tuberculosis in 93% (13/14) of AFB+ participants without culture-confirmed tuberculosis. The incremental yield of a second Xpert was 35%, identifying 2 additional participants with AFB+ sputum and 12 with AFB– sputum, increasing overall per-participant sensitivity to 88.1% (100% in AFB+ and 69.4% in AFB– participants).
Table 2.
Xpert MTB/RIF Assay Sensitivity and Specificity Resultsa
| Characteristic | Sensitivity |
Specificity |
PPV | NPV | ||
|---|---|---|---|---|---|---|
| TB Culture Positive | AFB Smear Positive/ TB Culture Positive | AFB Smear Negative/ TB Culture Positive | TB Culture Negative | |||
| All participants, first Xpert assay | 81.4% (175/215) | 98.5% (129/131) | 54.8% (46/84) | 98.7% (735/745) | 94.6% (175/185) | 94.8% (735/775) |
| [75.7%–86.0%] | [94.6%–99.6%] | [44.1%–65.0%] | [97.5%–99.3%] | [90.3%–97.0%] | [93.0%–96.2%] | |
| HIV status | ||||||
| HIV infected | 73.6% (64/87)b | 100.0% (39/39) | 52.1% (25/48) | 98.3% (339/345) | 91.4% (64/70) | 93.6% (339/362) |
| [63.4%–81.7%] | [91.0%–100.0%] | [38.3%–65.5%] | [96.3%–99.2%] | [82.5%–96.0%] | [90.6%–95.7%] | |
| HIV uninfected | 86.7% (111/128)b | 97.8% (90/92) | 58.3% (21/36) | 99.0% (396/400) | 96.5% (111/115) | 95.9% (396/413) |
| [79.8%–91.5%] | [92.4%–99.4%] | [42.2%–72.9%] | [97.5%–99.6%] | [1.4%–98.6%] | [93.5%–97.4%] | |
| Region | ||||||
| Low TB Prevalence (United states) | 85.2% (75/88) | 96.7% (59/61) | 59.3% (16/27) | 99.2% (526/530)c | 94.9% (75/79) | 97.6% (526/539) |
| [76.3%–91.2%] | [88.8%–99.1%] | [40.7%–75.5%] | [98.1%–99.7%] | [87.7%–98.0%] | [95.9%–98.6%] | |
| Higher TB prevalence (Brazil & South Africa) | 78.7% (100/127) | 100.0% (70/70) | 52.6% (30/57) | 97.2% (209/215)c | 94.3% (100/106) | 88.6% (209/236) |
| [70.8%–85.0%] | [94.8%–100.0%] | [39.9%–65.0%] | [94.0%–98.7%] | [88.2%–97.4%] | [83.9%–92.0%] | |
| Sputum collection | ||||||
| Induced | 86.8% (59/68) | 100.0% (50/50) | 50.0% (9/18) | 99.2% (254/256) | 96.7% (59/61) | 96.6% (254/263) |
| [76.7%–92.9%] | [92.9%–100.0%] | [29.0%–71.0%] | [97.2%–99.8%] | [88.8%–99.1%] | [93.6%–98.2%] | |
| Expectorated | 77.4% (103/133) | 97.2% (69/71) | 54.8% (34/62) | 98.2% (429/437) | 92.8% (103/111) | 93.5% (429/459) |
| [69.6%–83.7%] | [90.3%–99.2%] | [42.5%–66.6%] | [96.4%–99.1%] | [86.4%–96.3%] | [90.8%–95.4%] | |
| Sputum processing | ||||||
| Unprocessed | 78.0% (32/41) | 92.6% (25/27)d | 50.0% (7/14) | 99.1% (233/235) | 94.1% (32/34) | 96.3% (233/242) |
| [63.3%–88.0%] | [76.6%–97.9%] | [26.8%–73.2%] | [97.0%–99.8%] | [80.9%–98.4%] | [93.1%–98.0%] | |
| Concentrated | 82.2% (143/174) | 100.0% (104/104)d | 55.7% (39/70) | 98.4% (502/510) | 94.7% (143/151) | 94.2% (502/533) |
| [75.8%–87.2%] | [96.4%–100.0%] | [44.1%–66.8%] | [96.9%–99.2%] | [89.9%–97.3%] | [91.9%–95.9%] | |
| All participants, 2 Xpert assayse | 88.1% (192/218) | 100.0% (133/133) | 69.4% (59/85) | 97.9% (746/762) | 92.3% (192/208) | 96.6% (746/772) |
| [83.1%–91.7%] | [97.2%–100.0%] | [59.0%–78.2%] | [96.6%–98.7%] | [87.9%–95.2%] | [95.1%–97.7%] | |
Data are presented as % (no./No.) [95% confidence interval].
Abbreviations: AFB, acid-fast bacilli; HIV, human immunodeficiency virus; NPV, negative predictive value; PPV, positive predictive value; TB, tuberculosis.
a Only positive and negative culture and Xpert results were included in these calculations. For the first Xpert assay, 3 failed cultures, 7 missing cultures due to site/laboratory error, 20 invalid Xpert results, and 2 missing Xpert results due to site/laboratory error were excluded. For 2 Xperts, 3 failed cultures, 7 missing cultures due to site/laboratory error, and 2 invalid Xpert results were excluded.
b Fisher exact P = .020 for comparison of sensitivity by HIV status.
c Fisher exact P = .038 for comparison of specificity by region.
d Fisher exact P = .041 for comparison of sensitivity by sputum processing within AFB smear positive results.
e Two Xperts are per-participant Xpert results based on 2 sputum samples.
The first Xpert was false positive in a total of 10 participants: 1 with AFB+ sputum (Mycobacterium avium complex [MAC] growth in 2 mycobacteria growth indicator tubes [MGIT] and 2 solid cultures) and 9 participants with AFB– sputum (Supplementary Appendix Table 1). Of the 9 participants with AFB– sputum, 5 had no mycobacterial growth and 4 had NTM growth (1 M. abscessus-chelonae, 1 MAC, and 2 NTM not identified to species level), 2 of whom had M. tuberculosis detected with a line probe assay (but not by FDA-approved Accuprobe). Testing with 2 Xpert assays yielded a total of 16 false-positive results, 10 with tuberculosis detected on the first Xpert assay as well. Of the 16 participants with 1 or more positive Xpert results without M. tuberculosis growth, 3 had prior tuberculosis treatment (completed >6 months prior to Xpert testing). The first Xpert was falsely negative in 2 participants with AFB+/culture-positive tuberculosis and 38 participants with AFB–/culture-positive tuberculosis; 2 of these participants had mixed growth with M. tuberculosis and NTM.
Low- Versus Higher-Prevalence Settings
In the 618 US participants with both Xpert and mycobacterial culture results available, 1 Xpert assay demonstrated a sensitivity of 96.7% in AFB+ participants and 59.3% in AFB– participants, with a specificity of 99.2%. A second Xpert assay increased overall sensitivity from 85.2% to 91.1%. In the 342 participants from Brazil and South Africa, sensitivity was 100% (P = .21) in AFB+ participants and 52.6% (P = .64) in AFB– participants, and specificity was 97.2% (P = .038) (Table 2).
Xpert Performance in US Respiratory Isolation Algorithm
In comparison to AFB smear as part of the respiratory isolation algorithm, 2 Xpert assays identified all 62 AFB+/culture-positive tuberculosis cases, whereas the first Xpert assay identified 96.7% (59/61) of AFB+/culture-positive tuberculosis cases (Table 3). In 2 instances of AFB+/culture-positive tuberculosis missed by initial Xpert, testing was performed on an unconcentrated sputum specimen distinct from the AFB+ specimen that grew M. tuberculosis. In a third case, Xpert was invalid for a concentrated sputum specimen distinct from the AFB+ specimen that grew M. tuberculosis. In the subset of 361 US participants (58%) with 3 AFB smears available, 1 and 2 Xpert assays identified 82% (41/50) and 88.5% (46/52), respectively, of culture-positive tuberculosis, compared with 62.0% (31/50) and 61.5% (32/52) of culture-positive tuberculosis identified with 3 AFB smears, respectively (Table 3). One Xpert assay identified more than half of the AFB–/culture-positive tuberculosis cases (11/19), which would be missed entirely with initial AFB smears. In a secondary post hoc analysis, a single negative Xpert assay predicted the absence of AFB+/culture-positive tuberculosis with a negative predictive value (NPV) of 99.7% (99.6% in the United States and 100% outside the United States); the NPV was 100% for 2 negative Xpert assays.
Table 3.
Sensitivity of Xpert MTB/RIF Assay Versus 2 or 3 Acid-Fast Bacilli Smears for Identification of US Culture-Confirmed Tuberculosis Cases
| Smear | AFB Positive | 1 Xpert Positive Results | P Value | 2 Xpert Results, at Least 1 Positive | P Value |
|---|---|---|---|---|---|
| 2 AFB smears (n = 91a) | |||||
| All | 68.1%b (62/91) [58.0%–76.8%] |
85.2% (75/88)c [76.3%–91.2%] |
.001 | 91.1% (82/90)d [83.4%–95.4%] |
<.001 |
| AFB positive | 96.7% (59/61)d [88.8%–99.1%] |
100% (62/62) [94.2%–100%] |
|||
| AFB negative | 59.3% (16/27)e [40.7%–75.5%] |
71.4% (20/28)d [52.9%–84.7%] |
|||
| 3 AFB smears (n = 53) | |||||
| All | 60.4% (32/53)f [46.9%–71.4%] |
82.0% (41/50)c [69.2%–90.2%] |
.006 | 88.5% (46/52)d [77.0%–94.6%] |
<.001 |
| AFB positive | 96.8% (30/31)d [83.8%–99.4%] |
100% (32/32) [89.3%–100%] |
|||
| AFB negative | 57.9% (11/19)e [36.3%–76.9%] |
70.0% (14/20)d [48.1%–85.5%] |
|||
Data are presented as % (no./No.) [95% confidence interval]. P values are for comparison of AFB smear vs Xpert assay, using Fisher exact test.
Abbreviation: AFB, acid-fast bacilli.
a One missing Xpert due to site/laboratory error.
b AFB+ in 61 of 88 (69.3%) for 1 Xpert assay comparison and 62 of 90 (68.9%) for 2 Xpert assays comparison.
c Three invalid Xpert assays.
d One invalid Xpert assay.
e Two invalid Xpert assays.
f AFB+ in 31 of 50 (62.0%) for 1 Xpert assay comparison and 32 of 52 (61.5%) for 2 Xpert assays comparison.
Rifampin Resistance
Of the 200 culture-confirmed cases with rifampin susceptibility results available, 3.5% (7/200) had rifampin resistance: 2 from the United States, 4 from South Africa, and 1 from Brazil. The first Xpert detected rifampin resistance in 5 of 7 (71.4%; 95% CIs, 35.9–91.8%) culture-confirmed rifampin resistance cases. Xpert did not detect either rifampin resistance or tuberculosis in 2 participants with AFB–/culture-positive tuberculosis whose M. tuberculosis from cultures were phenotypically resistant to rifampin and had mutations in rpoB associated with rifampin resistance (Supplementary Appendix Table 2). Testing with 1 Xpert assay had a specificity for rifampin resistance of 99.5% (186/187; 95% CIs, 97.0–99.9%) and an NPV of 98.9% (186/188; 95% CIs, 96.2–99.7%). In the 1 participant with false-positive Xpert rifampin resistance testing, the first Xpert indicated rifampin resistance, but the second did not. Sequencing of the cultured isolate did not show rpoB mutations.
Xpert Performance in HIV-Infected Participants
Xpert sensitivity was significantly lower in HIV-infected participants (73.6%) than in HIV-uninfected participants (86.7%) (P = .020; Table 2). However, when evaluated by AFB status, sensitivity in AFB+ sputum (100% vs 97.8%) and AFB– sputum (52.1% vs 58.3%) in HIV-infected and -uninfected persons, respectively, did not differ (both P > .5). Overall specificity also did not differ significantly between HIV-infected and -uninfected participants (98.3% vs 99.0%; P = .53). Among 53 HIV-infected participants with AFB– sputum, there was no evidence that Xpert sensitivity was affected by CD4+ cell count (first Xpert assay, P = .90; 2 Xpert assays, P = .58).
DISCUSSION
In the largest study to date evaluating the Xpert assay in settings of low tuberculosis prevalence, the diagnostic performance of Xpert in the United States was similar to higher-tuberculosis prevalence sites in Brazil and South Africa, and was comparable to other studies in higher-tuberculosis prevalence locations [12]. These data support the use of Xpert as a diagnostic tool in US patients undergoing initial evaluation for tuberculosis.
The current US algorithm for suspected tuberculosis recommends respiratory isolation until 3 sputum smears collected at least 8 hours apart are confirmed to be AFB– [13]. In our study, 1 Xpert assay identified 96% of AFB+/culture-positive tuberculosis cases, and 2 Xpert assays identified all AFB+/culture-positive tuberculosis cases. One Xpert predicted the absence of culture-positive tuberculosis with an NPV of 97.6%, and predicted the absence of smear-positive tuberculosis with an NPV of 99.7%. Importantly, 1 Xpert assay was significantly more sensitive than 3 AFB smears in the subset for whom 3 smears were available (82.0% vs 61.5%; P < .001). A single Xpert assay identified more than half of the AFB–/culture-positive tuberculosis cases, which were missed entirely by AFB smears alone.
The benefit of a second Xpert to determine a need for continued respiratory isolation depends on the risk of a false-negative initial Xpert result, the incremental yield of a second Xpert result, and the risk of undetected infectious tuberculosis. Cost-benefit analysis may identify settings in which 1 Xpert alone is adequate and others where a second Xpert is appropriate. For those with HIV infection, a second Xpert may be quite important for prompt diagnosis regardless of smear status given the morbidity and mortality associated with HIV/tuberculosis coinfection. In addition, the impact of failure to identify even a small number of patients with highly infectious smear-positive tuberculosis must be considered in hospital settings where a large number of more susceptible persons may be exposed.
In the United States, Xpert performance in the presence of NTM is crucial, given the relatively high prevalence of NTM; in this study, 12% of US participants had NTM growth. Xpert performed well despite the presence of NTM; 1 Xpert assay demonstrated specificity of 92% among participants with NTM and excluded tuberculosis in 13 of 14 AFB+ participants without culture-proven M. tuberculosis who commonly would initiate tuberculosis treatment on the basis of AFB detection. Previous data have indicated no cross-reactivity of Xpert with multiple species of NTM [14], suggesting that the 5 false-positive Xpert results may indeed have had tuberculosis present that was overgrown in culture by NTM. The contribution of excess NTM DNA masking M. tuberculosis DNA polymerase chain reaction amplification in the 2 participants with NTM and false-negative Xpert results is not clear and merits further evaluation.
Rifampin resistance was uncommon in this study, limiting our ability to evaluate Xpert to detect rifampin resistance. However, Xpert demonstrated a high NPV of 98.9% for exclusion of rifampin resistance. Given the importance of prompt, accurate identification of rifampin resistance, rapid molecular testing is recommend to confirm rifampin resistance detected by Xpert, and culture-based drug susceptibility testing is recommended in all US tuberculosis cases [15].
It must be emphasized that 1 or more negative Xpert results, like AFB smear [16], and even mycobacterial culture [17], does not exclude the possibility of pulmonary tuberculosis. In addition to molecular testing, US guidelines recommend collection of at least 3 respiratory samples for AFB smear and mycobacterial culture during initial evaluation of suspected respiratory tuberculosis, and drug susceptibility testing when M. tuberculosis is recovered [15, 18].
In summary, these results demonstrate that the Xpert assay is a valuable addition to the tuberculosis armamentarium in the United States and, potentially, other lower-prevalence settings where the assay can facilitate initial diagnosis of tuberculosis and can be used in conjunction with other clinical information as part of the algorithm for initial respiratory isolation decisions.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The team gratefully acknowledges the contribution of the participants and their families, the enrolling sites and staff, and the laboratories and staff who made this study possible. The Centers for Disease Control and Prevention (CDC) Reference Laboratory, Division of Tuberculosis Elimination, conducted rpoB sequencing and rifampin drug susceptibility testing. We acknowledge the field representatives Jay Dwyer and Nancy Reilly; laboratory technologist Joan Dragavon; laboratory data managers Oswald Dadson, Anthony Bloom, and Angela Ellis; data manager Linda Wieclaw; and community representative Flavia Nakayima Miiro; the GeneXpert dried culture spot external quality assurance program led by Wendy Stevens and Lesley Scott; the Tuberculosis Trials Consortium (TBTC) Training Advisory Committee, especially Amy Kerrigan and Donna Conwell; and Westat representatives Nancy Dianis, Kathleen Robergeau-Hunt, Fatima Jones, Bert Arevalo, Vicki Sadique, and Jennifer Green. Please see the Supplementary Appendix for individual site and laboratory acknowledgments.
Author contributions. A. F. L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: D. A., D. A. B., C. F., B. G., D. V. H., M. A. K., A. F. L., G. H. M., I. M. S., S. S., X. W. Acquisition of data: D. A., D. A. B., C. F., B. G., D. V. H., M. A. K., A. F. L., G. H. M., B. M., I. M. S., S. S., X. W. Analysis and interpretation of data: D. A., D. A. B., C. F., B. G., D. V. H., M. A. K., A. F. L., B. M., G. H. M., S. S., X. W. Drafting of the manuscript: D. A., D. A. B., C. F., D. V. H., M. A. K., G. H. M., X. W. Critical revision of the manuscript for important intellectual content: D. A., R. A., D. A. B., M. F., C. F., B. G., E. G., P. J., D. V. H., M. A. K., A. F. L., G. H. M., B. M., I. M. S., F. S., S. S., E. T., Y. F. W., M. W., X. W. Statistical analysis: M. A. K., X. W. Administrative, technical, or material support: P. J., B. M., Y. F. W. Study supervision: D. A., D. A. B., C. F., B. G., D. V. H., M. A. K., A. F. L., G. H. M., S. S., X. W.
Disclaimer. The AIDS Clinical Trials Group (ACTG) and TBTC were responsible for design and conduct of the study and preparation, review, and approval of the manuscript. Cepheid contributed to study design, provided material support (Xpert cartridges, GeneXpert machines, technical support, and funding for specimen shipping and some laboratory activities), and conducted a review of the study manuscript. References in this manuscript to any specific commercial products, process, service, manufacturer, or company do not constitute its endorsement or recommendation by the US government. The content is solely the responsibility of the authors and does not necessarily represent the official views of any US government agency.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases through the ACTG (grant numbers AI068634, U01AI068636, and UM1 AI106701); the CDC through the TBTC and the Division of Tuberculosis Elimination, National Center for HIV, Viral Hepatitis, STD, and TB Prevention; the National Institute of Mental Health, and the National Institute of Dental and Craniofacial Research.
Potential conflicts of interest. D. A. has received contracts for research from Cepheid, and is one of a group of coinvestigators who invented molecular beacon technology and who receive income from licensees, including a license to Cepheid for M. tuberculosis detection. However, the income attributable to the Xpert MTB/RIF assay which he may receive has been irrevocably capped at $5000 per year as a management of this conflict of interest. P. J. is an employee of Cepheid. D. A., D. A. B., C. F., A. F. L., and Y. F. W. have received research grant support from Cepheid. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; 1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Policy statement. Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. FDA permits marketing of first U.S. test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm362602.htm Accessed 1 December 2015. [PubMed]
- 4.US Food and Drug Administration. New data shows test can help physicians remove patients with suspected TB from isolation earlier. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm434226.htm Accessed 12 February 2015.
- 5.Bevilaqua AA. Boletim tuberculose 2014 [in Portugese]. Available at: http://www.riocomsaude.rj.gov.br/Publico/MostrarArquivo.aspx?C=wXJ%2BKouHyII%3D Accessed 28 August 2015.
- 6.Nanoo A, Izu A, Ismail NA et al. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004–12: a time series analysis. Lancet Infect Dis 2015; 15:1066–76. [DOI] [PubMed] [Google Scholar]
- 7.Kent P, Kubica G. Public health mycobacteriology: a guide for the level III laboratory. Atlanta, GA: Centers for Disease Control and Prevention, 1985. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Laboratory user guide for U.S. public health laboratories: molecular detection of drug resistance (MDDR) in Mycobacterium tuberculosis complex by DNA sequencing (version 2.0). Available at: http://www.cdc.gov/tb/topic/laboratory/mddrusersguide.pdf Accessed 30 March 2015.
- 9.Cepheid. Xpert MTB/RIF. Package insert. Cepheid: Sunnyvale, CA, July 2013.
- 10.Boehme CC, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossuyt PM, Reitsma JB, Bruns DE; Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003; 138:W1–12. [DOI] [PubMed] [Google Scholar]
- 12.Steingart KR, Sohn H, Schiller I et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2013; 1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141. [PubMed] [Google Scholar]
- 14.Blakemore R, Story E, Helb D et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 2010; 48:2495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62:821–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NS, Cavanaugh JS, Pratt R et al. Epidemiology of smear-negative pulmonary tuberculosis in the United States, 1993–2008. Int J Tuberc Lung Dis 2012; 16:1234–40. [DOI] [PubMed] [Google Scholar]
- 17.Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis 1989; 139:867–70. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration, Division of Microbiology Devices, Office of In Vitro Diagnostics and Radiological Health, Center for Devices Radiological Health. Revised device labeling for the Cepheid Xpert MTB/RIF assay for detecting Mycobacterium tuberculosis. MMWR Morb Mortal Wkly Rep 2015; 64:193. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.