In this meta-analysis of pertussis vaccine efficacy/effectiveness studies focusing on formulations currently on the market, the overall short-term (<3 years after completion of the primary series) protective effect against WHO-defined pertussis was lower for acellular vaccines than for whole-cell vaccines.
Keywords: pertussis, vaccine efficacy/effectiveness, acellular, whole-cell, meta-analysis
Abstract
Background. Acellular pertussis (aP) and whole-cell (wP) pertussis vaccines are presumed to have similar short-term (<3 years after completion of the primary series) efficacy. However, vaccine effect varies between individual pertussis vaccine formulations, and many originally studied formulations are now unavailable. An updated analysis of the short-term protective effect of pertussis vaccines limited to formulations currently on the market in developed countries is needed.
Methods. We conducted a systematic review and meta-analysis of published studies that evaluated pertussis vaccine efficacy or effectiveness within 3 years after completion (>3 doses) of a primary series of a currently available aP or wP vaccine formulation. The primary outcome was based on the World Health Organization (WHO) clinical case definitions for pertussis. Study quality was assessed using the approach developed by the Child Health Epidemiology Research Group. We determined overall effect sizes using random-effects meta-analyses, stratified by vaccine (aP or wP) and study (efficacy or effectiveness) type.
Results. Meta-analysis of 2 aP vaccine efficacy studies (assessing the 3-component GlaxoSmithKline and 5-component Sanofi-Pasteur formulations) yielded an overall aP vaccine efficacy of 84% (95% confidence interval [CI], 81%–87%). Meta-analysis of 3 wP vaccine effectiveness studies (assessing the Behringwerke, Pasteur/Mérieux, and SmithKline Beecham formulations) yielded an overall wP vaccine effectiveness of 94% (95% CI, 88%–97%) (both I2 = 0%).
Conclusions. Although all contemporary aP and wP formulations protect against pertussis disease, in this meta-analysis the point estimate for short-term protective effect against WHO-defined pertussis in young children was lower for currently available aP vaccines than wP vaccines.
Despite high rates of vaccine coverage, pertussis incidence has recently increased dramatically in many countries that previously had achieved good control of pertussis [1, 2]. Some of the largest increases in incidence have been among age groups that have completed a childhood pertussis vaccine series [3]. This shift in the burden of disease has been attributed, in part, to the fact that immunity to pertussis is not lifelong, whether after natural infection or vaccination [4]. Multiple observational studies have indicated that the immunity conferred by childhood acellular pertussis (aP) vaccines rapidly wanes in the first few years after completion of the childhood series [5–9]. In contrast, receipt of even a single dose of whole-cell pertussis (wP) vaccine as part of the childhood series confers more durable protection against pertussis [10, 11].
Acellular pertussis vaccines were initially licensed based on data suggesting that, as a group, they had similar short-term (<3 years after completion of a primary series) efficacy as wP formulations [12], but were less reactogenic [13]. These data came from multiple large vaccine efficacy and effectiveness studies that evaluated multiple comparator aP and wP formulations, whose individual performance varied substantially between studies, and many of which are no longer commercially available [14–21]. Consequently, modern assumptions regarding both the absolute and relative protective effects of these vaccines may not be valid [22]. Evidence-based estimates are needed to provide context for modeling studies of population-level disease risk due to waning immunity [23] and for policy decisions regarding pertussis vaccine implementation. They can also serve as a performance benchmark for clinical trials and for novel pertussis vaccines currently in development.
In this systematic review and meta-analysis, we address this critical knowledge gap by assessing the protective effect of only those pertussis vaccines currently on the market. Our review applied standards developed by the Child Health Epidemiology Reference Group (CHERG) allowing for the systematic classification of evidence indicating the effect of various interventions on child morbidity and mortality. We generated estimates of the protective effect of both aP and wP vaccine against child morbidity, particularly as modeled by the Lives Saved Tool (LiST), a resource developed to allow countries to model the impact of maternal, neonatal, and child health interventions prior to implementation [24].
METHODS
Literature Search
We conducted our systematic review and meta-analysis according to the standards for intervention reviews developed by CHERG [25]. We searched PubMed, the Cochrane Library, Web of Science, EMBASE, and the World Health Organization (WHO) Regional Databases, with no date restrictions, for English-language studies using the following search terms: pertussis, whooping cough, DTwP, DTaP, vaccine, efficacy, morbidity, and mortality. Following de-duplication, one reviewer (T. R. F.) screened all titles and abstracts to assess eligibility for inclusion. Any uncertainty during the screening process was resolved by discussion between the reviewer and principal investigator (S. B. O.).
Study Selection
Publications were included if they produced either (1) a pertussis vaccine efficacy or effectiveness estimate against a relevant outcome (all-cause mortality, pertussis-specific mortality, pertussis-specific hospitalization, or incidence or risk of typical or severe pertussis disease); or (2) an estimate of risk or odds of acquisition of pertussis with respect to time since completion of vaccination series. After our initial search, a meta-analysis on the duration of protection after childhood aP immunization was published [23]. For this analysis, we focused on the studies identified during our search that reported estimates of short-term efficacy or effectiveness to generate point estimates of aP and wP vaccine effect. We did not exclude publications based on type of study, which included randomized controlled trials (RCTs) and observational designs, including case-control, cohort, household contact, and screening studies (vaccine effectiveness computed on the basis of population coverage data and the proportion of cases vaccinated [26]). Studies that did not evaluate absolute efficacy (ie, compared to a placebo or an unvaccinated comparison population) were included if they contained data on the protective effect of wP or aP vaccine against severe pertussis or hospitalizations due to pertussis. Studies conducting an intent-to-treat analysis were included if data against a relevant outcome were available and if >1 dose of pertussis vaccine was administered; these studies underwent additional examination to ensure consistency of assumptions and strength of evidence.
The included studies evaluated pertussis incidence, odds or risk of pertussis infection, and vaccine efficacy and effectiveness estimates for children <6 years of age within 3 years after completion (>3 doses, with or without booster doses) of a primary pertussis vaccine series. No minimum duration of follow-up was required for study inclusion. Study outcomes were required to be based on the current WHO definition of (1) “typical” pertussis (>14 days of cough with at least one of the following: paroxysmal cough, inspiratory whoop, or posttussive vomiting, in addition to laboratory confirmation [27]); or (2) “severe” pertussis (>21 days of paroxysmal cough with laboratory confirmation of Bordetella pertussis infection, or epidemiological linkage [28]). Studies using less stringent clinical criteria were also included if their laboratory criteria provided a high level of confidence for pertussis infection (eg, positive culture or polymerase chain reaction assay for B. pertussis).
Only studies that evaluated aP or wP formulations that were available at the time of our search (November 2013) were included (Tables 1 and 2). Market availability of pertussis vaccines was assessed by cross-referencing the formulations used in studies against 3 sources of market information (when available): standard reference texts [29, 30], manufacturer product information pages, and/or inserts available online. Any uncertainty regarding the availability of a particular vaccine formulation was resolved through direct contact with the manufacturer.
Table 1.
Acellular Pertussis Vaccine Preparations Studied in Vaccine Efficacy/Effectiveness Trials
| Brand Name(s) | Manufacturer | Pertussis Antigens in Vaccine |
|---|---|---|
| Availablea | ||
| Daptacel (Tripacel) | Sanofi-Pasteur (Canada) | PT, FHA, PRN, FIM-2/FIM-3 |
| Infanrix | GlaxoSmithKline (Belgium) | PT, FHA, PRN |
| Triavax | Sanofi (France) | PT, FHA (not available standalone, see Pentavac) |
| Pentavac | Sanofi (France) | PT, FHA + IPV + Hib |
| Tripedia | Sanofi (United States) | PT, FHA |
| No longer availablea | ||
| Acel-Imune | Wyeth-Lederle (United States) | PT, FHA, PRN, FIM-2 |
| Acelluvax (Triacelluvax) | Chiron (United States) | PT, FHA, PRN |
Abbreviations: FHA, filamentous hemagglutinin; FIM, fimbrial protein; Hib, Haemophilus influenzae type b vaccine; IPV, inactivated polio vaccine; PRN, pertactin; PT, pertussis toxin.
a As of 2013.
Table 2.
Whole-Cell Pertussis Vaccine Preparations Studied in Vaccine Efficacy/Effectiveness Trials (Formulations Currently on the Market)
| Manufacturer | Manufacturer Country of Origin (at Time of Original Study) |
|---|---|
| Availablea | |
| Behringwerke | Germany |
| Pasteur/Mérieux | France |
| SmithKline Beecham | United Kingdom |
| Merck Sharp & Dohme | United States |
| CSL Limited | Australia |
Abbreviation: CSL, Commonwealth Serum Laboratories.
a As of 2013.
Data Extraction
Key variables were abstracted to grade each study according to the CHERG adaptation of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) technique [25]. Effect measures from both efficacy and effectiveness trials were abstracted, with vaccine effectiveness calculated as 1 minus the odds ratio or 1 minus the relative risk following the methodology used by the respective study. We defined vaccine efficacy as an estimate of protective effect generated from an RCT, and vaccine effectiveness as any estimate of protective effect measured by studies other than RCTs. For studies publishing multiple measures against a range of outcomes (eg, absolute aP efficacy vs aP efficacy relative to wP, or vaccine effectiveness for multiple vaccine formulations or age groups), only values related to the outcomes of interest were abstracted. Similarly, we only abstracted effect measures for the population that received all scheduled doses of the vaccine. We did not compare endpoints based on the number or schedule of doses received.
All research studies meeting the inclusion criteria and the relevant variables were abstracted into an Excel spreadsheet by one reviewer (T. R. F.).
Quality Assessment
We employed the CHERG-adapted GRADE process in ranking the quality of study evidence by 4 criteria: study design, study quality, relevance to the objectives of the review, and consistency across studies [25].
Statistical Analysis
Studies yielding sufficient quality of evidence and sharing characteristics with other studies that would make them appropriate for a pooled analysis underwent fixed- and random-effects meta-analyses. Meta-analyses were stratified on type of vaccine (aP or wP) and type of study (efficacy or effectiveness). Per-protocol and intent-to-treat data were pooled within these strata. Heterogeneity between studies was evaluated using the I2 statistic. Studies demonstrating low heterogeneity (I2 < 50%) were pooled, and we took a conservative approach and presented overall effect estimates from the random-effects model rather than the fixed-effects model [31] to account for known and potential heterogeneity in pertussis vaccine performance. We then applied the CHERG rules for generating estimated intervention effects for use in LiST as appropriate. We did not perform comparative analyses of protective effect among the different aP vaccines.
We conducted 2 sensitivity analyses, first removing the lowest GRADE-ranked study from the meta-analysis and assessing changes in effect size and significance, then removing any study contributing >50% of the total weight to determine impact on pooled effect size.
Meta-analyses were performed, and forest plots generated, using Microsoft Excel 2013 and Review Manager 5.2 software.
RESULTS
Literature Search Results
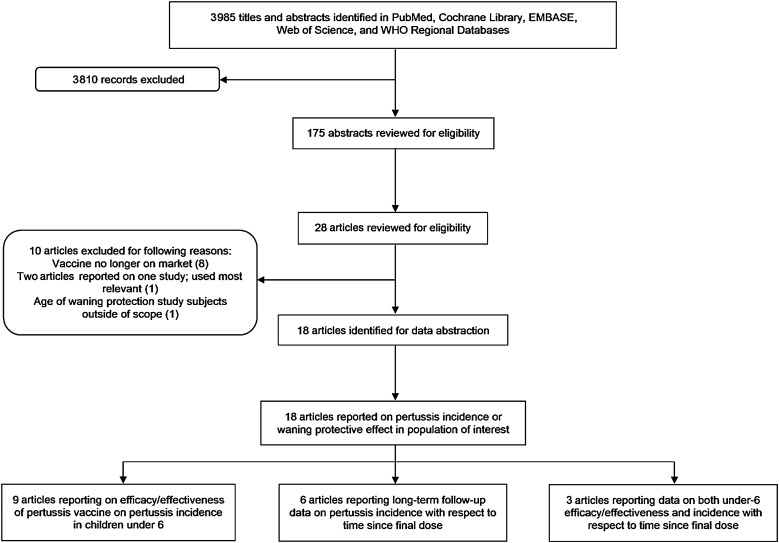
The initial literature search yielded 3985 titles. Of these, 175 abstracts were reviewed for eligibility; from these, 28 full articles were assessed for eligibility (Figure 1). Ten articles were considered ineligible for various reasons, principally because the vaccines studied were no longer on the market. There were 6 articles with data on longer-term protective effect; however, for this analysis we focused on the 12 articles that evaluated the short-term (<3 years after the primary series) effect of pertussis vaccines (Table 3). Following convention for CHERG intervention reviews, details of each of these studies and key variables were abstracted into a spreadsheet (Supplementary Table).
Figure 1.
Pertussis study selection flowchart. Abbreviation: WHO, World Health Organization.
Table 3.
Key Characteristics of Studies Identified by the Search Strategy Used for This Systematic Review and Meta-analysis
| Study, First Author and Year | Design | Location | Intervention(s) | Dosage Schedule | Outcome/Case Definition | Method for Case Ascertainment | Duration of Follow-up (Mean) | Included in Meta-analysis | Included in Pooled Estimate |
|---|---|---|---|---|---|---|---|---|---|
| Efficacy studies | |||||||||
| Greco 1996 [14] | RCT | Italy | aP: Infanrix (GSK 3-component) or Acelluvax (Chiron 3-component) wP: Connaught | 3 doses at 2–12 wk, 13–20 wk, 21–28 wk | Illness with >21 d of cough with culture- or serologically confirmed Bordetella pertussis infection | Active surveillance nurse follow-up every month | 17.2 mo | Yes | Yes (for aP efficacy only) |
| Gustafsson 1996 [15] | RCT | Sweden | aP: SKB (2-component) or Daptacel (Connaught 5-component) wP: Connaught | 3 doses at 2 mo, 4 mo, 6 mo | Illness with >21 d of cough with culture- or serologically confirmed B. pertussis infection | Active surveillance with nurse follow-up every 6–8 wk | 21–23.5 mo | Yes | Yes (for aP efficacy only) |
| Effectiveness studies | |||||||||
| Bisgard 2005 [32] | Matched case-control | Multisite in US | aP: Infanrix (GSK 3-component), Triavax (Sanofi 2-component), Acel-Imune (Wyeth-Lederle 4-component), or Daptacel (Sanofi-Pasteur 5-component) wP: Connaught, Wyeth-Lederle | 5 doses at 2 mo, 4 mo, 6 mo, 15–18 mo, 4–6 y | Illness with >1 d of cough with culture-confirmed B. pertussis infection, or illness with >14 d of cough with PCR-confirmed B. pertussis infection or epidemiologic linkage with a laboratory-confirmed case | Confirmed cases reported to public health authorities | …a | Yes | No |
| Juretzko 2002 [34] | Screening study | Germany | aP: Infanrix (GSK 3-component) or Tetravac/Pentavac (Sanofi 2-component) wP: Various | >3 doses at 2 mo, 3 mo, 4 mo, 12–15 mo | At least 1 of: typical clinical symptoms (illness with >14 d of cough or paroxysmal cough with >4 d of whoop), positive serology, or positive culture, PCR or direct immunofluorescence test for B. pertussis | Hospital-based surveillance for pertussis cases | …a | No | … |
| Liese 1997 [18] | Matched case-control | Germany | aP: Tripedia (Sanofi 2-component) wP: Behringwerke | 3 doses at 2 mo, 4 mo, 6 mo | Illness with >21 d of paroxysmal cough with culture-confirmed B. pertussis infection or epidemiologic linkage to a laboratory-confirmed case | Passive surveillance of cough illnesses lasting >7 d presenting for physician evaluation | …a | Yes | Yes (for wP effectiveness only) |
| Misegades 2012 [6] | Matched case-control | Multisite in US (California) | aP: Infanrix (GSK 3-component) or Daptacel (Sanofi-Pasteur 5 component) | 5 doses at 2 mo, 4 mo, 6 mo, 15–18 mo, 4–6 y | Illness with >1 d of cough with culture-confirmed B. pertussis infection, or illness with >14 d of cough with PCR-confirmed B. pertussis infection or epidemiologic linkage with a laboratory-confirmed case | Suspected, probable, and confirmed cases reported to public health authorities | …a | Yes | No |
| Preziosi 2003 [35] | Prospective cohort | Senegal | aP: Triavax (Sanofi 2-component) wP: Pasteur/Mérieux | 3 doses at 2 mo, 4 mo, 6 mo | Severe pertussis (study-defined) with laboratory confirmation (culture or serology) of B. pertussis infection or epidemiologic linkage to a culture-confirmed case | Active surveillance with field worker follow-up every week | None listed, cohort followed since 1983 | No | … |
| Rendi-Wagner 2006 [33] | Screening study | Austria | aP: Tetravac (Sanofi 2 component), Infanrix (GSK 3-component), or Hexavac (Sanofi-Pasteur 2-component) wP: Merck Sharp & Dohme | 3 doses (given between 3 and 5 mo) | Illness with >14 d of cough with at least 1 of: paroxysmal cough, whoop, or posttussive vomiting with culture- or serologically confirmed B. pertussis infection | Hospital-based surveillance for pertussis cases | …a | No | … |
| Schmitt 1996 [21] | Prospective cohort | Germany | aP: Infanrix (GSK 3-component) wP: Behringwerke or SKB | 3 doses at 3 mo, 4 mo, 5 mo | Illness with >21 d of paroxysmal cough with culture- or serologically confirmed B. pertussis infection | Active surveillance with medical field supervisor follow-up every week | 23 mo | Yes | Yes (for wP effectiveness only) |
| Simondon 1997 [20] | Case-contact nested in RCT | Senegal | aP: Triavax (Sanofi 2-component) wP: Pasteur/Mérieux | 3 doses at 2 mo, 4 mo, 6 mo | Illness with >21 d of paroxysmal cough with culture- or serologically confirmed B. pertussis infection or epidemiologic linkage to a culture-confirmed case | Active surveillance with field worker follow-up every week | 1.73 y | Yes | Yes (for wP effectiveness only) |
| Torvaldsen 2003 [36] | Screening study | Australia | wP: CSL Ltd | 3 doses at 2 mo, 4 mo, 6 mo | Typical clinical symptoms (illness with >14 d of cough with at least 1 of: paroxysmal cough, whoop or posttussive vomiting), culture- or serologically proven B. pertussis infection, or cough illness lasting >14 d with epidemiologic linkage to a laboratory-confirmed case | Confirmed cases reported to public health authorities | …a | No | … |
| Zielinski 2004 [37] | Screening study | Poland | wP: formulation/brand not given | 3 or 4 doses by age 2 years; no intervals given | Culture or serologically confirmed B. pertussis | Laboratory-confirmed cases reported to public health authorities | …a | No | … |
Abbreviations: aP, acellular pertussis vaccine; CSL, Commonwealth Serum Laboratories; GSK, GlaxoSmithKline; PCR, polymerase chain reaction; RCT, randomized controlled trial; SKB, SmithKline Beecham; wP, whole-cell pertussis vaccine.
a Data not available.
Ten studies evaluated vaccine efficacy or effectiveness of aP formulations currently on the market. Among these were 2 aP efficacy studies—these were RCTs conducted in Italy [14] and Sweden [15] that used similar vaccine dose schedules, methods, and duration of follow-up, and laboratory assays for pertussis infection, and reported vaccine efficacy against an identical outcome (ie, WHO-defined pertussis). There were no vaccine efficacy studies of on-market wP formulations (the Connaught wP formulation evaluated in the Italian [14] and Swedish [15] efficacy trials is no longer on the market). There were 8 aP effectiveness [6, 18, 20, 21, 32–35] and 7 wP effectiveness [18, 20, 21, 33, 35–37] studies; these studies varied in design (case-control, prospective cohort, or screening), number and schedule of vaccine doses evaluated (ie, before or after booster doses), duration of follow-up, and methods for case ascertainment (eg, hospital-based surveillance, cases reported to public health authorities). All studies evaluated outcomes corresponding to the WHO clinical case definitions for pertussis; however, there were slight differences in the specific definitions used in each study—for example, one study assessed effectiveness against laboratory-confirmed “severe pertussis” according to a protocol-defined severity scale [35].
Applying the CHERG-adapted GRADE procedure to each study (Table 4) and averaging the adjusted scores over each outcome resulted in the assignment of an overall outcome-specific quality of evidence grade of “high” to aP efficacy trials, “moderate-low” to aP effectiveness trials, and “moderate-low” to wP effectiveness trials (Table 5 and Supplementary Table).
Table 4.
Quality Assessment of Studies Exploring Pertussis Vaccine Efficacy or Effectiveness
| Study, First Author and Year | Quality Assessment |
Summary of Findings |
Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Design | Limitationsa | Consistencyb | Directness |
No. of Events/No. of Children |
Effect |
||||
| Generalizability to Population of Interest | Generalizability to Intervention of Interest | Intervention | Control | Vaccine Effectiveness (95% CI) | |||||
| Outcome: acellular vaccine efficacy/effectiveness among children <6 y | |||||||||
| Efficacy against typical pertussis (outcome-specific quality: high) | |||||||||
| Greco 1996 [14] | RCT | None | No concerns | Multisite trial conducted in Europe (Italy) | Healthy infants receive primary series at 2–12 wk, 13–20 wk, 21–28 wk | 37/4481 | 74/1470 | 84% (76%–89%) | |
| Gustafsson 1996 [15] | RCT | None | No concerns | Multisite trial conducted in Europe (Sweden) | Healthy infants receive primary series at 2 mo, 4 mo, 6 mo | 59/2551 | 371/2538 | 85% (81%–89%) | |
| Effectiveness against typical pertussis (outcome-specific quality: moderate-low) | |||||||||
| Bisgard 2005 [32] | Matched case-control | Effect estimates of multiple aP vaccines combined | No concerns | Multisite conducted in multiple states in US | Healthy infants receive primary series at 2, 4, 6 mo; booster at 15–18 mo and 4–6 y | 20/146 | 48/62 | 97% (91%–99%) | |
| Juretzko 2002 [34] | Screening study | Hospital cases only; effect estimates of multiple aP vaccines combined | Not included in meta-analysis | Nationwide screening conducted in Germany | Healthy infants receive primary series at 2, 3, 4 mo with booster between 12–15 mo | 1/…c | …/…c | 100% (99%–100%) | |
| Liese 1997 [18] | Matched case-control | Low number of cases and controls | No concerns | Multisite conducted in Germany | Healthy infants receive primary series at 2, 4, 6 mo with booster between 15–24 mo | 4/290 | 81/987 | 93% (63%–99%) | |
| Misegades 2012 [6] | Matched case-control | Effect estimates of multiple aP vaccines combined | No concerns | Multisite conducted in US (California) | Healthy infants receive primary series at 2, 4, 6 mo; booster at 15–18 mo and 4–6 y | 629/2626 | 53/72 | 89% (79%–94%) | |
| Preziosi 2003 [35] | Prospective cohort | Combined wP and aP effectiveness estimate | Not included in meta-analysis | Rural, conducted in Senegal; applicability to developing contexts | Healthy infants receive primary series at 2 mo, 4 mo, 6 mo | 190/594 | 149/243 | 48% (39%–55%) | Follow-up study from Senegal RCT ([20]); evaluates effect of aP in reducing clinical severity |
| Rendi-Wagner 2006 [33] | Screening study | Hospital cases only; effect estimates of multiple aP vaccines combined | Not included in meta-analysis | Nationwide screening conducted in Austria | Healthy infants administered 3 doses between 3–5 mo of age and booster at 2 y | 65/…c | …/…c | 92% (no CI given) | |
| Schmitt 1996 [21] | Prospective cohort | Low number of cases and controls | No concerns | Multisite conducted in Germany | Healthy infants receive primary series at 3, 4, 5 mo | 7/112 | 96/173 | 89% (77%–95%) | |
| Simondon 1997 [20] | Case-contact nested in RCT | Low number of cases and controls | Borderline heterogeneity from meta-analysis (P = .11) | Rural, conducted in Senegal; applicability to developing contexts | Healthy infants receive primary series at 2 mo, 4 mo, 6 mo | 24/197 | 8/17 | 74% (51%–86%) | |
| Outcome: whole-cell vaccine efficacy/effectiveness among children <6 y | |||||||||
| Effectiveness against typical pertussis (outcome-specific quality: moderate-low) | |||||||||
| Liese 1997 [18] | Matched case-control | Low number of cases and controls | No concerns | Multisite conducted in Germany | Healthy infants receive primary series at 2, 4, 6 mo with booster between 15–24 mo | 1/196 | 81/987 | 97% (79%–100%) | |
| Preziosi 2003 [35] | Prospective cohort | Combined wP and aP effectiveness estimate | Not included in meta-analysis | Rural, conducted in Senegal; applicability to developing contexts | Healthy infants receive primary series at 2 mo, 4 mo, 6 mo | 190/594 | 149/243 | 48% (39%–55%) | Follow-up study from Senegal RCT ([20]); evaluates effect of aP in reducing clinical severity |
| Rendi-Wagner 2006 [33] | Screening study | Hospital cases only; effect estimate of multiple aP vaccines combined | Not included in meta-analysis | Nationwide screening conducted in Austria | Healthy infants administered 3 doses between 3–5 mo of age and booster at 2 y | 11/…c | …/…c | 79% (no CI given) | |
| Simondon 1997 [20] | Case-contact nested in RCT | Low number of cases and controls | No concerns | Rural, conducted in Senegal; applicability to developing contexts | Healthy infants receive primary series at 2 mo, 4 mo, 6 mo | 7/190 | 8/17 | 92% (81%–97%) | |
| Schmitt 1996 [21] | Prospective cohort | Low number of cases and controls | No concerns | Multisite conducted in Germany | Healthy infants receive primary series at 3, 4, 5 mo | 1/75 | 75/173 | 98% (83%–100%) | |
| Torvaldsen 2003 [36] | Screening study | None | Not included in meta-analysis | Multisite screening conducted in New South Wales, Australia | Healthy infants receive primary series at 2, 4, 6 mo; boosters at 18 mo and 4–5 y | 223/…c | 198/…c | 87% (83%–90%) | Confounding due to patients receiving 4 doses instead of intended 3 |
| Zielinski 2004 [37] | Screening study | None | Not included in meta-analysis | Nationwide screening conducted in Poland | Healthy infants complete primary series by age 2 years; no intervals given | 157/1 569 956 | 12/31 817 | 74% (52%–85%) | |
Abbreviations: aP, acellular pertussis vaccine; CI, confidence interval; RCT, randomized controlled trial; wP, whole-cell pertussis vaccine.
a Limitations listed are in addition to those inherent in study design.
b Applies only to studies included in meta-analysis.
c Data not available.
Table 5.
Application of Standardized Rules for Choice of Final Outcome to Estimate the Effect of Pertussis Vaccine on Pertussis-Specific Morbidity
| Outcome Measure | Studies | Effect Size | Application of Standard Rules |
|---|---|---|---|
| Acellular pertussis outcome measurea | |||
| All-cause mortality | 0 | NA | Rule 1: do not apply |
| Cause-specific mortality | 0 | NA | Rules 1, 2, 3, 4: do not apply |
| Incidence of severe pertussis (≥21 d paroxysmal cough) | 2 | 84% (81%–87%) | Rule 5: applyb |
| Whole-cell pertussis outcome measurec | |||
| All-cause mortality | 0 | NA | Rule 1: do not apply |
| Cause-specific mortality | 0 | NA | Rules 1, 2, 3, 4: do not apply |
| Incidence of severe pertussis (≥21 d paroxysmal cough) | 3 | 94% (88%–97%) | Rule 5: apply |
Abbreviation: NA, not applicable.
a Strong evidence of serious morbidity reduction with acellular vaccine: highly plausible.
b Pooled acellular pertussis vaccine efficacy estimate used (high heterogeneity in pooled acellular pertussis vaccine effectiveness estimate).
c Strong evidence of serious morbidity reduction with whole-cell vaccine: highly plausible.
Effects of Interventions
Acellular Vaccine Efficacy
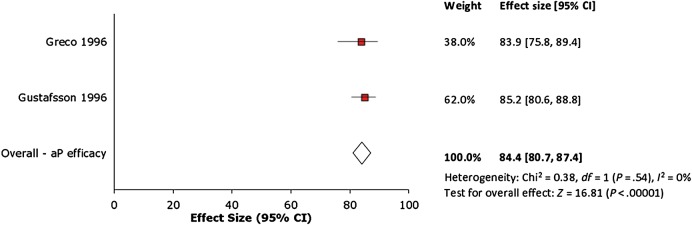
Meta-analysis of the 2 aP vaccine efficacy studies [14, 15] generated a random-effects pooled vaccine efficacy of 84% (95% confidence interval [CI], 81%–87%; P < .00001; Figure 2). Heterogeneity was not significant at I2 = 0%. This pooled analysis combined efficacy estimates for a complete 3-dose series of the GlaxoSmithKline 3-component (Infanrix) and Connaught 5-component (Daptacel) formulations.
Figure 2.
Forest plot of acellular pertussis vaccine efficacy studies. Abbreviations: aP, acellular pertussis; CI, confidence interval.
Acellular Vaccine Effectiveness
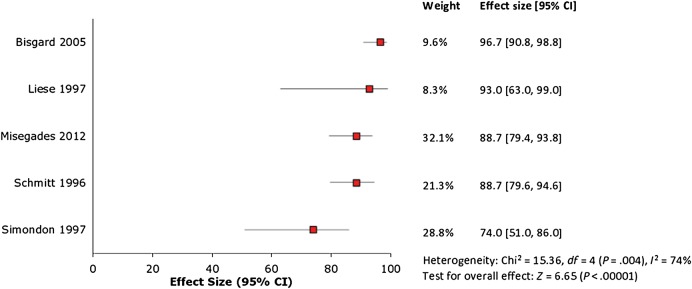
Five [6, 18, 20, 21, 32] of the 8 aP vaccine effectiveness studies were deemed appropriate for inclusion in further meta-analysis. Two of the 3 excluded studies [33, 34] computed aP vaccine effectiveness utilizing the screening method; these studies were considered low quality because of the potential for substantial bias in the input estimates of vaccine coverage and pertussis incidence on which the effectiveness calculation is based [26]. The third excluded study estimated vaccine effectiveness against a study-specific (not WHO-defined) outcome [35], which differed from all of the other studies. Meta-analysis of the 5 included aP vaccine effectiveness studies generated significant heterogeneity with I2 = 74% (Figure 3); therefore, we did not compute a pooled estimate. The estimates of vaccine effectiveness reported in these studies ranged from 74% (95% CI, 51%–86%) to 97% (95% CI, 91%–99%).
Figure 3.
Forest plot of acellular pertussis vaccine effectiveness studies. Abbreviation: CI, confidence interval.
Whole-Cell Vaccine Effectiveness
Three [18, 20, 21] of the 7 wP vaccine effectiveness were deemed appropriate for inclusion in further meta-analysis. Similar to the meta-analysis of aP effectiveness studies, we excluded 3 studies because they provided vaccine effectiveness utilizing the screening method [33, 36, 37], and the fourth was excluded because it provided aP vaccine effectiveness against a study-specific (not WHO-defined) outcome [35], different from the 3 included studies. Meta-analysis of the 3 included wP vaccine effectiveness studies generated a random-effects pooled vaccine effectiveness of 94% (95% CI, 88%–97%; P < .00001; Figure 4). Heterogeneity was not significant at I2 = 0%. This pooled analysis combined effect estimates for a complete 3-dose series of wP formulations from Pasteur/Mérieux, Behringwerke, and SmithKline Beecham.
Figure 4.
Forest plot of whole-cell pertussis vaccine effectiveness studies. Abbreviations: CI, confidence interval; wP, whole-cell pertussis.
On the strength of evidence of effect of aP and wP vaccines against WHO-defined pertussis, we applied Rule 5 of the CHERG rule set for generating estimated intervention effects for use in LiST to the pooled estimate of aP vaccine efficacy and wP vaccine effectiveness (Table 5) [25]. The estimated effects of aP vaccine and wP vaccine on WHO-defined pertussis in children aged <5 years recommended for LiST use were thus 84% (95% CI, 81%–87%) and 94% (95% CI, 88%–97%), respectively.
DISCUSSION
In this systematic review and meta-analysis, we found that a complete 3-dose initial series of currently available aP or wP vaccines provides short-term protection against WHO-defined pertussis disease in children <6 years of age. However, our meta-analysis yielded a lower pooled effect size for on-market aP vaccines than for wP vaccines. Our study differs from previous meta-analyses that have focused only on vaccine efficacy trials, safety studies, aP (but not wP) vaccines, or longer-term effectiveness, or have not restricted their evaluation to studies of vaccines that are currently available on the market, or generated point estimates [23, 38].
These point estimates for on-market aP and wP vaccine effect corroborate observations regarding their relative real-world performance, and provide context for the resurgence in pertussis seen in some countries. Multiple hypotheses have been put forward to explain why aP vaccines have not prevented, or have contributed to, this rise in pertussis incidence [22]. The lower initial potency of aP vaccines compared with wP vaccines—as reflected by the lower pooled estimate of protective effect—is probably one reason why pertussis has reemerged among age groups that have just completed their pertussis vaccine series, even before their immunity has begun to wane. Indeed, the lower primary efficacy of aP vaccines compared with that of wP vaccines may partly explain why the protection conferred by aP vaccines wanes more rapidly [23].
The results of this study have public health implications. From the standpoint of disease control, our point estimates for both aP and wP vaccine effect are below or just meet the projected crude herd immunity threshold for pertussis of 92%–94% [39]. Thus, although pertussis vaccines have had a dramatic impact on the overall disease burden in many countries, the currently available formulations will be insufficient to achieve complete elimination, even if vaccine coverage could be increased to 100%.
Our meta-analysis has some limitations. Pooled estimates for aP and wP vaccine effect may not be representative of all vaccine formulations, which may limit the generalizability of our effect estimates to vaccines that were not included in the meta-analysis. Heterogeneity in performance between individual aP and wP vaccines is well known. For example, the aP vaccine formulations used in the Italian [14] and Swedish [15] efficacy trials were superior to the comparator wP vaccine, a Connaught-developed wP preparation that is no longer on the market. This variability in aP and wP vaccine performance is one reason that previous systematic reviews have avoided estimating overall effect sizes for aP and wP vaccines [38]. In contrast, we focused only on currently available vaccines, and excluded studies from the pooled analysis that combined data from multiple aP or wP formulations (some of which are no longer available) prior to reporting effect estimates, as well as observational studies of insufficient consistency in methodology (eg, screening studies) or reporting (eg, nonstandard case definitions) to warrant inclusion in our analysis, even if they offered effect estimates for a wider range of vaccines. As a result, we were able to generate pooled estimates that are both precise (nonoverlapping confidence intervals) and relevant.
We did not compute a pooled estimate for the 5 aP vaccine effectiveness studies included in the meta-analysis due to significant heterogeneity (I2 = 74%). These studies exhibited variability in study design, dosage schedules, methods for case ascertainment, and reporting of overall effect (Table 3), which may introduce substantial bias. Estimates from case-control (and case-contact) studies may have varying degrees of bias (eg, classification and/or ascertainment) due to differences in the approach to identifying vaccination status and pertussis cases. Three of these 5 studies evaluated 3 doses of vaccine, whereas the other 2 assessed >3 doses [32] or 5 doses [6]. Two studies combined data from multiple aP formulations and reported only a single estimate of vaccine effectiveness [6, 32], precluding evaluation of any individual formulation; furthermore, one of these combined estimates included data from a formulation that is no longer available [32]. Finally, 3 studies [18, 20, 21] had a small number of cases, resulting in wide confidence intervals for the effect estimate.
Another limitation is that most of the studies represented in the pooled estimate utilized a 2-, 4-, 6-month dosing schedule (only one study [21] used a 3-, 4-, 5-month schedule). Similarly, there were too few studies with outcome data after <3 doses to adequately evaluate an incomplete vaccine series. As such, our pooled estimates may not be generalizable to populations exposed to alternative dose schedules or to <3 vaccine doses. Only one of the studies included in the meta-analyses assessed aP vaccine effectiveness in a low-income country (Senegal) [20], and this difference in setting may impact pertussis vaccine performance and thereby contribute to statistical heterogeneity. This could be attributable to regional differences in pertussis epidemiology or other factors known to impact the immunogenicity or effectiveness of childhood vaccines (eg, interference by maternal antibodies, nutritional status, household size). Notably, the Senegal study also found that aP vaccine was inferior to the comparator wP vaccine [20], similar to our overall findings. More data are needed from a broader range of countries employing a variety of dose schedules to provide a more complete assessment of pertussis vaccination strategies.
In conclusion, in this systematic review and meta-analysis focusing on the short-term protective effect of currently available childhood pertussis vaccines, we determined an overall protective effect of 84% for aP vaccines and 94% for wP vaccines. These results provide context for the observed resurgence in pertussis and the apparent rapidly waning immunity to pertussis in the population, and present a standard against which newer immunization strategies or vaccine formulations should be evaluated. Improved vaccines may be needed to achieve pertussis control.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author contributions. Study concept and design: T. R. F., S. B. O. Acquisition, analysis, or interpretation of data: T. R. F., V. K. P., W. A. O., A. R. H., W. D. J., S. B. O. Drafting of the manuscript: T. R. F., V. K. P., S. B. O. Critical revision of the manuscript for important intellectual content: T. R. F., V. K. P., W. A. O., A. R. H., W. D. J., S. B. O. Administrative, technical, or material support: S. B. O. Study supervision: S. B. O.
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health.
Financial support. This work was supported by a subgrant from the US Fund for UNICEF to Emory University from a grant awarded to the US Fund for UNICEF by the Bill & Melinda Gates Foundation (grant number 50140). V. K. P. is supported by the Emory Vaccinology Training Program (award number T32AI074492 from the NIAID).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Adams DA, Jajosky RA, Ajani U et al. Summary of notifiable diseases—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 61:1–121. [PubMed] [Google Scholar]
- 2.NNDSS Annual Report Writing Group. Australia's notifiable disease status, 2012: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep 2015; 39:E46–136. [DOI] [PubMed] [Google Scholar]
- 3.Tan TQ, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatr Infect Dis J 2005; 24(5 suppl):S10–8. [DOI] [PubMed] [Google Scholar]
- 4.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 2005; 24(5 suppl):S58–61. [DOI] [PubMed] [Google Scholar]
- 5.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 2012; 367:1012–9. [DOI] [PubMed] [Google Scholar]
- 6.Misegades LK, Winter K, Harriman K et al. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA 2012; 308:2126–32. [DOI] [PubMed] [Google Scholar]
- 7.Tartof SY, Lewis M, Kenyon C et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 2013; 131:e1047–52. [DOI] [PubMed] [Google Scholar]
- 8.Sin MA, Zenke R, Ronckendorf R, Littmann M, Jorgensen P, Hellenbrand W. Pertussis outbreak in primary and secondary schools in Ludwigslust, Germany demonstrating the role of waning immunity. Pediatr Infect Dis J 2009; 28:242–4. [DOI] [PubMed] [Google Scholar]
- 9.Lai FY, Thoon KC, Ang LW et al. Comparative seroepidemiology of pertussis, diphtheria and poliovirus antibodies in Singapore: waning pertussis immunity in a highly immunized population and the need for adolescent booster doses. Vaccine 2012; 30:3566–71. [DOI] [PubMed] [Google Scholar]
- 10.Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 2013; 56:1248–54. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 2012; 308:454–6. [DOI] [PubMed] [Google Scholar]
- 12.Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1997; 46:1–25. [PubMed] [Google Scholar]
- 13.Decker MD, Edwards KM, Steinhoff MC et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995; 96(3 II suppl):557–66. [PubMed] [Google Scholar]
- 14.Greco D, Salmaso S, Mastrantonio P et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N Engl J Med 1996; 334:341–8. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 1996; 334:349–55. [DOI] [PubMed] [Google Scholar]
- 16.Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Ad Hoc Group for the Study of Pertussis Vaccines. Lancet 1997; 350:1569–77. [DOI] [PubMed] [Google Scholar]
- 17.Trollfors B, Taranger J, Lagergard T et al. A placebo-controlled trial of a pertussis-toxoid vaccine. N Engl J Med 1995; 333:1045–50. [DOI] [PubMed] [Google Scholar]
- 18.Liese JG, Meschievitz CK, Harzer E et al. Efficacy of a two-component acellular pertussis vaccine in infants. Pediatr Infect Dis J 1997; 16:1038–44. [DOI] [PubMed] [Google Scholar]
- 19.Stehr K, Cherry JD, Heininger U et al. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 1998; 101:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Simondon F, Preziosi MP, Yam A et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997; 15:1606–12. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt HJ, Von Konig CHW, Neiss A et al. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA 1996; 275:37–41. [PubMed] [Google Scholar]
- 22.Cherry JD. Why do pertussis vaccines fail? Pediatrics 2012; 129:968–70. [DOI] [PubMed] [Google Scholar]
- 23.McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics 2015; 135:331–43. [DOI] [PubMed] [Google Scholar]
- 24.Boschi-Pinto C, Young M, Black RE. The Child Health Epidemiology Reference Group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. Int J Epidemiol 2010; 39(suppl 1):i3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol 2010; 39(suppl 1):i21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orenstein WA, Bernier RH, Dondero TJ et al. Field evaluation of vaccine efficacy. Bull World Health Organ 1985; 63:1055–68. [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO-recommended standards for surveillance of selected vaccine-preventable diseases. Available at: http://apps.who.int/iris/bitstream/10665/68334/1/WHO_V-B_03.01_eng.pdf Accessed 18 December 2015.
- 28.World Health Organization, Division of Communicable Diseases . WHO meeting on case definition of pertussis, Geneva, 10–11 January 1991. Geneva, Switzerland: WHO, 1991. [Google Scholar]
- 29.Edwards KM, Decker MD. Pertussis vaccines. In: Plotkin SA, Orenstein WA, Offit PA. Vaccines. 6th ed Philadelphia, PA: Elsevier Saunders, 2013. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Pertussis. In: Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Atlanta, GA, 2015. [Google Scholar]
- 31.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011; 342:d549. [DOI] [PubMed] [Google Scholar]
- 32.Bisgard KM, Rhodes P, Connelly BL et al. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998–2001. Pediatrics 2005; 116:e285–94. [DOI] [PubMed] [Google Scholar]
- 33.Rendi-Wagner P, Kundi M, Mikolasek A, Vecsei A, Fruhwirth M, Kollaritsch H. Hospital-based active surveillance of childhood pertussis in Austria from 1996 to 2003: estimates of incidence and vaccine effectiveness of whole-cell and acellular vaccine. Vaccine 2006; 24:5960–5. [DOI] [PubMed] [Google Scholar]
- 34.Juretzko P, Von Kries R, Hermann M et al. Effectiveness of acellular pertussis vaccine assessed by hospital-based active surveillance in Germany. Clin Infect Dis 2002; 35:162–7. [DOI] [PubMed] [Google Scholar]
- 35.Preziosi MP, Halloran ME. Effects of pertussis vaccination on disease: vaccine efficacy in reducing clinical severity. Clin Infect Dis 2003; 37:772–9. [DOI] [PubMed] [Google Scholar]
- 36.Torvaldsen S, Simpson JM, McIntyre PB. Effectiveness of pertussis vaccination in New South Wales, Australia, 1996–1998. Eur J Epidemiol 2003; 18:63–9. [DOI] [PubMed] [Google Scholar]
- 37.Zielinski A, Rosinska M, Czarkowski M, Rudowska J. The effectiveness of vaccination with whole-cell pertussis vaccine by age group in Poland 1996–2001. Scand J Infect Dis 2004; 36:114–8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev 2014; 9:CD001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fine PE, Mulholland K. Community immunity. In: Plotkin SA, Orenstein WA, Offit PA. Vaccines. 6th ed Elsevier Saunders, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.