The persistence of vaccine-induced antibody responses to tetanus and diphtheria were studied in 546 adults. Immunity was long-lived in both men and women and remained durable regardless of advanced age, suggesting that the 10-year adult booster vaccination schedule be reexamined.
Keywords: tetanus, diphtheria, immunological memory, cross-sectional analysis
Abstract
Background. Many adult immunization schedules recommend that tetanus and diphtheria vaccination be performed every 10 years. In light of current epidemiological trends of disease incidence and rates of vaccine-associated adverse events, the 10-year revaccination schedule has come into question.
Methods. We performed cross-sectional analysis of serum antibody titers in 546 adult subjects stratified by age or sex. All serological results were converted to international units after calibration with international serum standards.
Results. Approximately 97% of the population was seropositive to tetanus and diphtheria as defined by a protective serum antibody titer of ≥0.01 IU/mL. Mean antibody titers were 3.6 and 0.35 IU/mL against tetanus and diphtheria, respectively. Antibody responses to tetanus declined with an estimated half-life of 14 years (95% confidence interval, 11–17 years), whereas antibody responses to diphtheria were more long-lived and declined with an estimated half-life of 27 years (18–51 years). Mathematical models combining antibody magnitude and duration predict that 95% of the population will remain protected against tetanus and diphtheria for ≥30 years without requiring further booster vaccination.
Conclusions. These studies demonstrate that durable levels of protective antitoxin immunity exist in the majority of vaccinated individuals. Together, this suggests that it may no longer be necessary to administer booster vaccinations every 10 years and that the current adult vaccination schedule for tetanus and diphtheria should be revisited.
Vaccination against tetanus and diphtheria has resulted in a significant decline in the incidence of these 2 serious diseases. Deaths attributable to tetanus have declined by 99% since the prevaccine era, and diphtheria is virtually nonexistent in the United States despite as many as approximately 21 000 cases and approximately 1800 diphtheria-related deaths recorded each year before introduction of routine vaccination [1]. These vaccines have a long safety history, but nevertheless, 50%–85% of patients experience injection site pain or tenderness, and 25%–30% experience edema and erythema [2]. Higher preexisting anti-tetanus antibody levels are also associated with higher reactogenicity of greater severity [2]. Anaphylaxis after tetanus vaccination represents a rare but potentially serious adverse event, with an incidence of 1.6 cases per million distributed doses [3]. Concerns about vaccine-associated adverse events when immunizations were performed at short intervals led to revision of the tetanus/diphtheria vaccination schedule in 1966 to once every 10 years for patients >6 years of age [4, 5].
Understanding the durability of vaccine-induced immunity is critical for making informed decisions on the recommended time interval between booster vaccinations. In longitudinal studies involving 7 [6] or 45 [7] subjects, tetanus-specific antibodies declined with a half-life of 11 years (95% confidence interval [CI], 10–16) [7]. Likewise, longitudinal analysis indicated that diphtheria-specific antibody titers declined with a half-life of 19 years (95% CI, 14–33) [7]. Although these studies provided provocative details indicating that immunity to tetanus and diphtheria could be long-lived, the number of subjects followed up was too small to inform clinical decisions on the overall proportion of persons who have antibody titers above a protective threshold or the duration of serological immunity that may exist in the context of a broader population. Although many countries, including the United States [8] and Canada [9], continue to recommend booster vaccination every 10 years, the United Kingdom recommends no adult booster vaccinations after the initial 5-dose childhood immunization series [10]. The safety and success of more moderate European vaccination programs with longer intervals between booster vaccinations [10–13] indicate that the current 10-year booster vaccination schedule should be reexamined. In the current study, we measured the magnitude and duration of immunity to these vaccine antigens to determine whether antibody responses declined more rapidly in an aged population and to provide an evidence-based evaluation of current tetanus and diphtheria vaccine programs.
MATERIALS AND METHODS
Subjects and Study Design
Subjects (n = 546) were recruited from 2002 to 2008 (526 in 2002–2004) to study the duration of immunity after vaccination against smallpox [14], tetanus and diphtheria. Further details are provided in the Supplementary Data. All studies were approved by the institutional review board of Oregon Health & Science University.
Analysis of Toxin-Specific Antibody Titers
Tetanus and diphtheria toxin-specific antibodies were measured using enzyme-linked immunosorbent assay (ELISA) [7, 14], double-antigen tetanus ELISA [15], or diphtheria neutralization assays [16] after calibration with international serum standards (see Supplementary Data).
Statistics
The duration of antibody-mediated protection by cross-sectional analysis was examined with a regression model using diphtheria- or tetanus-specific antibody levels at >1 year after vaccination as the dependent variable and time after vaccination (years) as the independent variable in the following equation: log(antibody titer) = α + β * years + ε, where α represents mean log titer at time of vaccination; β, decay rate or average log titer change per year; and ε, error term. In this model, the half-life is independent of the reference time point and is estimated as log(0.5)/β. For longitudinal analysis, we used the random intercept and slope model to account for correlated errors within the same individual subject, as described elsewhere [7]. Statistical analyses were performed using Statistical Analysis System software (SAS; version 9.3), with further details provided in the Supplementary Data.
RESULTS
Magnitude and Duration of Immunity to Tetanus
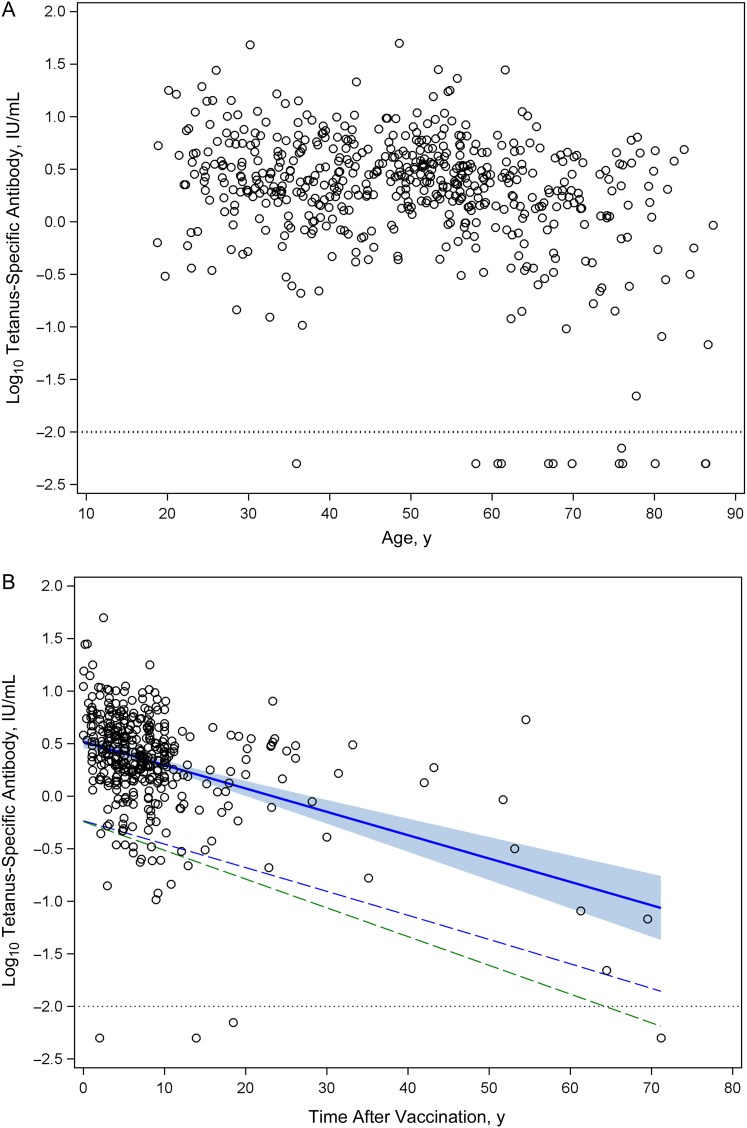
Tetanus vaccines became commercially available in the United States in 1938, but routine vaccination was not widely practiced until the mid-1940s [2]. To determine the magnitude and persistence of tetanus-specific antibody responses in contemporary populations, we measured the levels of immunity as a function of age (Figure 1A) or time after vaccination (Figure 1B). In general, tetanus-specific antibody levels were high, averaging 3.6 IU/mL for the total population, and there was no significant difference (P = .36) in titers between men and women (3.89 and 3.46 IU/mL, respectively) (Table 1). In terms of protective immunity, 99% of subjects <60 years of age (and 97% of the overall population) demonstrated tetanus-specific antibody responses above the protective level of 0.01 IU/mL.
Figure 1.
Humoral immunity to tetanus as a function of age and time after vaccination. Tetanus-specific serum antibody responses were measured in adult subjects and plotted versus age (A) or time after vaccination (B). Dotted line in each panel represents level of antibody required for protection, equivalent to 0.01 IU/mL. B, Solid blue line is the fitted regression line representing the antibody half-life decay rate, and the shaded blue region represents the upper and lower bound of 95% confidence interval (CI) for the cross-sectional antibody half-life estimation. Dashed blue line represents a 1-sided lower bound 95% CI based on a 14-year half-life and indicates when tetanus-specific antibody titers would decline to 95% seroprotection by crossing the protective threshold of 0.01 IU/mL (ie, −2 log10 IU/mL) at 72 years after vaccination. Dashed green line is based on an estimated 11-year half-life [7] and indicates that 95% of the population will remain protected against tetanus for 64 years after vaccination.
Table 1.
Comparison of Antibody Responses to Tetanus and Diphtheria According to Sexa
| Study Population | DT Ab Titer, Mean (SD) IU/mL | DT Ab Half-life (95% CI), y | TT Ab Titer, Mean (SD), IU/mL | TT Ab Half-life (95% CI), y |
|---|---|---|---|---|
| All | 0.35 (0.6) | 27 (18–51) | 3.6 (4.6) | 14 (11–17) |
| Men | 0.31 (0.39) | 21 (14–46) | 3.89 (5.24) | 14 (11–19) |
| Women | 0.37 (0.68) | 42 (20–∞) | 3.46 (4.26) | 13 (10–18) |
Abbreviations: Ab, antibody; CI, confidence interval; DT, diphtheria toxin; SD, standard deviation; TT, tetanus toxin.
a Antibody titers to DT or TT were determined from 546 subjects (187 men and 359 women). Antibody half-life estimations (in years) were based on 392 subjects from whom data was available (134 men and 258 women).
We next determined the half-life of tetanus-specific antibody as a function of time after last vaccination (Figure 1B). The overall half-life was found to be 14 years (95% CI, 11–17). There was no significant difference (P = .59) in antibody decay rates between men and women (half-life, 14 and 13 years, respectively) (Table 1). The duration of protective immunity is a function of the magnitude of the serum antibody response and the time it takes for antibody titers to decline to nonprotective levels. Based on these parameters, the model predicts that 95% of the population will remain seroprotected against tetanus for up to 72 years without further booster vaccination (Figure 1B). In a previous longitudinal study, the tetanus-specific antibody half-life was estimated to be 11 years [7]. If this more conservative antibody half-life is applied to the current population-based data set, we find that 95% of the subjects will remain seroprotected for 64 years without requiring further vaccination.
Magnitude and Duration of Immunity to Diphtheria
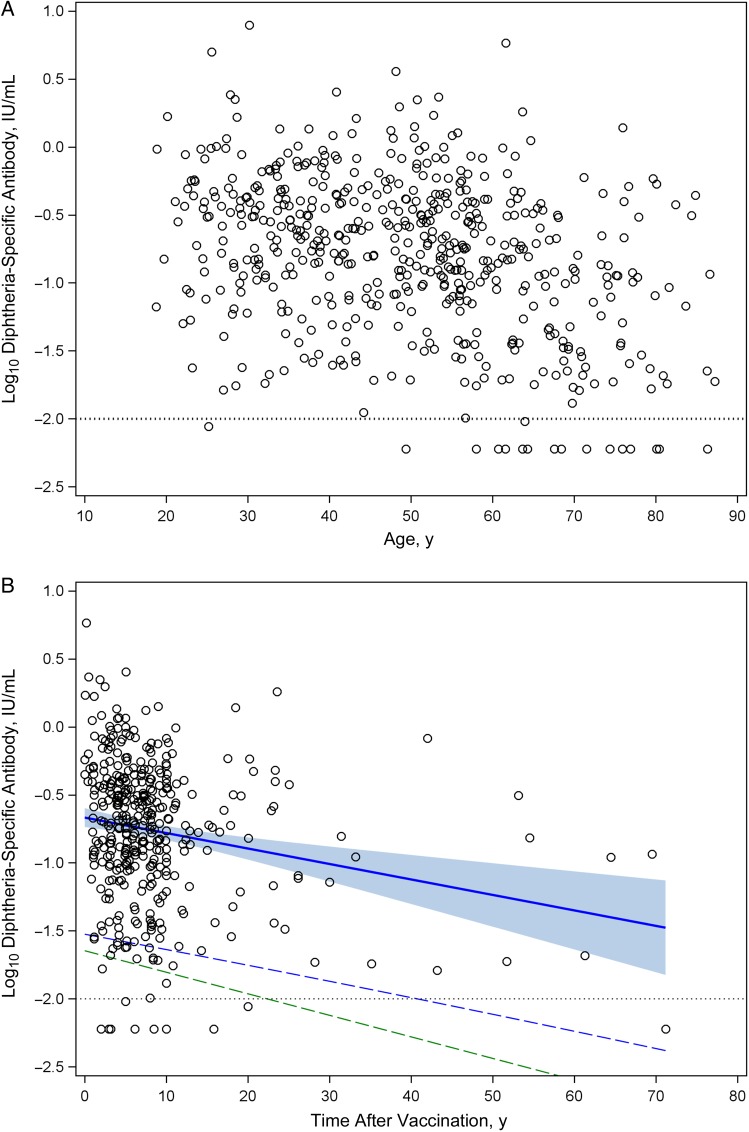
In contrast to tetanus, diphtheria represents a communicable disease and although relatively rare in developed countries, it is believed that vaccine coverage of 80%–85% must be maintained to reduce the threat of an outbreak [17]. Diphtheria toxoid is commonly administered as a combined vaccine with tetanus toxoid and we measured the duration of immunity to diphtheria to determine if protective levels of immunological memory were similar to that observed for tetanus (Figure 2). The mean antibody titer to diphtheria was 0.35 IU/mL, and there were no significant differences (P = .23) in titers between men and women (0.31 and 0.37 IU/mL, respectively) (Table 1). Approximately 99% of subjects <60 years of age (and 97% of the overall population) showed diphtheria-specific antibody responses that were above the protective level of 0.01 IU/mL.
Figure 2.
Humoral immunity to diphtheria as a function of age and time after vaccination. Diphtheria-specific serum antibody responses were measured in adult subjects and plotted versus age (A) or time after vaccination (B). Dotted line in each panel represents level of antibody required for protection, equivalent to 0.01 IU/mL. B, Solid blue line is the fitted regression line representing the antibody half-life decay rate, and the shaded blue region represents the upper and lower bound of 95% confidence interval (CI) for the antibody half-life estimation. Dashed blue line represents a 1-sided lower bound 95% CI based on a 27-year half-life and indicates when diphtheria-specific antibody titers would decline to 95% seroprotection by crossing the protective threshold of 0.01 IU/mL (ie, −2 log10 IU/mL) at 42 years after vaccination. Dashed green line is based on an estimated 19-year half-life [7] and indicates that 95% of the population will remain protected against diphtheria for 30 years after vaccination.
Based on analysis of antibody levels as a function of time after vaccination (Figure 2B), we found that diphtheria-specific immunity declined with a 27-year half-life (95% CI, 18–51 years) (Table 1). Although the overall antibody levels to diphtheria were lower than observed with tetanus, the antibody response to diphtheria showed a longer half-life, and 95% of the population is predicted to maintain seroprotection for 42 years without requiring further booster vaccination (Figure 2B). Diphtheria-specific antibody was shown elsewhere to have a 19-year half-life when measured longitudinally [7], and if this half-life is applied to the data set, 95% of adults are predicted to remain seroprotected for 30 years without additional booster vaccinations.
Impact of Age on Serological Memory to Tetanus and Diphtheria
Potential age-associated defects in immunological function represent an important parameter when measuring herd immunity because this may alter the interpretation of the data set and the levels of protective immunity within a given cohort. Of the 13 subjects lacking tetanus-specific immunity (<0.01 IU/mL), 11 of 13 (85%) were >60 years old (mean age, 69 years; median, 70 years; range, 36–86 years). Likewise, of the 17 subjects lacking diphtheria-specific immunity (<0.01 IU/mL), 14 of 17 (82%) were >60 years old (mean age 67 years; median, 68 years; range, 25–86 years). Four subjects (aged 58, 61, 80, and 86 years) lacked immunity to both toxins. These results indicate that individuals older than 60 years have an increased likelihood of low immunity to tetanus or diphtheria.
When stratified by age, cross-sectional analysis (Figures 1A and 2A) indicated that older subjects (age, ≥50 years) had lower antibody titers to tetanus and diphtheria than younger subjects (age, <50 years) (Table 2), and this is consistent with findings of prior studies [18, 19]. To determine whether differences in antitoxin immunity were due to more rapid antibody decay rates in older individuals, we used cross-sectional analysis to compare antibody half-life estimates between subjects <50 years and those ≥50 years of age (Table 2). Tetanus-specific antibody decay rates were essentially the same in the younger and older age groups (half-life [95% CI], 11 [8–20] and 14 [10–20] years, respectively; P = .11). Similar results were observed when comparing subjects aged <60 (n = 407) or ≥60 (n = 133) years (half-life [95% CI], 15 [11–24] vs 16 [11–26] years).
Table 2.
Comparison of Antibody Responses to Tetanus and Diphtheria According to Agea
| Age Group, y | DT Ab Titer, Mean (SD), IU/mL |
DT Ab Half-life (95% CI), y |
TT Ab Titer, Mean (SD), IU/mL |
TT Ab Half-life (95% CI), y |
||
|---|---|---|---|---|---|---|
| Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | |||
| <50 | 0.43 (0.70) | 24 (12–∞) | 19 (15–25) | 4.16 (5.41) | 11 (8–20) | 11 (10–13) |
| ≥50 | 0.28 (0.48) | 30 (18–84) | 21 (15–34) | 3.05 (3.62) | 14 (11–20) | 15 (11–24) |
Abbreviations: Ab, antibody; CI, confidence interval; DT, diphtheria toxin; SD, standard deviation; TT, tetanus toxin.
a Antibody titers to DT or TT were determined after stratifying the cohort of 540 subjects of known age into age groups of <50 or ≥50 years (both n = 270). Antibody half-life estimates (in years) were determined after stratifying 388 subjects of known age and estimated time after vaccination into age groups of <50 (n = 177) or ≥50 (n = 211) years. Longitudinal data [7] were stratified by age for comparison with the cross-sectional analysis.
When the durability of immunity against diphtheria was compared, antibody half-life was also similar between individuals <50 or ≥50 years of age (half-life [95% CI], 24 [12 years to ∞] and 30 [18–84] years, respectively), although the modest increase in antibody half-life among older subjects was statistically significant (P = .02). Diphtheria-specific immunity was also long-lived among subjects <60 or ≥60 years of age (half-life [95% CI], 23 [13–76] vs 57 [23 to ∞]). Together, these results indicate that serological memory to these 2 bacterial toxins does not decay faster in older populations.
DISCUSSION
We examined the levels and duration of serological memory after vaccination against tetanus and diphtheria toxins in a cross-sectional analysis of >500 adults. Protective levels of antitoxin antibodies were observed in 99% of subjects <60 years old (approximately 97% of the total all-age population), and vaccine-induced antibody responses declined with estimated half-lives of 14 years for tetanus and 27 years for diphtheria. Mathematical analysis of the magnitude and decay rate of antitoxin antibody responses predicts that 95% of the adult population remain protected for ≥30 years after vaccination.
Serosurveys conducted with samples obtained >20 years ago in the United States indicated that only about 58% [20], 70% [18] or 72% [19] of adults had protective immunity to tetanus, and only 51%–61% showed protective immunity to diphtheria [19, 20]. One key difference between these prior publications and our current study is the working definition of protective immunity. In prior studies [18–20], a protective antibody titer was defined as ≥0.15 IU/mL for tetanus and ≥0.10 IU/mL for diphtheria [19, 20]. These threshold values are likely to underestimate the levels of protection because studies in humans and animal models have shown that 0.01 IU/mL is a protective level of immunity for tetanus [2, 21–23] or diphtheria [17, 24–26]. For comparison with prior serosurveys [18–20], if we use ≥0.15 IU/mL as the protective threshold for tetanus and ≥0.10 IU/mL for diphtheria, we found that 96% of the population would be protected against tetanus, and 69% against diphtheria.
These proportions of protected individuals are higher than observed in prior studies [18–20] performed with samples obtained in the late 1980s and early 1990s but more comparable to findings in recent studies showing similar durability of anti-tetanus immunity among European American and African American military personnel [27]. Interestingly, seroprotection rates in Finland showed similar improvements over time [28]; the percentage of individuals with protective immunity to diphtheria (0.01 IU/mL) within the 30–39-year age group increased from 77% to 92% from the 1980s to the 1990s and further improved to 98% protection in 2000–2001. This change was believed to be due to improved vaccination coverage during these periods of time, and it highlights the importance of reevaluating population serostatus to different vaccines, which may change over time in parallel with changes in vaccination policies and improvements in vaccination coverage.
In concordance with prior epidemiological studies [18–20], we found that immunity to tetanus and diphtheria was lower in older subjects (Figures 1A and 2A; Table 2). These findings could be due to a “cohort effect,” in which older subjects born in the 1920s and 1930s (ie, before initiation of routine vaccination) may not have received the full childhood vaccination series. Alternatively, lower antibody titers in older subjects could result from age-related immune senescence in which antibody responses decay more rapidly with advanced age. We were able to examine this question in more detail by dividing adults into 2 age groups and measuring antibody half-life longitudinally as well as cross-sectionally (Table 2). This analysis was informative because we discovered that the duration of antitoxin immunity (ie, antibody half-life) was not affected by age regardless of whether we measured immunity to tetanus or to diphtheria or if we stratified the cohorts according to an age cutoff of 50 or 60 years.
Tetanus is rare in the United States, with approximately 27 cases reported annually from 2008 to 2012 [29]. Although tetanus is highly lethal in unvaccinated patients, disease severity is sharply reduced in fully vaccinated individuals [2, 30–33]. Of 124 cases of tetanus, all 14 deaths occurred in patients who had received <3 doses of vaccine or had unknown vaccination status, whereas all 110 patients (100%) who received ≥3 doses of vaccine survived [31, 32]. From 2001 to 2008, the Centers for Disease Control and Prevention (CDC) identified 233 tetanus cases (29 cases per year) for an annual incidence of 0.1 case per 1 million persons [33]. The overall case-fatality rate among the vaccinated, the unvaccinated, and those with unknown vaccination history was estimated at 13.2%. This indicates that tetanus is exceedingly rare, with a mortality rate of approximately 3 deaths per year among a population of >300 million.
Diphtheria is nearly nonexistent in the United States, with no cases reported from 2008 to 2012 [29]. This indicates that it is less common than other rare reportable bacterial diseases, including tularemia, plague, cholera, or anthrax [29]. Serious adverse events after vaccination against tetanus and diphtheria are uncommon, but anaphylaxis is estimated at 1.6 cases per million doses, and brachial plexus neuropathy may occur at a rate of 5–10 cases per million doses [3]. When multiplied by an estimated 16 million doses of tetanus and diphtheria (Td) vaccine administered annually in the United States [32], severe adverse events include approximately 25 severe allergic events and 80–160 cases of brachial plexus neuritis. Because overimmunization provides a negligible increase in protection [32], this suggests that the risk-benefit ratio of a decennial adult booster vaccination schedule should be reexamined.
The data provided in this study indicate that adult booster vaccination with Td every 10 years may no longer be necessary to maintain protective immunity. Others have questioned the decennial vaccination schedule [32, 34, 35], and countries such as the United Kingdom do not vaccinate adults after they have received their childhood vaccination series [10]. Although the impact of advanced age was not determined, a recent cross-sectional study performed in US military personnel also found immunity to tetanus to be long-lived (antibody half-life, 51.6 years) [27]. Indeed, the World Health Organization recommends only a single booster to be given at the time of first pregnancy or during military service [36]. If a revised adult Td vaccination schedule were implemented, we believe that a simplified age-based vaccination plan could be designed to involve a single vaccination at age 30 years and again at age 60 years. By substituting tetanus, diphtheria, and acellular pertussis (Tdap) for Td at age 30 years, this would not affect the currently recommended 1-time dose of Tdap for adults. Likewise, Tdap vaccination of pregnant women should be continued as a preventive step toward protecting infants from Bordatella pertussis. Vaccination of adults at age 60 years is important, because the majority of unprotected individuals reside within this age group [2]. The current adult vaccination guidelines already recommend varicella zoster virus vaccination at age 60–64 years, and it is possible that these 2 vaccines could be administered on the same visit to further increase patient compliance.
The large diphtheria outbreaks that occurred in the former Soviet Union in the 1990s provide several lessons on the importance of maintaining herd immunity through broad vaccination coverage [37–39]. In the mid-1980s, diphtheria outbreaks that occurred mainly in Russia ranged from 839 to nearly 2000 cases per year before reaching the peak of the epidemic in 1994–1995, with >98 000 cases and 3400 deaths reported in the Newly Independent States of the former Soviet Union [37]. Universal childhood immunization began in the late 1950s, but by the 1980s, the number of booster doses was reduced [37, 39], and with decreased public support of childhood vaccination and a vocal antivaccine movement [39] coverage of infants fell to between 60% and 80% or as low as 18%–59% in some areas [38, 39]. In general, adult immunization was not recommended [37], and vaccine coverage was estimated at 20% in 1990 [38]. The combination of low vaccination coverage among both children and adults resulted in a population that was susceptible to explosive outbreaks of diphtheria.
The circumstances leading to these large outbreaks in the former Soviet Union, however, are very different from those facing the United States and many other developed nations today. In contrast to hundreds of diphtheria cases reported each year in the former Soviet Union in the pre-epidemic 1980s, only 5 cases of diphtheria have been reported in the United States in the last 15 years [40]. Vaccination coverage of children is >90%, and if antivaccine sentiment can be held in check through continued education of healthcare workers and the general public, this high level of herd immunity will be maintained. Likewise, unlike the former Soviet Union in which there was a highly susceptible unvaccinated adult population, our results show that approximately 97% of adults have protective levels of anti-diphtheria antibodies (Figure 2A). Based on longitudinal and cross-sectional analysis of antibody half-life (Tables 1 and 2) and a model of booster vaccination every 30 years, protective immunity is predicted to remain at ≥95% (Figure 2B).
Modification of decennial Td vaccination may have a substantial impact on healthcare costs. Among adults aged 19–64 years, 63%–64% self-report that they comply with the decennial Td revaccination schedule [41, 42]. Of 234 million adults (2010 census), this would indicate that approximately 150 million adults have been vaccinated within the last 10 years, or approximately 15 million doses administered per year, similar to previous estimates [32]. This is also in line with the number of adult Td vaccine doses distributed by the CDC (eg, approximately 15.2 million doses distributed in 1998) [37]. At a cost of $28 per dose (CDC adult vaccine price list [43], this equals $420 million per year spent on adult Td booster vaccination. If this were changed to a 30-year schedule, then the costs would be reduced by two-thirds, equating to a reduction of approximately $280 million per year in healthcare costs (ie, >$1 billion in cost savings within 4 years). As noted elsewhere [32], in addition to substantial cost savings, other advantages of modifying the adult Td booster vaccination interval include (1) improved compliance with age-based recommendations and a simplified age-specific vaccination schedule, (2) better acceptance of recommendations based on immunological and epidemiological data and current risk-benefit analyses rather than conformity to historical convention [32], and (3) reduction of vaccine-associated adverse events owing to overimmunization.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the study subjects for their time and participation in this research study and M. W. Lewis for blood collection and processing.
Author contributions. M. K. S. designed the study. E. H., A. T., E. A. P., I. J. A., and A. E. R. performed assays and analyzed the data. I. J. A., M. M., and Z. C. performed the statistical analysis. All authors contributed to the writing of the manuscript. M. K. S. had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported in part by the National Institutes of Health (Public Health Service grant R01 AI098723 to E. H., A. T., E. A. P., A. E. R., and M. K. S. and grant U01 AI082196 to E. H., A. T., E. A. P., I. J. A., and M. K. S.), the Oregon Clinical & Translational Research Institute Biostatistics & Design Program (M. M. and Z. C.), and the Oregon National Primate Research Center (grant, 8P51 OD011092-53 to M. K. S.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Roush SW, Murphy TV. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007; 298:2155–63. [DOI] [PubMed] [Google Scholar]
- 2.Wassilak SG, Roper MH, Kretsinger K, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia: Saunders Elsevier, 2008. [Google Scholar]
- 3.Vaccine Safety Committee. Diphtheria and tetanus toxoids. Adverse events associated with childhood vaccines: evidence bearing on causality. In: Stratton KR, Howe CJ, Johnston RB, eds. Research strategies for assessing adverse effects associated with vaccines. Washington, DC: National Academy Press, 1994:67–117. [Google Scholar]
- 4.National Communicable Disease Center. Recommendation of Public Health Service Advisory Committee on Immunization Practices: diphtheria, tetanus, and pertussis vaccines: tetanus prophylaxis in wound management. MMWR Morb Mortal Wkly Rep 1966; 15:416–8. [Google Scholar]
- 5.Peebles TC, Levine L, Eldred MC, Edsall G. Tetanus-toxoid emergency boosters: a reappraisal. N Engl J Med 1969; 280:575–81. [DOI] [PubMed] [Google Scholar]
- 6.Bonsignori M, Moody MA, Parks RJ et al. . HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J Immunol 2009; 183:2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007; 357:1903–15. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Immunization schedules. Available at: http://www.cdc.gov/vaccines/schedules/ Accessed 6 January 2016. [Google Scholar]
- 9.Public Health Agency of Canada. Canadian immunization guide. Available at: http://www.phac-aspc.gc.ca/publicat/cig-gci/p03-02-eng.php Accessed 6 January 2016. [Google Scholar]
- 10.Wagner KS, White JM, Andrews NJ et al. . Immunity to tetanus and diphtheria in the UK in 2009. Vaccine 2012; 30:7111–7. [DOI] [PubMed] [Google Scholar]
- 11.The National Board of Health and Welfare. Regulations for the prevention of diphtheria and tetanus (SOSFS 1990-10-21,ISSN: 0346-6000) [in Swedish]. Stockholm, Sweden: Socialstyrelsen, 1990.
- 12.The National Board of Health and Welfare. Recommendations for the prevention of diphtheria and tetanus in adults. (2009-130-5) [in Swedish], 2009. Available at: https://www.folkhalsomyndigheten.se/pagefiles/20442/rekommendationer-for-profylax-till-vuxna-mot-difteri-och-stelkramp-2009-130-5.pdf. Accessed 17 February 2016.
- 13.Siegrist CA. New Swiss recommendations for adult boosters against pertussis, tetanus and diphtheria [in French]. Rev Med Suisse 2012; 8:125–8. [PubMed] [Google Scholar]
- 14.Hammarlund E, Lewis MW, Hansen SG et al. . Duration of antiviral immunity after smallpox vaccination. Nature Medicine 2003; 9:1131–7. [DOI] [PubMed] [Google Scholar]
- 15.Rosskopf U, Noeske K, Werner E. Efficacy demonstration of tetanus vaccines by double antigen ELISA. Pharmeuropa Bio 2005:31–52. [PubMed] [Google Scholar]
- 16.Usuwanthim K, Pootong A, Chaisri U et al. . Murine monoclonal antibodies neutralizing the cytotoxic activity of diphtheria toxin. Asian Pac J Allergy Immunol 2008; 26:47–55. [PubMed] [Google Scholar]
- 17.Vitek CR, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia: Saunders Elsevier, 2008. [Google Scholar]
- 18.Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med 1995; 332:761–6. [DOI] [PubMed] [Google Scholar]
- 19.McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002; 136:660–6. [DOI] [PubMed] [Google Scholar]
- 20.Kruszon-Moran DM, McQuillan GM, Chu SY. Tetanus and diphtheria immunity among females in the United States: are recommendations being followed? Am J Obstet Gynecol 2004; 190:1070–6. [DOI] [PubMed] [Google Scholar]
- 21.Wolters KL, Dehmel H. Abschliessende untersuchungen uber die Tetanus Prophylaxe durch active Immunisierung. Zeitschrift fur Hyeitschrift 1942; 124:326–32. [Google Scholar]
- 22.Scheibel I. The uses and results of active tetanus immunization. Bull World Health Organ 1955; 13:381–94. [PMC free article] [PubMed] [Google Scholar]
- 23.McComb JA. The prophylactic dose of homologous tetanus antitoxin. N Engl J Med 1964; 270:175–8. [DOI] [PubMed] [Google Scholar]
- 24.Pappenheimer AM., Jr The Schick test, 1913–1958. Int Arch Allergy Appl Immunol 1958; 12:35–41. [DOI] [PubMed] [Google Scholar]
- 25.Ipsen J. Circulating antitoxin at the onset of diphtheria in 425 patients. J Immunol 1946; 54:325–47. [PubMed] [Google Scholar]
- 26.Bjorkholm B, Bottiger M, Christenson B, Hagberg L. Antitoxin antibody levels and the outcome of illness during an outbreak of diphtheria among alcoholics. Scand J Infect Dis 1986; 18:235–9. [DOI] [PubMed] [Google Scholar]
- 27.Garman L, Vineyard AJ, Crowe SR et al. . Humoral responses to independent vaccinations are correlated in healthy boosted adults. Vaccine 2014; 32:5624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olander RM, Auranen K, Harkanen T, Leino T. High tetanus and diphtheria antitoxin concentrations in Finnish adults—time for new booster recommendations? Vaccine 2009; 27:5295–8. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Notifiable diseases and mortality tables. MMWR Morb Mortal Wkly Rep 2013; 62:ND-86. [PubMed] [Google Scholar]
- 30.Pascual FB, McGinley EL, Zanardi LR, Cortese MM, Murphy TV. Tetanus surveillance—United States, 1998–2000. MMWR Surveill Summ 2003; 52:1–8. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Tetanus Surveillance–United States, 1995–1997. MMWR Morb Mortal Wkly Rep 1998; 47(SS-2):1–13.9450721 [Google Scholar]
- 32.Gardner P. Issues related to the decennial tetanus-diphtheria toxoid booster recommendations in adults. Infect Dis Clin North Am 2001; 15:143–53, ix–x. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Tetanus surveillance—United States, 2001–2008. MMWR Morb Mortal Wkly Rep 2011; 60:365–9. [PubMed] [Google Scholar]
- 34.Balestra DJ, Littenberg B. Should adult tetanus immunization be given as a single vaccination at age 65? a cost-effectiveness analysis. J Gen Intern Med 1993; 8:405–12. [DOI] [PubMed] [Google Scholar]
- 35.LaForce FM. Routine tetanus immunizations for adults: once is enough. J Gen Intern Med 1993; 8:459–60. [DOI] [PubMed] [Google Scholar]
- 36.WHO. Tetanus vaccine WHO position paper. Wkly Epidemiol Rec 2006; 20:198–208. [Google Scholar]
- 37.Golaz A, Hardy IR, Strebel P et al. . Epidemic diphtheria in the Newly Independent States of the former Soviet Union: implications for diphtheria control in the United States. J Infect Dis 2000; 181(suppl 1):S237–43. [DOI] [PubMed] [Google Scholar]
- 38.Galazka A. Implications of the diphtheria epidemic in the former Soviet Union for immunization programs. J Infect Dis 2000; 181(suppl 1):S244–8. [DOI] [PubMed] [Google Scholar]
- 39.Dittmann S, Wharton M, Vitek C et al. . Successful control of epidemic diphtheria in the states of the former Union of Soviet Socialist Republics: lessons learned. J Infect Dis 2000; 181(suppl 1):S10–22. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Diphtheria: epidemiology and prevention of vaccine-preventable diseases. The pink book: course textbook. 13th ed 2015. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/dip.html Accessed 8 January 2016.
- 41.Williams WW, Lu PJ, O'Halloran A et al. . Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102. [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Adult vaccination coverage—United States, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:66–72. [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. CDC vaccine price list. Available at: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html#f1 Accessed 6 January 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.