Experts attending tuberculous meningitis meeting ask World Health Organization to revise current recommendations regarding tuberculous meningitis.
Keywords: tuberculous, meningitis, diagnosis, guideline, perspective
Abstract
Tuberculous meningitis (TBM) is the most severe form of tuberculous with substantial mortality. In May 2015, 54 researchers from 10 countries met in Da Lat, Vietnam, to discuss advances in TBM. Among the attendees were researchers involved in pivotal studies on the use of Xpert MTB/Rif for TBM diagnosis. Attendees discussed the 2014 World Health Organization strong recommendation favoring the use of Xpert “in preference to conventional microscopy and culture as the initial diagnostic test for cerebrospinal fluid (CSF) if the sample volume is low or if additional specimens cannot be obtained to make a quick diagnosis.” Attendees were concerned that the limitations of Xpert testing for TBM are not emphasized. Clear guidance is needed for the investigational pathway for TBM, including recommendations on the diagnostic package of investigations, which does not stop with Xpert testing. Second, emphasis on the large CSF volumes (ideally 8–10 mL) needed for Xpert testing is required. Guidelines should also emphasize that TBM is a medical emergency and early treatment reduces mortality.
Tuberculous meningitis (TBM) is the most severe form of tuberculous with substantial mortality. In May 2015, 54 TBM researchers from 10 countries met in Da Lat, Vietnam, to discuss advances in TBM. Among the attendees were researchers involved in pivotal studies on the use of Xpert MTB/Rif (Xpert, Cepheid, Sunnyvale, California) for TBM diagnosis [1, 2]. During the meeting, the World Health Organization (WHO) strong recommendation favoring the use of Xpert “in preference to conventional microscopy and culture as the initial diagnostic test for cerebrospinal fluid” (CSF) if the sample volume is low or if additional specimens cannot be obtained to make a quick diagnosis” was discussed [3, 4]. In agreement with previous concerns [5], attendees concurred that caution is required in interpreting the recommendations of the 2014 WHO Xpert MTB/Rif implementation manual [4], as the limitations of Xpert testing for TBM are not emphasized.
Rapid TBM diagnosis and treatment is a strong prognostic indicator for reduced death and neurologic deficit. Available TBM diagnostic tests are inadequate, for the most part due to low bacillary loads in CSF [6]. In locations where TBM is less common, missed diagnosis and delayed treatment are common [7]. CSF Ziehl-Neelsen staining is rapid, but sensitivity is poor (approximately 10%–20%) [2, 8]. Although mycobacterial culture is more sensitive (approximately 60%–70%), culture often takes ≥2 weeks [1, 8]. Clinical diagnostic algorithms have been developed, but their use outside of populations in which algorithms were derived is largely untested. A consensus clinical TBM case definition was developed to standardize research but was not intended to aid diagnosis in clinical practice [9]. Clinicians are therefore often left with a diagnostic dilemma [10], and TBM diagnosis can only be made at autopsy.
Given the lack of adequate TBM diagnostics, Xpert appeals as a new diagnostic tool. However, PCR testing of CSF for TBM diagnosis has important limitations. Three cohort studies have evaluated the use of Xpert on CSF in TBM suspects [1, 2, 8]. The first study, from South Africa, found 67% sensitivity for Xpert detecting microbiologically proven TBM but only 36% sensitivity against a clinical case definition in a predominantly human immunodeficiency virus (HIV)-infected population [2]. In a subset of 27 TBM cases using higher volumes (3 mL vs 1 mL), Xpert exhibited 82% sensitivity [2].
In Vietnam, Nhu and colleagues found 59% sensitivity for Xpert used on CSF vs a clinical TBM case definition [1]. This study routinely centrifuged CSF and then divided the pellet apportioning 40% for Xpert testing and 20% for each of microscopy, culture, and storage [1]. The volumes used for Xpert testing were modest with 78% of persons having an effective volume used for Xpert testing of ≤2 mL.
The WHO 2013 meta-analysis to assess the diagnostic performance of Xpert for TBM included the cohorts by Patel and by Nhu and numerous studies with small numbers of meningitis cases among extrapulmonary tuberculous cohorts (total 117 cases in 16 studies) [1–3]. The pooled Xpert sensitivity in this meta-analysis was 79.5% (95% confidence interval [CI], 62.0%–90.2%) with culture as a reference gold standard. When a clinical gold standard was used, sensitivity was only 55% with 84% negative predictive value (NPV). In high prevalence TBM settings, this NPV equates to 1 in 6 persons with TBM tested by Xpert being missed.
A 2015 Ugandan study (not included in the WHO meta-analysis) showed with small numbers (n = 18) that centrifugation of larger CSF volumes (median: 6 mL, interquartile range: 4–10 mL) resulted in 72% (13/18) sensitivity, which was equivalent to culture (71%, 12/17) [8]. Using 2 mL of un-centrifuged CSF had only 28% sensitivity (5/18) [8]. The reference standard was any positive polymerase chain reaction or culture. Importantly, neither Xpert nor culture of centrifuged CSF would have detected each case of microbiologically-proven TBM, with only 39% of TBM cases being positive both by culture and Xpert testing [8]. These findings emphasize “missed cases” will occur should clinicians rely solely on Xpert to diagnose TBM. Even when using large volumes of centrifuged CSF, the NPV was 94% (77/82) in a moderate tuberculous setting [8]. As Boyles and Thwaites noted [5], for a test to adequately rule out a life-threatening disease, a high NPV is needed. Such a TBM test does not exist. Even next generation molecular tests (eg, Xpert Ultra) are unlikely to exceed the sensitivity of culture.
Considering the current evidence [1, 2, 8], we wish to caution against the use of Xpert as the sole diagnostic test for TBM, as this practice may lead to missed TBM cases and deaths. We encourage clinicians to use large CSF volumes with Xpert, but to remember that a negative Xpert test does not exclude TBM. Xpert should be used in combination with other diagnostic tests (eg, cryptococcal antigen of blood and CSF), clinical findings, and when possible, radiologic data to inform their overall suspicion for TBM.
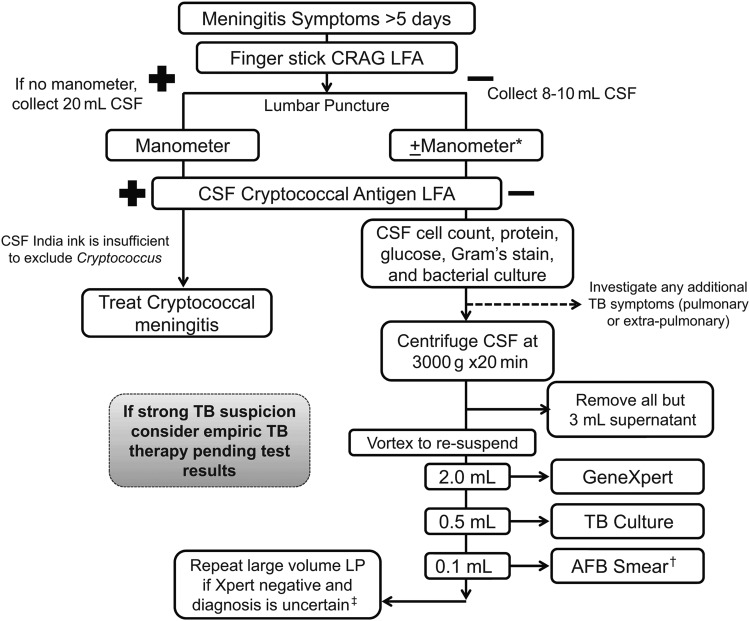
In Figure 1, we describe our preferred methodology for investigating meningitis suspects with symptoms for >5 days in settings with moderate to high endemic levels of tuberculous and HIV. As stated above, we encourage the early use of the cryptococcal antigen lateral flow assay on blood and CSF given the high sensitivity, ease of use, and low cost. If symptoms of tuberculous exist they should be investigated as able (eg, chest radiograph and sputum isolation for pulmonary tuberculous, lymph node biopsy for suspected tuberculous lymphadenitis, etc.). Basic CSF testing such as total protein, cell count, and glucose should be performed with the idea of risk stratification—not definite diagnosis. Finally, we recommend obtaining 8–10 mL of CSF if possible (recognizing this may be difficult) and using centrifugation to concentrate the specimen. All but approximately 2.5 mL supernatant should be drawn off and the remaining volume reconstituted with 2 mL being used for Xpert and 0.5 mL for culture (and 0.1 mL for acid-fast bacillus [AFB] stain if included). We strongly advocate empiric therapy in the case of high suspicion at the time of testing and retesting if strong suspicion in the face of negative testing.
Figure 1.
Suggested algorithm for diagnosis of Subacute Meningitis in population with high human immunodeficiency virus (HIV) and tuberculous (TB) prevalence. The algorithm is the suggested method of the authors and is meant as guidance. The circumstances of any individual clinical situation may render deviation from this algorithm preferable, and expert physician opinion remains vital. The figure describes our preferred method for investigating meningitis suspects with symptoms for >5 days in settings with moderate to high endemic levels of TB and HIV. *If possible use a manometer in these cases as well; however, if cost is a limitation a manometer is of most use in those suspected to have cryptococcal meningitis and so could be omitted in cases not suspected to have cryptococcal meningitis. †In areas of high utility (eg, southeast Asia), acid-fast bacillus (AFB) microscopy should be included if laboratory technicians have adequate time to perform a thorough investigation. However, the AFB microscopy should be omitted in other settings where sensitivity is much lower. ‡Tests to exclude other causes for neurologic syndromes should be performed as clinically indicated and available (eg, cerebrospinal fluid [CSF] syphilis serology, viral polymerase chain reaction testing, cytology, anti-N-methyl D-aspartate [NMDA] receptor antibodies). Consider brain imaging (computerized tomography/magnetic resonance imaging) as available for focal neurologic deficit(s) and/or to aid in tuberculous meningitis diagnosis.
Abbreviations: CRAG, cryptococcal antigen; LFA, lateral flow assay; LP, lumbar puncture.
We would encourage WHO to revise the current recommendations to first emphasize the limitations of Xpert for TBM; second, provide clear guidance on the investigational pathway for TBM, including recommendations on the diagnostic package of investigations; and third, emphasize the need for large CSF volumes (>5 mL). Guidelines should also emphasize that TBM must be considered a medical emergency and treatment initiated immediately (and often empirically) to reduce mortality.
Notes
Acknowledgments. The Tuberculous Meningitis International Meeting was convened by the Oxford University Clinical Research Unit and the Clinical Infectious Diseases research Initiative of the University of Cape Town with support from the Li Ka Shing foundation and Wellcome Trust, UK. N. C. B. and D. R. B. are supported by the National Institute of Health (R01NS086312, T32AI055433), S. M. and R. J. W. are supported by the Wellcome Trust (097254; 104803 and 084323 respectively).
Tuberculous Meningitis International Research Consortium members include. Rob Aarnoutse, Reinout Van Crevel, Aarjan van Laarhoven, Sofiati Dian (Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands); Nathan C Bahr (University of Kansas Medical Center, Kansas City, USA); David R Boulware (University of Minnesota, Minneapolis, USA); Maxine Caws (Liverpool School of Tropical Medicine, Liverpool, United Kingdom); Mark R Cronan, David Tobin (Duke University School of Medicine, Durham, USA); Kelly Dooley (John Hopkins University School of Medicine, Baltimore, USA); Sarah Dunstan (University of Melbourne, Melbourne, Australia); Guo-dong Feng, Xiaodan Shi, Ting Wang (Fourth Military Medical University, Xi′an, People's Republic of China); Anthony Figaji, Suzaan Marais, Helen McIlleron, Graeme Meintjes, Ursula Rohlwink (University of Cape Town, South Africa); Ahmad Rizal, Rovina Ruslami (Padjadjaran University, Bandung, Indonesia); Ravindra K Garg (King George Medical University, Lucknow, India); Mudit Gupta, Rakesh K Gupta (Fortis Memorial Research Institute, Gurgaon, India); Sneha Gupta, Rada Savic (University of California, San Francisco, USA); Anna D Heemskerk, Thương Thụy Thương Nguyễn, Mai Thị Hoàng Nguyễn, Vijay Srinivasan, Guy Thwaites, Trâm Thị Bích Trần, Thịnh Thi Vân Trần, Anh Thi Ngọc Trần, Trang Hồng Yêng Võ, Marcel Wolbers (Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam); Jayantee Kalita, Usha K Misra (Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India); Rachel Lai (The Francis Crick Institute, London, United Kingdom); Ben J Marais, Mai Quỳnh Trinh (University of Sydney, Sydney, Australia); Bằng Đức Nguyễn, Yến Bích Nguyễn (Pham Ngoc Thach Hospital for Tuberculous & Lung Diseases, Ho Chi Minh City, Vietnam); Vinod Patel (University of KwaZulu-Natal, Durban, South Africa); Thomas Pouplin (Mahidol-Oxford Tropical Medicine Research Unit, Bangkok, Thailand); Lalita Ramakrishnan (University of Cambridge, Cambridge, United Kingdom); Johan F Schoeman, Regan Solomons, Ronald Van Toorn (University of Stellenbosch, South Africa); James Seddon (Imperial College, London, United Kingdom); Javeed Shah (University of Washington, Washington, USA); Jaya S Tyagi (All India Institute of Medical Sciences, New Delhi, India); Douwe H Visser (VU University Medical Center, Amsterdam, The Netherlands); Robert J Wilkinson (Imperial College and The Francis Crick Institute, London, United Kingdom and University of Cape Town, South Africa).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: on behalf of the Tuberculous Meningitis International Research Consortium, Rob Aarnoutse, Reinout Van Crevel, Aarjan van Laarhoven, Sofiati Dian, Nathan C. Bahr, David R. Boulware, Maxine Caws, Mark R. Cronan, David Tobin, Kelly Dooley, Sarah Dunstan, Guo-dong Feng, Xiaodan Shi, Ting Wang, Anthony Figaji, Suzaan Marais, Helen McIlleron, Graeme Meintjes, Ursula Rohlwink, Ahmad Rizal, Rovina Ruslami, Ravindra K. Garg, Mudit Gupta, Rakesh K. Gupta, Sneha Gupta, Rada Savic, Anna D. Heemskerk, Thương Thụy Thương Nguyễn, Mai Thị Hoàng Nguyễn, Vijay Srinivasan, Guy Thwaites, Trâm Thị Bích Trần, Thịnh Thi Vân Trần, Anh Thi Ngọc Trần, Trang Hồng Yêng Võ, Marcel Wolbers, Jayantee Kalita, Usha K. Misra, Rachel Lai, Ben J. Marais, Mai Quỳnh Trinh, Bằng Đức Nguyễn, Yến Bích Nguyễn, Vinod Patel, Thomas Pouplin, Lalita Ramakrishnan, Johan F. Schoeman, Regan Solomons, Ronald Van Toorn, James Seddon, Javeed Shah, Jaya S. Tyagi, Douwe H. Visser, and Robert J. Wilkinson
References
- 1.Nhu NT, Heemskerk D, Thu do DA et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol 2014; 52:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel VB, Theron G, Lenders L et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med 2013; 10:e1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Policy update: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Available at: www.who.int/tb/laboratory/xpert_launchupdate Accessed 7 June 2015.
- 4.World Health Organization. Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations. Available at: http://who.int/tb/publications/xpert_implem_manual Accessed 7 June 2015. [PubMed]
- 5.Boyles TH, Thwaites GE. Appropriate use of the Xpert(R) MTB/RIF assay in suspected tuberculous meningitis. Int J Tuberc Lung Dis 2015; 19:276–7. [DOI] [PubMed] [Google Scholar]
- 6.Ho J, Marais BJ, Gilbert GL, Ralph AP. Diagnosing tuberculous meningitis - have we made any progress? Trop Med Int Health 2013; 18:783–93. [DOI] [PubMed] [Google Scholar]
- 7.Sheu JJ, Yuan RY, Yang CC. Predictors for outcome and treatment delay in patients with tuberculous meningitis. Am J Med Sci 2009; 338:134–9. [DOI] [PubMed] [Google Scholar]
- 8.Bahr NC, Tugume L, Rajasingham R et al. Improved diagnostic sensitivity for TB meningitis with Xpert MTB/Rif testing of centrifuged CSF: a prospective study. Int J Tuberc Lung Dis 2015; 19:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marais S, Thwaites G, Schoeman JF et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10:803–12. [DOI] [PubMed] [Google Scholar]
- 10.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013; 12:999–1010. [DOI] [PubMed] [Google Scholar]