High-dose inactivated split-virus influenza vaccine showed higher immunogenicity and relative efficacy compared with standard-dose inactivated split-virus influenza vaccine, irrespective of type of vaccine used the preceding year. The safety profile was also unaffected by previous-year vaccine.
Keywords: human influenza vaccines, inactivated vaccines, phase 3 clinical trial, aged, age 80 and older
Abstract
Background. High-dose inactivated influenza vaccine (IIV-HD) is an alternative to the standard-dose inactivated influenza vaccine (IIV-SD) in the United States for influenza prevention in older adults. IIV-HD improved efficacy relative to IIV-SD in a randomized controlled trial. Recent observational studies suggest that previous influenza vaccination may influence the immunogenicity and effectiveness of current-season vaccination.
Methods. The original study was a double-blind, randomized trial comparing IIV-HD to IIV-SD in adults aged ≥65 years over 2 influenza seasons. A subset of year 1 (Y1) participants reenrolled in year 2 (Y2), receiving vaccine by random assignment in both years. We evaluated the effect of Y1 vaccination on Y2 relative vaccine efficacy (VE), immunogenicity (hemagglutination inhibition [HAI] titers), and safety among reenrolled participants.
Results. Of 14 500 Y1 participants, 7643 reenrolled in Y2. Relative to participants who received IIV-SD both seasons, VE was higher for IIV-HD vaccinees in Y2 (28.3% overall; 25.1% for Y1 IIV-HD, Y2 IIV-HD; and 31.6% for Y1 IIV-SD, Y2 IIV-HD). In multivariate logistic regression models, Y1 vaccine was not a significant modifier of Y2 VE (P = .43), whereas Y2 IIV-HD remained significantly associated with lower influenza risk (P = .043). Compared to administration of IIV-SD in both years, postvaccination HAI titers were significantly higher for patterns that included IIV-HD in Y2. No safety concerns were raised with IIV-HD revaccination.
Conclusions. IIV-HD is likely to provide clinical benefit over IIV-SD irrespective of previous-season vaccination with IIV-HD or IIV-SD. IIV-HD consistently improved immune responses, and no safety concerns emerged in the context of IIV-HD revaccination.
Influenza vaccines are recommended annually in the United States for all persons ≥6 months of age who do not have contraindications [1]. This practice is supported by data from a randomized controlled trial (RCT) performed nearly 3 decades ago over 5 influenza seasons that did not find differences in efficacy between primary and repeated vaccinations [2]. However, this study was carried out with inactivated whole-virus influenza vaccines, and its generalizability to other vaccines (including currently used inactivated split-virus influenza vaccines) remains uncertain. In addition, considerable data from recent observational studies [3–7] suggest that previous influenza vaccination may influence the immunogenicity and effectiveness of current-season vaccination, resulting in renewed interest in the topic within the scientific community [8].
A recently completed double-blind RCT (NCT01427309) demonstrated that a high-dose inactivated split-virus influenza vaccine (IIV-HD) was more efficacious than a standard-dose inactivated split-virus influenza vaccine (IIV-SD) in preventing laboratory-confirmed influenza illness in adults ≥65 years of age [9]. The study was performed over 2 consecutive influenza seasons. Reenrollment of first-year participants into the second year was allowed. Reenrolled subjects were rerandomized in the second year; thus, the investigation of carryover effects may be regarded as a nested double-blind RCT allowing the unbiased evaluation of the impact of previous-year influenza vaccine (IIV-HD or IIV-SD) on the effect of IIV-HD (compared to IIV-SD) on safety, immunogenicity, and efficacy in older adults [10].
METHODS
Overall Study Design
Details of the original study design and participant eligibility criteria are presented elsewhere [9]. In brief, the study was a phase 3b/4, multicenter, randomized, double-blind trial comparing IIV-HD vs IIV-SD in medically stable adults ≥65 years of age at 126 centers in the United States and Canada. Enrollment occurred during the fall seasons of 2011 (year 1 [Y1]) and 2012 (year 2 [Y2]).
A subset of Y1 participants reenrolled in Y2, thus providing the opportunity to investigate whether there were any carryover effects on Y2 outcomes from the vaccine they received in Y1.
Vaccines
The split-virus vaccines used in the study were formulated according to US Food and Drug Administration recommendations. IIV-SD (Fluzone vaccine, Sanofi Pasteur, Swiftwater, Pennsylvania) contained 15 µg of hemagglutinin (HA) per strain. IIV-HD (Fluzone High-Dose vaccine, Sanofi Pasteur) contained 60 µg of HA per strain. Both vaccines contained A/California/7/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Brisbane/60/2008 strains during Y1 and A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Texas/6/2011 (B/Wisconsin/1/2010-like virus) strains during Y2. The vaccines were provided in prefilled syringes and administered as a 0.5-mL dose intramuscularly into the deltoid.
Treatment Allocation and Assignment
Y1 study participants who reenrolled in Y2 were randomly assigned again in a 1:1 ratio to receive IIV-HD or IIV-SD during the second year. The study used concealed allocation through an interactive voice response system that centrally assigned participants based on computer-generated block randomization. Approximately one-third of participants were selected randomly for immunogenicity assessments. Participants, investigators, and the sponsor's study staff were blinded.
Surveillance and Influenza Case Ascertainment
Details about illness surveillance, illness definitions, and influenza testing have been provided elsewhere [9]. Respiratory illnesses detected by active and passive surveillance triggered the collection of nasopharyngeal swab for influenza confirmation.
A protocol-defined influenza-like illness (PD-ILI) was defined as an acute illness with ≥1 of the following respiratory symptoms: sore throat, cough, sputum production, wheezing, or difficulty breathing; concurrent with ≥1 of the following systemic signs or symptoms: temperature >37.2°C (>99.0°F), chills, tiredness, headaches, or myalgia.
Laboratory confirmation of influenza in nasopharyngeal specimens was defined as a positive result on tissue culture and/or polymerase chain reaction (PCR). Methods utilized to determine whether a positive influenza sample was similar (matched) to a vaccine strain have been previously described [9].
Immunogenicity
Blood samples were collected for measurement of hemagglutination inhibition (HAI) titers approximately 28 days postvaccination in the immunogenicity subset; baseline samples were not obtained. HAI titers were measured using a standard assay [11], and testing was performed by a single laboratory (Focus Diagnostics, Inc, Cypress, California).
Safety
Safety surveillance extended from vaccination (October or November) to approximately 15 May of the following year. Collection of safety data was limited to serious adverse events (SAEs) occurring at any time during study follow-up (6–8 months), defined as events leading to death or hospitalization (or its prolongation), considered as life-threatening or medically important, or resulting in disability.
Statistical Analysis
Statistical analyses of carryover effects were conducted on reenrolled participants in the Y2 full analysis set, which comprised vaccinated individuals. Analyses were conducted according to each of the following 4 vaccination patterns: IIV-HD in both years (Y1 IIV-HD, Y2 IIV-HD), IIV-SD in Y1 and IIV-HD in Y2 (Y1 IIV-SD, Y2 IIV-HD), IIV-HD in Y1 and IIV-SD in Y2 (Y1 IIV-HD, Y2 IIV-SD), or IIV-SD in both years (Y1 IIV-SD, Y2 IIV-SD). A fifth vaccination pattern—IIV-SD or IIV-HD in Y1, IIV-HD in Y2—was included to illustrate the pooled effect of IIV-HD in Y2. For efficacy and immunogenicity analyses, participants were grouped according to the actual vaccine received in Y1 and the Y2 vaccine to which they were randomly assigned (intent-to-treat [ITT]). For safety analyses, participants were grouped according to the vaccine actually received in both years.
Using the vaccination pattern of IIV-SD in both years as the reference, vaccine efficacy (VE) relative to this referent pattern was estimated for each of the other vaccination patterns, calculated as 1 – relative risk, where risk was defined as the proportion of participants developing influenza in Y2. Logistic regression was used to model the probability of influenza as a function of Y2 vaccine, Y1 vaccine, and the interaction (effect modification) between Y1 and Y2 vaccines. The primary case definition as defined in the original study was laboratory-confirmed influenza associated with a PD-ILI caused by any strain (regardless of matching). Three additional case definitions were evaluated as supportive: (1) culture-confirmed influenza associated with a PD-ILI caused by any strain; (2) laboratory-confirmed influenza associated with a PD-ILI caused by vaccine-similar (matched) strains; and (3) culture-confirmed influenza associated with a PD-ILI caused by vaccine-similar (matched) strains.
Statistical significance for VE and immunogenicity was defined by a 95% confidence interval [CI] excluding the null value (0 for relative VE; 1 for geometric mean titer [GMT] ratios) or by a P value <.05. For the evaluation of associations and interaction in logistic regression models, statistical significance was defined as a Wald test P < .05.
RESULTS
Study Groups and Participant Characteristics
Of the 14 500 participants vaccinated in Y1 of the full study [9], 7643 reenrolled in Y2. The number of individuals included in each of the possible Y1 and Y2 vaccination patterns was approximately 1900 per group, ranging between 1880 and 1943 for the ITT analyses.
The demographic and baseline characteristics of the reenrolled participants are shown in Table 1; these were similar to those reported for participants in the full study [9]. There were no striking differences among the vaccination patterns.
Table 1.
Demographic and Baseline Characteristicsa of Reenrolled Study Participants
| Characteristic | Vaccination Patternb |
||||
|---|---|---|---|---|---|
| Y1 IIV-HD, Y2 IIV-HD (n = 1943) | Y1 IIV-SD, Y2 IIV-HD (n = 1880) | Y1 IIV-HD or -SD, Y2 IIV-HD (n = 3823) | Y1 IIV-HD, Y2 IIV-SD (n = 1890) | Y1 IIV-SD, Y2 IIV-SD (n = 1930) | |
| Female sex | 1125 (57.9) | 1086 (57.8) | 2211 (57.8) | 1070 (56.6) | 1119 (58.0) |
| Age, y, mean (SD) | 74.3 (5.6) | 74.1 (5.5) | 74.2 (5.6) | 74.2 (5.7) | 74.3 (5.7) |
| Race | |||||
| White | 1855 (95.5) | 1789 (95.2) | 3644 (95.3) | 1814 (96.0) | 1856 (96.2) |
| Asian | 10 (0.5) | 11 (0.6) | 21 (0.5) | 9 (0.5) | 10 (0.5) |
| Black/African American | 71 (3.7) | 73 (3.9) | 144 (3.8) | 59 (3.1) | 50 (2.6) |
| Other | 7 (0.4) | 7 (0.4) | 14 (0.4) | 8 (0.4) | 14 (0.7) |
| Hispanic/Latino ethnicity | 79 (4.1) | 75 (4.0) | 154 (4.0) | 77 (4.1) | 90 (4.7) |
| At least 1 prespecified chronic comorbidity | 1321 (68.0) | 1246 (66.3) | 2567 (67.1) | 1281 (67.8) | 1287 (66.7) |
| At least 2 prespecified chronic comorbidities | 654 (33.7) | 631 (33.6) | 1285 (33.6) | 652 (34.5) | 631 (32.7) |
| Cardiac and respiratory disorders | |||||
| Coronary artery disease | 327 (16.8) | 308 (16.4) | 635 (16.6) | 354 (18.7) | 329 (17.0) |
| Atrial fibrillation | 145 (7.5) | 132 (7.0) | 277 (7.2) | 137 (7.2) | 138 (7.2) |
| Valvular heart disease | 82 (4.2) | 82 (4.4) | 164 (4.3) | 93 (4.9) | 92 (4.8) |
| Congestive heart failure | 52 (2.7) | 42 (2.2) | 94 (2.5) | 51 (2.7) | 64 (3.3) |
| COPD | 195 (10.0) | 156 (8.3) | 351 (9.2) | 174 (9.2) | 180 (9.3) |
| Asthma | 185 (9.5) | 152 (8.1) | 337 (8.8) | 163 (8.6) | 176 (9.1) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: COPD, chronic obstructive pulmonary disease; IIV-HD, high-dose inactivated influenza vaccine; IIV-SD, standard-dose inactivated influenza vaccine; SD, standard deviation; Y1, year 1; Y2, year 2.
a As reported at Y2 enrollment.
b Based on vaccine assigned on randomization in Y2, and vaccine actually received in Y1.
Efficacy
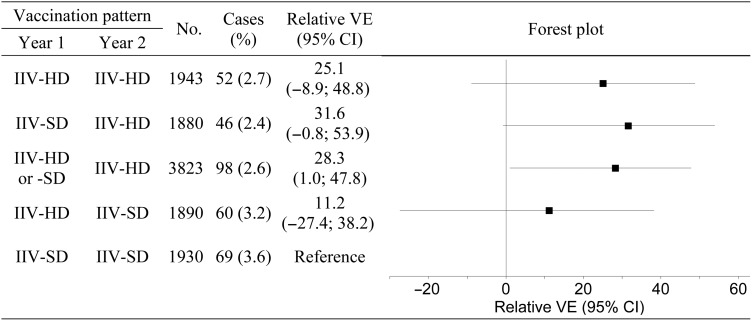
The numbers and percentages of participants developing influenza in Y2 according to each of the 4 case definitions and for each vaccination pattern are shown in Table 2. More than 80% of Y2 cases for which the strain subtype or lineage was determined were A(H3N2) [9]. The VE relative to the Y1 IIV-SD, Y2 IIV-SD vaccination pattern is also shown. Among the participants who received IIV-HD in Y2, relative VE point estimates were slightly higher for those who received IIV-SD in Y1, compared to those who received IIV-HD in Y1, for both laboratory- and culture-confirmed influenza caused by any strain; however, for both laboratory- and culture-confirmed influenza caused by similar (vaccine-matched) strains, relative VE point estimates were slightly higher for those who received IIV-HD in both years, compared to the Y1 IIV-SD, Y2 IIV-HD vaccination pattern. A forest plot of the relative efficacies for the primary case definition is shown in Figure 1.
Table 2.
Reenrolled Participants Developing Influenza and Relative Vaccine Efficacy
| Vaccination Patterna |
Laboratory-Confirmed Influenzab, Any Strain |
Culture-Confirmed Influenzab, Any Strain |
Laboratory-Confirmed Influenzab, Vaccine-Similar Strains |
Culture-Confirmed Influenzab, Vaccine-Similar Strains |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | No. | Cases, No. (%) | Relative VE, %c (95% CI) | Cases, No. (%) | Relative VE, %c (95% CI) | Cases, No. (%) | Relative VE, %c (95% CI) | Cases, No. (%) | Relative VE, %c (95% CI) |
| IIV-HD | IIV-HD | 1943 | 52 (2.7) | 25.1 (−8.9 to 48.8) | 47 (2.4) | 28.2 (−6.2 to 51.7) | 17 (0.9) | 23.2 (−51.4 to 61.7) | 16 (0.8) | 27.8 (−44.0 to 64.5) |
| IIV-SD | IIV-HD | 1880 | 46 (2.4) | 31.6 (−.8 to 53.9) | 43 (2.3) | 32.1 (−1.4 to 54.9) | 17 (0.9) | 20.7 (−56.4 to 60.5) | 16 (0.9) | 25.3 (−48.8 to 63.3) |
| IIV-HD or -SD | IIV-HD | 3823 | 98 (2.6) | 28.3 (1.0–47.8) | 90 (2.4) | 30.1 (2.3–49.8) | 34 (0.9) | 22.0 (−40.0 to 55.7) | 32 (0.8) | 26.6 (−32.6 to 58.6) |
| IIV-HD | IIV-SD | 1890 | 60 (3.2) | 11.2 (−27.4 to 38.2) | 55 (2.9) | 13.6 (−25.7 to 40.8) | 27 (1.4) | −25.3 (−131 to 31.3) | 26 (1.4) | −20.7 (−123 to 34.2) |
| IIV-SD | IIV-SD | 1930 | 69 (3.6) | Reference | 65 (3.4) | Reference | 22 (1.1) | Reference | 22 (1.1) | Reference |
Abbreviations: CI, confidence interval; IIV-HD, high-dose inactivated influenza vaccine; IIV-SD, standard-dose inactivated influenza vaccine; VE, vaccine efficacy; Y1, year 1; Y2, year 2.
a Based on vaccine assigned on randomization in Y2, and vaccine actually received in Y1.
b Confirmed influenza associated with protocol-defined influenza-like illness in Y2.
c Efficacy relative to Y1 IIV-SD, Y2 IIV-SD vaccination pattern. Confidence intervals for relative VE were calculated by the Clopper–Pearson exact method conditional on the total number of cases.
Figure 1.
Forest plot of relative efficacy against laboratory-confirmed influenza illness caused by any strain. Illness corresponds to protocol-defined influenza-like illness in year 2. Vaccine efficacy (VE) is the percent efficacy of the different vaccination patterns relative to the year 1 (Y1) standard-dose inactivated influenza vaccine (IIV-SD), year 2 (Y2) IIV-SD vaccination pattern. Vaccination patterns are based on vaccine assigned at randomization in Y2, and vaccine actually received in Y1. The plot on the right depicts the relative VE estimates for the vaccination patterns; horizontal lines represent the 95% confidence intervals (CIs), and solid squares represent the point estimates. All estimates to the right of the null value of 0 favor the corresponding pattern over the referent. CIs that do not intersect with the null value are statistically significant. Abbreviation: IIV-HD, high-dose inactivated influenza vaccine.
Results of the logistic regression of the probability of Y2 influenza as a function of Y2 vaccine and Y1 vaccine for the 4 case definitions are shown in the Supplementary Appendix. When an interaction term was included (Supplementary Table 1), the interaction was not statistically significant (P values ranging from .43 to .63 for the 4 case definitions), whereas Y2 IIV-HD was significantly associated with protection against influenza in Y2 for both laboratory- and culture-confirmed influenza caused by any strain (P = .043 and P = .046, respectively). There was no significant association between Y1 vaccine and influenza occurrence in Y2 in these models. After omitting the interaction term (Supplementary Table 2), the effect of Y2 vaccination remained statistically significant for both laboratory- and culture-confirmed influenza caused by any strain, and approached statistical significance for both laboratory- and culture-confirmed influenza caused by vaccine-similar strains (P = .098 and P = .073, respectively); of note, the numbers of cases for these latter 2 outcomes were considerably smaller. There was no evidence of an association between Y1 vaccine and Y2 influenza occurrence in the models without the interaction term.
Immunogenicity
GMTs by HAI assay are shown in Table 3. Among participants who received IIV-HD in Y2, GMTs were higher for those who received IIV-SD in Y1, compared with those who received IIV-HD in Y1, for all influenza types/subtypes. In contrast, among participants who received IIV-SD in Y2, GMTs were neither consistently higher nor consistently lower for those who received IIV-SD in Y1, compared with those who received IIV-HD in Y1.
Table 3.
Immunogenicity: Geometric Mean Titer of Reenrolled Participants by Hemagglutination Inhibition Assay
| Influenza Type/Subtype | Vaccination Patterna |
||||
|---|---|---|---|---|---|
| Y1 IIV-HD, Y2 IIV-HD (n = 643) | Y1 IIV-SD, Y2 IIV-HD (n = 605) | Y1 IIV-HD or IIV-SD, Y2 IIV-HD (n = 1248) | Y1 IIV-HD, Y2 IIV-SD (n = 639) | Y1 IIV-SD, Y2 IIV-SD (n = 617) | |
| A(H1N1) | 322.1 (295.0–351.6) | 416.7 (382.6–453.9) | 364.9 (343.1–388.1) | 207.9 (188.2–229.7) | 198.6 (180.1–219.1) |
| A(H3N2) | 400.6 (366.7–437.7) | 459.3 (419.1–503.4) | 428.1 (401.7–456.2) | 242.4 (220.6–266.4) | 243.0 (220.8–267.5) |
| B | 88.2 (81.1–95.8) | 93.2 (85.8–101.2) | 90.5 (85.4–96.0) | 52.3 (48.2–56.7) | 57.9 (53.3–62.8) |
Data are presented as geometric mean titer (95% confidence interval), calculated using the t distribution applied to log-transformed titers. The n values represent reenrolled subjects in the immunogenicity subset with assay results. Samples were obtained approximately 28 days after year 2 vaccination.
Abbreviations: IIV-HD, high-dose inactivated influenza vaccine; IIV-SD, standard-dose inactivated influenza vaccine; Y1, year 1; Y2, year 2.
a Based on vaccine assigned on randomization in Y2, and vaccine actually received in Y1.
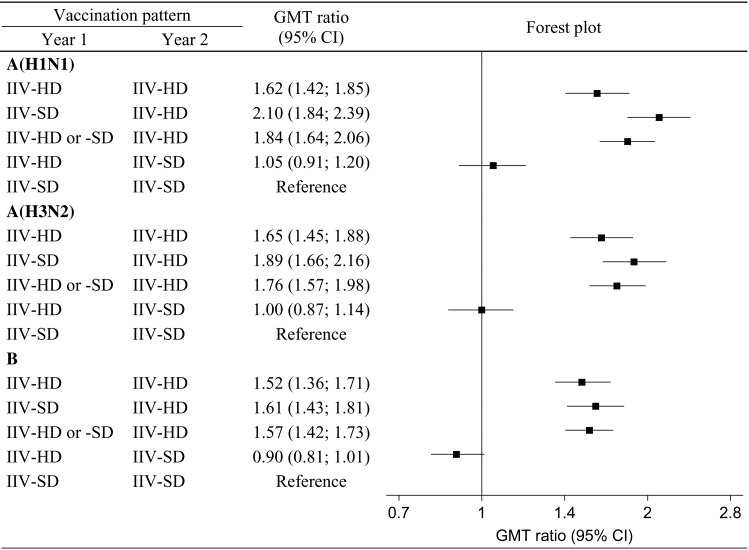
The ratios of the GMTs of other vaccination patterns relative to that of the Y1 IIV-SD, Y2 IIV-SD vaccination pattern are shown in Figure 2. The GMTs for participants who received IIV-HD in Y2 were substantially and significantly higher than for those who received IIV-SD.
Figure 2.
Geometric mean titer (GMT) ratios and forest plot. GMT ratios presented are for each vaccination pattern relative to the year 1 (Y1) standard-dose inactivated influenza vaccine (IIV-SD), year 2 (Y2) IIV-SD vaccination pattern. Vaccination patterns are based on vaccine assigned at randomization in Y2, and vaccine actually received in Y1. Samples for assaying were collected approximately 28 days after Y2 vaccination. The plot on the right depicts GMT ratios for each vaccination pattern; horizontal lines represent the 95% confidence intervals (CIs), and solid squares represent the point estimates. All estimates to the right of the null value of 1 favor the corresponding pattern over the referent. CIs that do not intersect with the null value are statistically significant. Abbreviation: IIV-HD, high-dose inactivated influenza vaccine.
Safety
The numbers and percentages of reenrolled participants experiencing SAEs within 30 days and 180 days of vaccination are shown in Table 4. The absolute number of serious pneumonias and hospitalizations within 180 days of Y2 vaccination were lower in vaccination patterns that included IIV-HD in Y2 vs the vaccination patterns that included IIV-SD in Y2.
Table 4.
Reenrolled Study Participants With Serious Adverse Events
| Event | Vaccination Patterna |
||||
|---|---|---|---|---|---|
| Y1 IIV-HD, Y2 IIV-HD (n = 1942) | Y1 IIV-SD, Y2 IIV-HD (n = 1881) | Y1 IIV-HD or -SD, Y2 IIV-HD (n = 3823) | Y1 IIV-HD, Y2 IIV-SD (n = 1891) | Y1 IIV-SD, Y2 IIV-SD (n = 1929) | |
| Subjects with SAEs within 30 d of Y2 vaccination | |||||
| Any SAE | 29 (1.5) | 15 (0.8) | 44 (1.2) | 12 (0.6) | 27 (1.4) |
| Death | 0 (0.0) | 1 (0.1) | 1 (0.0) | 0 (0.0) | 0 (0.0) |
| Pneumonia | 0 (0.0) | 3 (0.2) | 3 (0.1) | 3 (0.2) | 0 (0.0) |
| Hospitalization | 27 (1.4) | 13 (0.7) | 40 (1.0) | 11 (0.6) | 25 (1.3) |
| Subjects with SAEs within 180 d of Y2 vaccination | |||||
| Any SAE | 128 (6.6) | 122 (6.5) | 250 (6.5) | 141 (7.5) | 140 (7.3) |
| Death | 2 (0.1) | 7 (0.4) | 9 (0.2) | 6 (0.3) | 6 (0.3) |
| Pneumonia | 6 (0.3) | 8 (0.4) | 14 (0.4) | 16 (0.8) | 12 (0.6) |
| Hospitalization | 118 (6.1) | 113 (6.0) | 231 (6.0) | 134 (7.1) | 132 (6.8) |
Data are presented as No. (%).
Abbreviations: IIV-HD, high-dose inactivated influenza vaccine; IIV-SD, standard-dose inactivated influenza vaccine; SAE, serious adverse event; Y1, year 1; Y2, year 2.
a Based on vaccines actually received in year 1 and year 2.
DISCUSSION
IIV-HD was licensed in the United States in December 2009 and has been available for use since the 2010–2011 northern hemisphere influenza season as an alternative to IIV-SD for the prevention of influenza in the elderly. This study evaluated the impact of previous-year influenza vaccine type (IIV-HD or IIV-SD) on current-season vaccine effects.
Our analysis did not find significant evidence of modification of the relative VE of IIV-HD by type of previous-year vaccination. Overall, point estimates favored IIV-HD over IIV-SD in Y2, irrespective of Y1 vaccine. The relative VE point estimates were similar between individuals receiving IIV-HD both years and those who received IIV-HD the second year and IIV-SD the first year. IIV-HD vaccination in Y2 was associated with significant protection against influenza, independent of Y1 vaccination for the primary case definition. Positive point estimates for analysis of efficacy against any influenza strain regardless of matching may suggest some residual protection from IIV-HD received in Y1 in individuals who received IIV-SD in Y2, compared with those who received IIV-SD both years; however, this was not observed in analyses restricted to efficacy against vaccine-matched strains.
A landmark RCT performed in the 1980s over 5 influenza seasons did not report a meaningful difference in efficacy in participants who received repeated vaccination with an inactivated whole-virus vaccine compared with those who received the same vaccine for the first time [2]. Moreover, this conclusion was supported by a meta-analysis of 7 field studies, the majority of which were randomized trials [12]. In contrast, a prospective observational cohort study evaluating vaccine effectiveness in households during the 2011–2012 northern hemisphere influenza season found that vaccine effectiveness was affected by previous-year vaccination, with no effectiveness observed in those reporting prior vaccination and statistically significant effectiveness in those with no prior vaccination [3]. Similar findings were reported from another observational study performed during the 2011 and 2012 southern hemisphere influenza seasons, but in this study the authors caution that the findings appear more extreme if the analyses stratify on prior vaccination status only, and advised that estimates should be reported with reference to people vaccinated in neither season [4]. Subsequent studies have incorporated this referent group [5–7], allowing the assessment of previous-year vaccination effect in terms of residual protection and/or reduced vaccine response. A second prospective observational cohort study evaluating vaccine effectiveness in households during the 2012–2013 influenza season reported similar findings [6], and suggested residual protection from vaccination in the prior season, leading to the conclusion that current-season vaccine effectiveness may be modified by both residual protection from previous-season vaccine and reduced response to the current-season vaccine. A test-negative case-control study performed over 8 influenza seasons found that current-season vaccination was effective against medically attended influenza illness regardless of previous year vaccination [7]. The investigators also reported a residual benefit, with significant protection observed among persons vaccinated during the previous season but not the current season (compared with those vaccinated in neither season). However, in the analysis using 5 years of historical influenza vaccination data, current-season effectiveness against H3N2 was higher among vaccinated individuals with no prior vaccination history compared with vaccinated individuals with a frequent vaccination history. This suggests that a full understanding of the impacts of repeated influenza vaccination may require accounting for vaccination exposure beyond just the previous influenza season.
The lack of evidence for effect modification by previous-year vaccine in our study is consistent with most previous reports that used a randomized trial design [2, 12], but inconsistent with several recent observational studies [3–6]. In addition to the design, several features of our study may partially explain divergent findings: Our study evaluated outcomes during a northern hemisphere influenza season characterized by mismatch between predominant circulating strains and the vaccines tested in the study [13, 14], whereas matching between vaccine and circulating viruses was adequate for several of the previous observational studies [3–5]; our study used vaccine components that differed for 2 of 3 strains between seasons, whereas several observational studies used vaccine components that remained constant across seasons [3–5]; importantly, our study was restricted to older adults, an age group generally underrepresented in previous studies on the topic; finally, all participants in our study received an influenza vaccine in both study years, whereas previous reports evaluated the effect of previous-year vaccination or no vaccine on current-year VE compared to no vaccine. Of note, laboratory confirmation of influenza in our study was performed using currently accepted methods (ie, culture and PCR), in contrast to some previous studies [2, 12] in which laboratory confirmation was based on serology. Serologic confirmation is currently disfavored because of the so-called “ceiling effect” [15], which may result in overestimation of VE.
Our immunogenicity results reveal consistently and significantly higher HAI titers in participants who received IIV-HD in the second year compared with those who received IIV-SD, irrespective of Y1 vaccine. Previous studies have reported that prior-year influenza vaccination is associated with sustained higher HAI titers 1 year later but lower antibody responses to new influenza vaccination [16]. This was also described in one of the observational studies discussed above [6], supporting their conclusion that previous-year vaccination may influence current-season vaccine effect either by residual protective immunity from previous vaccine or by suboptimal response to current-season vaccine. Immune responses to influenza vaccine based on single or repeated vaccination were also evaluated in a meta-analysis of serologic studies [12], which reported wide variation in individual study results; however, the summary estimate for the pooled effect was close to the null value, supporting the authors' conclusion that repeated vaccination elicits protective immune responses that are as adequate as those following single vaccination [12]. The GMT and GMT ratio point estimates obtained in our analysis may suggest that the HAI responses after IIV-HD in Y2 could have been blunted to some extent when the vaccine received in Y1 was IIV-HD instead of IIV-SD, particularly for the H1N1 strain. However, our VE estimates do not suggest any meaningful deleterious effect on influenza protection from marginally lower HAI titers, especially when VE analyses were restricted to matched strains. Of note, 2 of the 3 vaccine components changed from Y1 to Y2, with only the H1N1 strain remaining constant over the 2 seasons, and H1N1 being the least frequent infecting strain in the study. Antigenic distance between sequential vaccine strains and between vaccine and circulating strains may both play a role in the variability of influenza vaccine effect by previous-year vaccine [17].
Assessments of SAE frequency did not raise safety concerns with IIV-HD revaccination. There were fewer all-cause SAEs, serious pneumonias, and all-cause hospitalizations in Y2 in vaccination patterns that included IIV-HD that year, compared to the vaccination patterns that included IIV-SD in the second season. Although the number of these events in the present analysis was limited, this observation is consistent with the report of significantly lower frequency of all-cause SAEs and serious pneumonia in the entire study cohort [18].
Our analysis has the major advantage of the double-blind, randomized design, which minimizes any source of bias. However, it also has important limitations. As influenza vaccine was administered to all participants, it was not possible to evaluate the effect of the vaccines in the context of no previous influenza vaccination. The study only spanned 2 influenza seasons, precluding assessments of the impact of frequent influenza vaccination over longer preceding periods. Only the vaccine component corresponding to the least frequent influenza subtype circulating in Y2 was maintained over the 2 seasons, not allowing evaluation of carryover effects in the setting of unchanged vaccine components from year to year. Finally, the original study was not powered to address the objectives of this supplementary analysis; therefore, several estimates presented here for different vaccination patterns do not have sufficient precision to demonstrate statistical significance.
In conclusion, this analysis indicates that IIV-HD vaccination is likely to provide clinical benefit over IIV-SD vaccination, irrespective of previous-season exposure to IIV-HD or IIV-SD. This benefit is further supported by significantly improved immune responses, regardless of previous-year vaccine type, and no safety concerns in the context of IIV-HD revaccination.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors thank the study participants, the investigators from the 126 participating research sites, and the sponsor study team for their contributions to the original study.
Financial support. This work was supported by Sanofi Pasteur.
Potential conflicts of interest. C. A. D., A. J. D., C. A. R., V. L., and D. P. G. are employees of Sanofi Pasteur. H. K. T has received research funding from MedImmune, Sanofi Pasteur, and Gilead and has served as an advisor for MedImmune, Novartis, and VaxInnate. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fiore AE, Uyeki TM, Broder K et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 2.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114–22. [DOI] [PubMed] [Google Scholar]
- 3.Ohmit SE, Petrie JG, Malosh RE et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis 2013; 57:474–6. [DOI] [PubMed] [Google Scholar]
- 5.Ohmit SE, Thompson MG, Petrie JG et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS. Influenza vaccine effectiveness in households with children during the 2012-2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis 2015; 211:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean HQ, Thompson MG, Sundaram ME et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuzil KM. How can we solve the enigma of influenza vaccine-induced protection? J Infect Dis 2015; 211:1517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiazGranados CA, Dunning AJ, Kimmel M et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 10.Dunning AJ, Reeves J. Control of type 1 error in a hybrid complete two-period vaccine efficacy trial. Pharm Stat 2014; 13:397–402. [DOI] [PubMed] [Google Scholar]
- 11.Kendal AP, Pereira MS, Skehel JJ. Hemagglutination inhibition. In: Kendal AP, Pereira MS, Skehel JJ, eds. Concepts and procedures for laboratory-based influenza surveillance. Atlanta, GA: Centers for Disease Control and Prevention and Pan-American Health Organization, 1982. [Google Scholar]
- 12.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med 1999; 159:182–8. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). Influenza activity—United States, 2012-13 season and composition of the 2013-14 influenza vaccine. MMWR Morb Mortal Wkly Rep 2013; 62:473–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis 2011; 203:1309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki S, He XS, Holmes TH et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 2008; 3:e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: a comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine 2015; 33:4988–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.