Abstract
Background
Liver diseases, the fifth most common cause of global death, can be metabolic, toxin-induced, or infective. Though approximately 35 Saudi medicinal plants are traditionally used to treat liver disorders, the hepatoprotective potential of Aerva javanica has not been explored.
Objective
To investigate the antioxidative and hepatoprotective effect of Aerva javanica.
Design
Total ethanol extract of A. javanica aerial parts was prepared and tested on DCFH-toxicated HepG2 cells ex vivo, and in CCl4-injured Wistar rats in vivo. MTT assay was used to determine cell viability and the serum biochemical markers of liver injury as well as histopathology was performed. In vitro 1,1-diphenyl-2-picrylhydrazyl and β-carotene free-radical scavenging assay and phytochemical screening of the extract were done. Furthermore, A. javanica total extract was standardized and validated by high-performance thin layer chromatographic method.
Results
MTT assay showed that, while DCFH-injured cells were recovered to ~56.7% by 100 µg/ml of the extract, a 200 µg/ml dose resulted in hepatocytes recovery by ~90.2%. Oral administration of the extract (100 and 200 mg/kg.bw/day) significantly normalized the serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, gamma-glutamyl transferase, alkaline phosphatase, bilirubin, cholesterol, high-density lipoprotein, low-density lipoprotein, very-low-density lipoprotein, triglyceride, and malondialdehyde levels, including tissue nonprotein sulfhydryl and total protein in CCl4-injured rats. In addition, the histopathology of dissected liver also revealed that A. javanica cured the tissue lesion compared to silymarin treatment. In vitro assays revealed strong free-radical scavenging ability of the extract and presence of alkaloids, flavonoids, tannins, sterols, and saponins where rutin, a well-known antioxidant flavonoid, was identified.
Conclusions
Our findings demonstrate the potential of A. javanica in the attenuation of ex vivo and in vivo hepatotoxicity and oxidative damage. This further suggests its therapeutic value in various liver diseases. However, isolations of the active principles, their mechanisms of action, and other therapeutic contributions remain to be addressed.
Keywords: Aerva javanica, hepatoprotection, oxidative stress, DCFH, CCl4, rutin, liver diseases
Liver is one of the most vital organs of the body, considered to be the metabolic ‘engine room’. Major functions of the liver include carbohydrate, protein, and fat metabolism as well as secretion of bile, and the storage and detoxification of several drugs and xenobiotics. Liver diseases can be classified as acute or chronic hepatitis (inflammatory liver diseases), hepatosis (non-inflammatory liver diseases), and cirrhosis. Liver disorders can be attributed to toxins like aflatoxin, chlorinated hydrocarbons, and certain drugs. Other factors include infectious pathogens such as hepatitis viruses (1) and alcohol consumption (2). Drug-induced liver toxicity accounts for approximately one-half of the cases of acute liver failure (3). Currently, there is a real need for effective therapeutic agents, especially natural products with a minimal incidence of toxic effects. Despite the significant popularity of several mono- and poly-herbal preparations for liver diseases (4), only few (e.g., silymarin) have been approved for protection or treatment of liver disorders (5). Nevertheless, in recent years, many phytoproducts have been investigated against hepatotoxin-induced liver damage (6–10).
The genus Aerva belongs to the plant family Amaranthaceae, and there are approximately 28 species in this genus (11). Aerva javanica is an erect, much-branched perennial herb that is a native of Africa and also distributed in various parts of the world. Different parts of the plant have wide applications in folk medicine, for example the whole plant is used for chest pain, diarrhea, and as a diuretic and demulcent. A decoction prepared from the aerial parts of A. javanica is used as a gargle to cure gum swelling and toothache. Though the seeds are used for headache relief, paste from the flowers and leaves is used externally to heal the wounds and the inflammation of joints (12). Also, it is used for dysentery, gonorrhea, and hyperglycemia (13). Previous biological screenings revealed the numerous biological activities of A. javanica. Its ethanolic and aqueous extracts showed significant anti-diarrheal activity at a dose level of 800 mg/kg in Wistar rats (14). In addition, A. javanica exhibited anti-bacterial, anti-fungal (15–17), smooth muscle relaxant, and anti-ulcer activities (13). The alcoholic extract of root of A. javanica was shown to possess significant nephroprotective activity against cisplatin- and gentamycin-induced renal toxicity in experimental animals (18).
A phytochemical analysis of A. javanica has revealed the presence of polyphenols, terpenoids, flavonoids, and alkaloids (11). Notably, four new ecdysteroids, aervecdysteroid A–D (1–4), and three new acylated flavone glycosides have been isolated from the flowers (19). In addition, heptacosane (3-allyl-6-methoxyphenol) and pentacosane were identified as the major components of the A. javanica seed oil (20). With this background information, the present study was intended to investigate the antioxidant and hepatoprotective activities of ethanolic extract of the aerial parts of A. javanica.
Materials and methods
Plant materials
Aerial parts (leaves, stems, and inflorescences) of A. javanica (Family: Amaranthaceae) were collected from Taif, Kingdom of Saudi Arabia. The plant was authenticated by an expert plant taxonomist at the herbarium of College of Pharmacy, King Saud University, Riyadh, and a voucher specimen (No. 16281) was deposited.
Preparation of A. javanica total ethanolic extract
The shade-dried powdered plant material (400 g) were soaked in 70% aqueous ethanol (Merck, Germany) for 2 days at room temperature and filtered. The extraction process was repeated twice with the same solvent. Then, the extracts were evaporated using a rotary evaporator (Buchi, Switzerland) under reduced pressure at 40°C. The obtained semi-solid extracts (37.68 g) were stored at −20°C until used for further study.
Human hepatoma cell culture and drugs
Human hepatoma cell line (HepG2) was grown in RPMI-1640 medium, supplemented with 10% heat-inactivated bovine serum (Gibco, USA), 1× penicillin–streptomycin, and 1× sodium pyruvate streptomycin (HyClone Laboratories, USA) at 37°C in a humified chamber with 5% CO2 supply. 2,7-Dichlorofluorescein (DCFH; Sigma, USA) was used to induce cytotoxicity in cultured HepG2 cells. Silymarin (Sigma, USA) was used as standard hepatoprotective drug in rats.
Ex vivo hepatoprotective assay of A. javanica total ethanolic extract
HepG2 cells were seeded (0.5×105 cells/well, in triplicate) in a 96-well flat-bottom plate (Becton-Dickinson Labware, USA) and grown over night. The cytotoxicity of DCFH was determined by using MTT assay (MTT-Cell proliferation Assay Kit, Tervigen). The concentration of DCFH that caused a 50% inhibition of HepG2 cell proliferation (IC50: 100 µg/ml) was used as a cytotoxic dose. A. javanica total extract was dissolved in DMSO (100 mg/ml), followed by dilution with RPMI-1640 media to four doses (25, 50, 100, and 200 µg/ml). The final concentration of DMSO used never exceeded >0.1% and was therefore tolerated by cultured cells. The culture monolayer was replenished with RPMI-1640 containing 100 µg/ml DCFH plus a dose of A. javanica, including untreated as well as DCFH-treated controls. The treated cells were incubated for 48 h at 37°C. Cells were treated with MTT reagent (10 µl/well) and further incubated for 3–4 h. Upon the appearance of a purple color, detergent solution (100 µl) was added to each well and further incubated for 1.5 h. The optical density (OD) was recorded at 570 nm in a microplate reader (BioTek, ELx800). Nonlinear regression analysis was performed in Excel software to determine the cell survival (%) using the following equation:
where OD(s), OD(b), and OD(c) are the absorbance of sample, blank, and negative control, respectively.
Microscopy
The morphological investigation of the cultured hepatoma cells was done under an inverted microscope (Optica, 40×, and 100×) to observe any changes in the cells cultured with different concentrations of A. javanica total extract and/or DCFH at 24 and 48 h.
In vivo hepatoprotective activity of A. javanica total extract: Experimental design and treatment
Thirty healthy male Wistar rats were obtained from the Experimental Animal Care Center (EACC) of the College of Pharmacy, King Saud University, Riyadh. Animals were kept in polycarbonate cages in a room free from any source of contamination, artificially illuminated (12 h dark/light cycle) at controlled temperature (25±2°C). After acclimatization, animals were randomized and divided into five groups (I–V) of six animals each. Group I served as untreated control and fed orally with normal saline 1 ml. Group II received CCl4 in liquid paraffin (1:1) 1.25 ml/kgbw intraperitoneally (i.p.) and served as negative control. Similarly, group (III, IV and V) also received CCl4. While groups III and IV were treated with A. javanica total extract at a dose of 100 mg/kg.bw and 200 mg/kgbw, respectively, group V was treated with the standard drug silymarin (21–23) at a dose of 10 mg/kgbw for 3 weeks. Blood was collected for serum biochemistry and animals were sacrificed for liver dissection. All animals received human care in compliance with the guidelines of the Ethics Committee of the Experimental Animal Care Society, College of Pharmacy, King Saud University, Riyadh.
Estimation of marker enzymes and bilirubin
Serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) (24), alkaline phosphatase (ALP) (25), gamma-glutamyl transferase (GGT) (26), and bilirubin (27) were determined using Reflotron Plus Analyzer and Roche kits (Roche Diagnostics GmbH, Mannheim, Germany).
Estimation of lipid profile
Total cholesterol (28), triglycerides (29) and high-density lipoproteins (HDL) (30) levels were estimated in serum using Roche diagnostic kits (Roche Diagnostics GmbH, Mannheim, Germany).
Determination of malondialdehyde (MDA)
The method reported by Utley et al. (31) was followed. In brief, the liver tissues were removed homogenized in 0.15 M KCl (at 4°C; Potter-Elvehjem type C homogenizer) to give a 10% w/v homogenate. The absorbance of the solution was then read at 532 nm. The content of MDA (nmol/g wet tissue) was then calculated, by reference to a standard curve of MDA solution.
Estimation of nonprotein sulfyhydryl (NP-SH)
Hepatic NP-SH was measured according to the method of Sedlak and Lindsay (32). The liver tissues were homogenized in ice-cold 0.02 mM ethylene diamine tetraacetic acid (EDTA). The absorbance was measured within 5 min of addition of 5,5′dithio-bis(2-nitrobenzoic acid) (DTNB) at 412 nm against a reagent blank.
Determination of total protein (TP)
Serum TP was estimated by the kit method (Crescent Diagnostics, Jeddah, Saudi Arabia). The absorbance (Abs) of the samples were recorded at 546 nm and concentration of serum TP was calculated using the following equation:
Histopathological evaluation
The animals were sacrificed under ethyl ether anesthesia and livers were quickly removed. Liver tissues were fixed in 10% neutral buffered formalin for 24 h and processed overnight for dehydration, clearing, and paraffin impregnation using an automatic tissue processor (Sakura, Japan). The specimens were embedded in paraffin blocks using embedding station (Sakura, Japan) and sections of 4 micron thickness were cut using rotary microtome (Leica-RM2245, Germany) and stained with hematoxylin and eosin (H&E) stains using routine procedures (33). The stained sections were observed under light microscopy, and the required images were taken with digital microscopic mounted camera (OMX1200C, Nikon, Japan).
Antioxidant assay of A. javanica total extract
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
Antioxidant activity was estimated by free-radical scavenging ability of the total extract against DPPH according to the method described elsewhere (34), but with minor modifications to suite 96-well microplate format. DPPH is a molecule containing a stable free radical, and in the presence of an antioxidant agents, which can donate an electron to the DPPH, the purple color typical for free DPPH radical decays, and the change in absorbance (λ=517 nm) is measured spectrophotometrically. In brief, 100 µl of different concentrations (31.25, 62.5, 125, 250, and 500 µg/ml) of the extract was mixed with 40 µl of DPPH (0.2 mM in methanol) in wells of a 96-well microplate. Appropriate control was prepared using the solvent only in addition to the same amount of DPPH reagent to get rid of any inherent solvent effect. Ascorbic acid was used as standard. After 30 min incubation at 25°C, the decrease in absorbance was measured using microplate reader. The test was carried out in triplicate. The radical-scavenging activity was calculated from the equation:
β-Carotene-linoleic acid bleaching assay
The antioxidant activity of A. javanica total extract was evaluated using the β-carotene bleaching method (35) with minor modifications for working with 96 well plate. Briefly, 0.25 mg β-carotene was dissolved in 0.5 ml of chloroform and added to flasks containing 12.5 µg of linoleic acid and 100 mg of Tween-40. The chloroform was evaporated at 43°C using speed vacuum concentrator (Savant, Thermo Electron Co.). The resultant mixture was immediately diluted to 25 ml with distilled water and shaken vigorously for 2–3 min to form an emulsion. A 200 µl aliquot of the emulsion was added to wells of a 96-well plate containing 50 µl of the extract or gallic acid (500 µg/ml). A control containing solvent instead of extract was also prepared. The plate was incubated at 50°C for 2 h. Absorbance was read at 470 nm at 30 min intervals using microplate spectrophotometer (BioRad). The test was carried out in triplicate. The antioxidant activity was estimated using two different methods where the kinetic curve was initially obtained by plotting absorbance of each sample against time. Then, antioxidant activity was expressed as % inhibition of lipid peroxidation using the formula:
where As(120) and Ac(120) are the absorbance of the sample and control, respectively, at time 120 min, and AC(0) is the absorbance of the control at time 0 min.
In vitro phytochemical screening of ethanolic extract of A. javanica
Qualitative phytochemical screening tests for major secondary metabolites, which included alkaloids, flavonoids, anthraquinones, tannins, saponins, and cardiac glycosides, were performed using standard procedures (36–38).
Standardization of ethanolic extract of A. javanica by HPTLC method
A high-performance thin layer chromatography (HPTLC) method was used to standardize the 70% ethanol extract of A. javanica as described elsewhere (39). The chromatography was carried out on 10×10 cm precoated silica gel F254 RP-HPTLC plate, using rutin as the reference standard. Several mobile phases were tried to get good resolution and separation of different compounds present in the A. javanica ethanol extract. Based on our observations, we selected acetonitrile and water in the ratio of 4:6 as suitable mobile phase to carry out the standardization of the extract. The standard along with samples was applied on the HPTLC plate by CAMAG Automatic TLC Sampler-4. The plate was developed under controlled condition in CAMAG Automated Developing Chamber-2 and scanned by CAMAG TLC Scanner-3 (λ=360 nm).
Statistical analysis
Results were expressed as mean±SEM. Total variation present in a set of data was estimated by one-way analysis of variance (ANOVA) followed by Dunnet's test. P <0.01 was considered significant.
Results
Effect of A. javanica on cell morphology
Microscopic observations showed considerable cytotoxic effect of DCFH on the HepG2 cells as reflected by altered morphology compared to untreated cells. Interestingly, the DCFH-treated cells supplemented with 100 and 200 µg/ml of A. javanica extract were morphologically different from those treated with DCFH alone but comparable to untreated cells (data not shown).
Hepatoprotective and cell proliferative effect of A. javanica
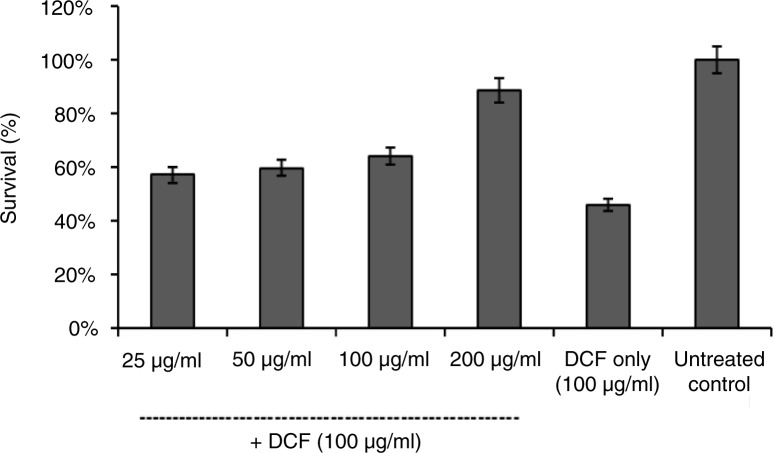
MTT test showed hepatoprotective effect of A. javanica ethanol extract in a dose-dependent manner against DCFH-toxicity. While DCFH-toxicated cells were recovered to about 56.7% after supplementation with 100 µg/ml of A. javanica extract, further doubling the dose (200 µg/ml) resulted in hepatocytes recovery by about 90.2% (Fig. 1).
Fig. 1.
MTT-cell proliferation assay showing hepatoprotective effect of A. javanica total ethanolic extract against DCFH-induced hepatotoxicity in cultured HepG2 cells.
In vivo effect of A. javanica extract on biochemical markers
Based on the ex vivo hepatoprotective activity on HepG2 cells, the effects of A. javanica extract were further examined in the experimental animal model. The administration of CCl4 dramatically elevated the serum SGOT, SGPT, GGT ALP, and bilirubin levels compared to the normal control group (P<0.0001), indicating significant hepatotoxicity of CCl4 treatment (Table 1). In contrast, oral administration of A. javanica extract significantly normalized the biochemical parameters in CCl4-treated rats, compared to the CCl4-treated group (Table 1). Moreover, CCl4-induced toxicity caused significant elevation in lipid profile including cholesterol, triglycerides, low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) and a reduction in the HDL levels in serum. The 3-week pretreatment of rats with two doses (250 and 500 mg/kg.bw/day) of A. javanica extract significantly reduced the cholesterol, triglycerides, LDL, and VLDL levels and improved the HDL level in a dose-dependent manner (Table 2). Silymarin, on the other hand, significantly prevented the CCl4-induced elevation in marker enzymes and lipid profile. Furthermore, our results indicated that treatment with A. javanica also resulted in a significant increase in MDA and decrease in NP-SH and TP concentration (Table 3). Oral supplementation of CCl4-treated rats with A. javanica extract dramatically diminished the levels of MDA and enhanced NP-SH and TP levels (Table 3).
Table 1.
Effect of A. javanica total ethanolic extract on CCl4-induced hepatotoxicity-related parameters in rats
| Treatment group | SGOT | SGPT (U/l) | ALP (U/l) | GGT (U/l) | Bilirubin (mg/dl) |
|---|---|---|---|---|---|
| Control | 107.45±5.31 | 28.83±2.20 | 321.66±13.88 | 4.06±0.32 | 0.54±0.01 |
| CCl4 | 294.83±8.33***a | 230.83±9.62***a | 515.16±13.70***a | 12.85±0.98***a | 2.16±0.08***a |
| A. javanica (100 mg) + CCl4 | 279.33±7.81b | 174.33±6.13***b | 437.00±8.66***b | 10.28±0.43*b | 1.81±0.05**b |
| A. javanica (200 mg) + CCl4 | 246.00±3.74***b | 140.16±4.04***b | 412.66±8.84***b | 6.86±0.33***b | 1.54±0.02***b |
| Silymarin (10 mg) + CCl4 | 136.66±6.00***b | 85.66±4.31***b | 396.33±7.62***b | 5.58±0.28***b | 1.06±0.06***b |
All values represent mean±SEM
P<0.05
P<0.01
P<0.001; ANOVA, followed by Dunnet's multiple comparison test
As compared with normal group.
As compared with CCl4-only group.
Table 2.
Effects of A. javanica total ethanolic extract on CCl4-induced lipid profile changes in rats
| Treatment group | TC (mg/dl) | TG (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) |
|---|---|---|---|---|---|
| Control | 109.83±3.94 | 59.01±2.74 | 55.18±2.40 | 42.84±3.22 | 11.80±0.54 |
| CCl4 | 206.00±4.53***a | 151.16±4.61***a | 26.25±1.79***a | 149.51±4.28***a | 30.23±0.92***a |
| A. javanica (100 mg) + CCl4 | 163.33±5.28***b | 105.85±5.20***b | 29.76±1.02b | 112.39±6.82***b | 21.17±1.04***b |
| A. javanica (200 mg) + CCl4 | 143.66±5.47***b | 82.61±3.65** b | 35.41±2.26**b | 91.72±6.25***b | 16.52±0.70***b |
| Silymarin (10 mg) + CCl4 | 147.66±4.88***b | 110.16±5.26***b | 40.41±2.97**b | 85.21±5.98***b | 22.02±1.05***b |
All values represent mean±SEM
P<0.05
P<0.01
P<0.001; ANOVA, followed by Dunnet's multiple comparison test.
As compared with normal group.
As compared with CCl4-only group.
Table 3.
Biochemical parameters of rat liver tissues treated with A. javanica total ethanolic extract
| Treatment group | MDA (nmol/g) | NP-SH (nmol/g) | TP (g/l) |
|---|---|---|---|
| Control | 0.50±0.02 | 7.39±0.53 | 113.76±2.81 |
| CCl4 | 4.82±0.29***a | 3.86±0.44***a | 49.11±1.82***a |
| A. javanica (100 mg) + CCl4 | 2.77±0.10***b | 5.67±0.47*b | 67.86±2.94***b |
| A. javanica (200 mg) + CCl4 | 1.79±0.14***b | 6.16±0.40**b | 82.23±4.44***b |
| Silymarin (10 mg) + CCl4 | 1.37±0.16***b | 6.52±0.31***b | 91.81±4.08***b |
All values represent mean±SEM
P<0.05
P<0.01
P<0.001; ANOVA, followed by Dunnet's multiple comparison test.
As compared with normal group.
As compared with CCl4-only group.
Histological improvement by A. javanica total extract
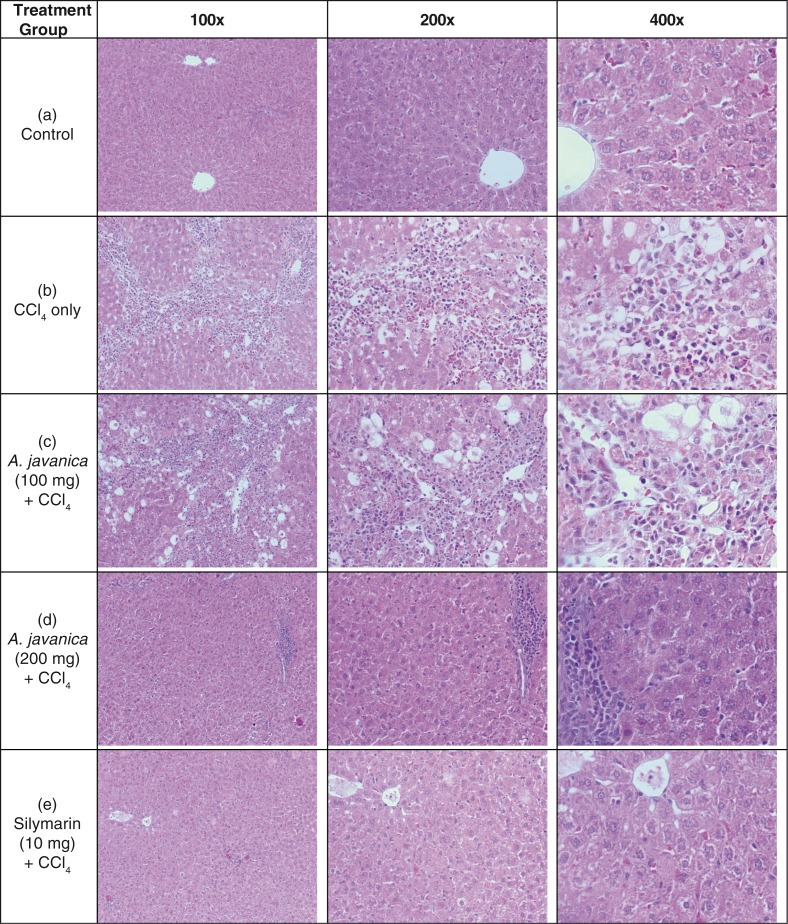
The histological examination of rat liver tissues revealed evidence of hepatic necrosis and fatty degenerative changes in CCl4-injured animals (Fig. 2a and b). Compared to this, the A. javanica-treated (100 mg/kg.bw/day) animals exhibited congested central vein with mild necrosis and fatty changes (Fig. 2c). On the other hand, the higher dose (200 mg/kg.bw/day) of A. javanica showed normal hepatocytes and central vein with full recovery that was comparable to silymarin administration (Fig. 2d and e). This finally confirmed the hepatoprotective efficacy of A. javanica total extract.
Fig. 2.
The histopathology of experimental rat liver at 100×, 200×, and 400× magnifications. Histograms showing the following: (a) healthy tissues with normal hepatocytes and central vein; (b) CCl4-injured tissue with necrosis and fatty degenerative changes; (c) tissue with congested central vein with necrosis and fatty changes after A. javanica (100 mg) + CCl4 treatment; (d) liver with normal hepatocytes and central vein with full recovery after A. javanica (200 mg) + CCl4 treatment; and (e) liver with normal hepatocytes and fully recovered central vein after silymarin (10 mg) + CCl4 treatment.
In vitro antioxidant activity of A. javanica
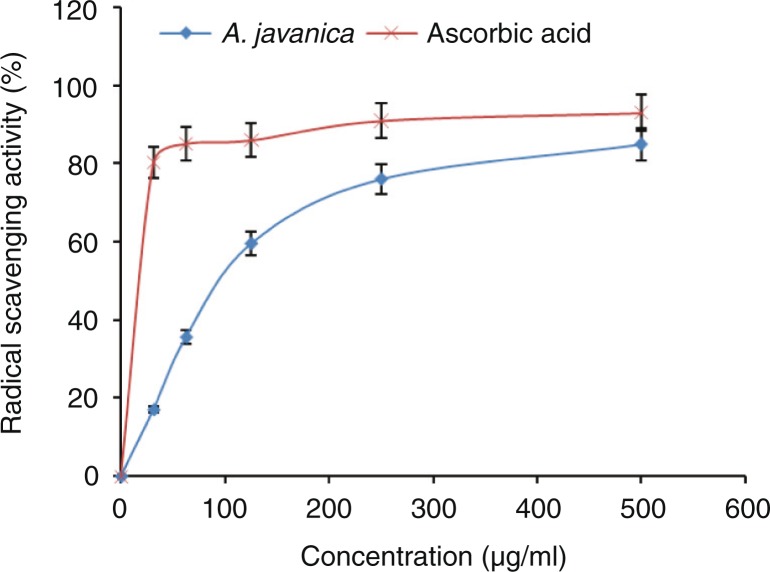
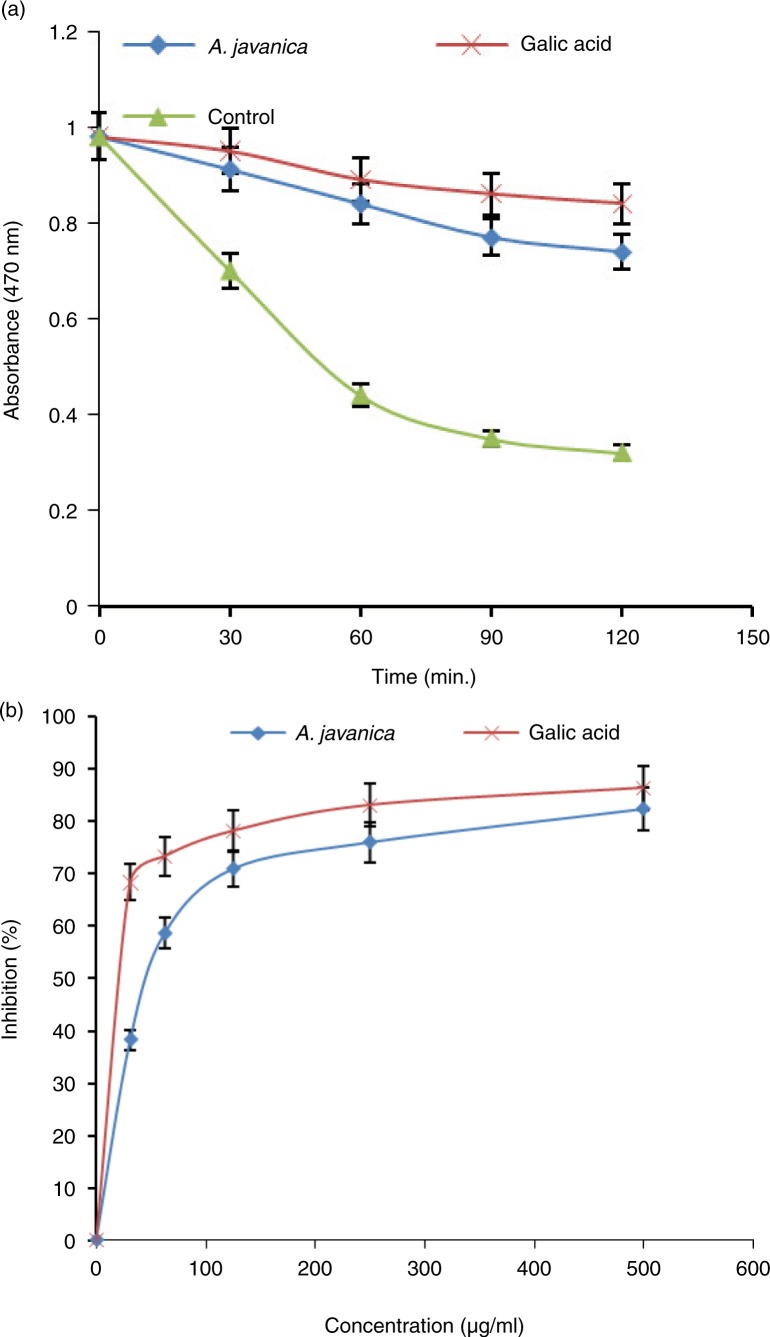
The total extract of A. javanica was able to reduce the stable free-radical DPPH to the yellow-colored DPPH (Fig. 3). At 500 µg/ml dose, the observed antioxidant activities were comparable to that of the positive control (ascorbic acid). In accordance with the DPPH radical scavenging assay results, A. javanica showed high antioxidant activity in β-carotene–linoleic acid bleaching assay (Fig. 4).
Fig. 3.
DPPH radical-scavenging activity of different concentrations (31.25–500 µg/ml) of total ethanolic extract of A. javanica and standard antioxidant (ascorbic acid).
Fig. 4.
Antioxidative activity of A. javanica total ethanolic extract assayed by the β-carotene bleaching method. (a) β-carotene bleaching rate in the presence of 500 µg/ml of the extract, gallic acid (reference antioxidant), or blank control. (b) % inhibition of lipid peroxidation by different concentrations (31.25–500 µg/ml) of the extract and gallic acid.
Phytochemical screening of the total extract
The qualitative phytochemical screening of the A. javanica total extract showed the presence of alkaloids, flavonoids, tannins, sterols, and saponins and the absence of anthraquinones and cardiac glycosides.
Identification of rutin, a biflavonoid in A. javanica extract
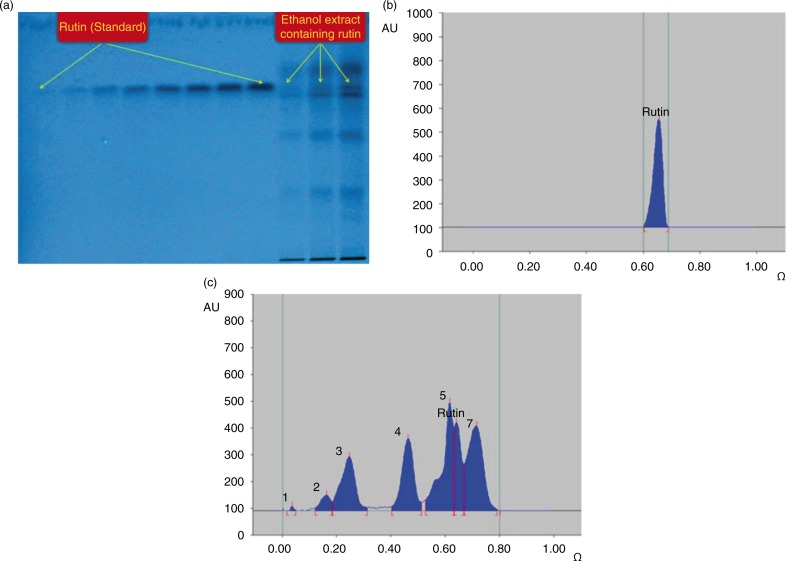
The HPTLC analysis revealed the presence of rutin, a well-known antioxidant flavonoid in the total extract (Fig. 5a). Using acetonitrile and water as suitable mobile phase, the compact spot of rutin was found at Rf=0.65 (Fig. 5b). Also, a good separation of different phytoconstituents present in the ethanol extract using the same mobile phase was achieved (Fig. 5c). The dried extract of A. javanica was found to contain 2.53 µg/mg of rutin.
Fig. 5.
Quantification of rutin in A. javanica total ethanolic extract by HPTLC. (a) Pictogram of developed RP-HPTLC plate at 254 nm; mobile phase – acetonitrile: water (4:6, v/v). (b) Chromatogram of standard rutin (1,000 ng spot−1), peak 1 (Rf=0.65) scanned at 360 nm; mobile phase – acetonitrile: water 4:6, v/v). (c) Chromatogram of the extract containing rutin (peak 6) scanned at 360 nm; mobile phase – acetonitrile: water 4:6, v/v).
Discussion
Among more than 100 species of medicinal plants documented in Saudi Arabia, approximately 35 plants are used in Saudi folk medicine for the treatment of liver disorders. However, most of them remain popular without scientific rationale. Of these, the hepatoprotective potential of Aerva javanica has been neither explored in traditional practices nor with experimental approaches. In this study, we have, therefore, evaluated the hepatoprotective and antioxidative potential of A. javanica total ethanolic extract against DCFH-induced cytotoxicity in HepG2 cells and CCl4-induced liver damage in rats, respectively. DCFH is generally used for the quantitative assessment of oxidative stress generated by free radicals through the principle of oxidation of DCFH to the fluorescent DCF (40). However, we used this agent because of its toxic effect on cultured cell, ex vivo. A. javanica total extract promoted HepG2 cell proliferation and recovery in a dose-dependent manner. These findings were further supported by microscopic examination of cell morphology and growth.
To further confirm the ex vivo cell proliferative effects, the in vivo hepatoprotective potential activity of A. javanica total extract was examined in CCl4-injured rats. CCl4 is a common hepatotoxin used in the experimental study of liver diseases that induces free-radical generation in liver tissues. In vivo, it is transformed to active free radicals that bind to macromolecules resulting in lipid peroxidation that causes cell injury (41, 42). Clinical symptoms of CCl4-induced acute liver injury include jaundice and elevated levels of serum SGOT, SGPT, and ALP, indicators of liver necrosis and inflammation (43). In the present study, pretreatment with two doses of A. javanica total extract (100 and 200 mg/kg.bw) showed its ability to reduce the SGOT, SGPT, GGT, ALP, and bilirubin levels significantly in a dose-dependent manner. In addition, A. javanica extract was able to normalize the serum cholesterol and triglycerides and HDL levels in CCl4-injured rats. The hepatoprotective effect of A. javanica at 200 mg/kg.bw was comparable to standard drugs silymarin (10 mg/kg.bw). The rise of serum enzyme SGOT and SGPT levels had indicated that hepatocyte cells were injured where the leakage of cell membrane had contributed to the accumulation of these enzymes into the plasma (44). The significant reduction in the levels of LDL, and total cholesterol in the A. javanica-treated rats and an increase in the HDL level further indicated the hepatoprotective potential of A. javanica.
MDA is produced during the peroxidation of polyunsaturated fatty acids, and the amount of MDA is a marker for cell membrane lipid peroxidation and cell damage (45). Treatment with A. javanica extract or silymarin reduced the level of MDA in livers with CCl4-induced damage, which suggests that the tested extract was protective and curative against liver toxicity.
NP-SH plays an important role in the defense against oxidative cellular damage (46). In our study, the liver NP-SH level in the CCl4-treated group was markedly reduced when compared with the control group. Pretreatment of rats with A. javanica total extract or silymarin replenished NP-SH concentration as compared with CCl4 only treated animals, suggesting free-radical scavenging activity of the tested extract.
The level of TP would be decreased in hepatotoxic conditions due to defective protein biosynthesis in liver. Thus, the reduction in TP level is a further indication of liver damage in CCl4-injured animals (47). In our study, the level of TP has been restored to the normal value, indicating the curative action of A. javanica total ethanolic extract. Moreover, most of the parameters in the animal group that received CCl4 plus A. javanica extract had values comparable to those of the group receiving CCl4 plus silymarin. This very clearly indicated that A. javanica was efficient to attenuate CCl4-induced hepatotoxicity in rats.
Conducting different assay methods for the evaluation of antioxidant activity (48, 49) is now commonly recommended. In our in vitro assays, the antioxidant activity of A. javanica ethanolic extract revealed strong antioxidant activities in both DPPH and β-carotene methods. In addition, our preliminary phytochemical screening of the A. javanica total extract showed the presence of alkaloids, flavonoids, tannins, sterols, and saponins. Taken together, the antioxidant activity of the A. javanica extract could be attributed to the presence of antioxidant and free-radical scavenging phytoconstituents, such as polyphenol, flavonoids, and saponins, which are known to have hepatoprotective activities. Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside), a biflavonoid found in many plants, has a wide range of pharmacological properties against various metabolic and infectious diseases (50). The reactive-species-mediated in vivo hepatotoxicity can be effectively attenuated by rutin possessing antioxidant, free-radical scavenger, and anti-lipid per oxidant activities (51). Recently, hepatoprotective mechanisms of rutin in CCl4-intoxicated BALB/cN mice has also been demonstrated (52). Our identification of rutin in A. javanica extract by validated HPTLC method further supports its therapeutic use in the prevention and treatment of liver diseases.
Very recently, A. Javanica-derived aervecdysteroid A–D (1–4) and acylated flavone glycosides have been shown to inhibit enzymatic activities of lipoxygenase and butyrylcholinesterase (53). In mammals, lipoxygenases catalyze the formation of hydroperoxides as the first step in the biosynthesis of several inflammatory mediators (54). Butyrylcholinesterase, also known as serum or plasma cholinesterase, is synthesized in the liver that hydrolyzes many different choline-based esters. In clinical assays, serum butyrylcholinesterase activity is used as a liver function test for acute and chronic liver damage, including other inflammatory disorder (55). Nevertheless, further biological analysis of these compounds, including identification of other bioactive constituents of A. javanica with antioxidative and hepatoprotective activities, remains to be explored.
Conclusions
Our investigation of A. javanica total ethanol extract revealed its promising antioxidative and hepatoprotective potential against chemically induced liver injury ex vivo as well as in vivo. This was supported by the in vitro phytochemical analysis and identification of rutin, a well-known antioxidant flavonoid. Our finding therefore, suggests the therapeutic potentiality of A. javanica in various liver diseases. However, further studies on main active ingredients of the extract, their mechanism of action, and other therapeutic contribution of these interventions would be necessary.
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (MED11-1585-02).
Conflicts of interest and funding
The authors declare that they do not have any conflicts of interest.
References
- 1.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–4. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 2.Liu J. Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World J Gastroenterol. 2014;20:14672–85. doi: 10.3748/wjg.v20.i40.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplowitz N. Drug-induced liver injury. Clin Infect Dis. 2004;1:S44–8. doi: 10.1086/381446. [DOI] [PubMed] [Google Scholar]
- 4.Aniya Y, Koyama T, Miyagi C, Miyahira M, Inomata C, Kinoshita S, et al. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol Pharm Bull. 2005;28:19–23. doi: 10.1248/bpb.28.19. [DOI] [PubMed] [Google Scholar]
- 5.Salam OMEA, Sleem AA, Omara EA, Hassan NS. Hepatoprotective effects of misoprostol and silymarin on carbon tetrachloride-induced hepatic damage in rats. Fund Clin Pharmacol. 2009;23:179–88. doi: 10.1111/j.1472-8206.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 6.Alqasoumi SI, Abdel-Kader MS. Screening of traditional Saudi plants for hepatoprotective effect. Planta Med. 2008;74:1139. [Google Scholar]
- 7.Dandagi PM, Patil MB, Mastiholimath VS, Gadad AP, Dhumansure RH. Development and evaluation of hepatoprotective polyherbal formulation containing some indigenous medicinal plants. Indian J Pharm Sci. 2008;70:265–8. doi: 10.4103/0250-474X.41474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinjo J, Hitoshi M, Tsuchihashi R, Korematsu Y, Miyakoshi M, Murakami T, et al. Hepatoprotective constituents in plants 15: protective effects of natural-occurring flavonoids and miscellaneous phenolic compounds as determined in an HepG2 cell cytotoxicity assay. J Nat Med. 2006;60:36–41. [Google Scholar]
- 9.Ganie SA, Zargar BA, Masood A, Zargar MA. Hepatoprotective and antioxidant activity of rhizome of Podophyllum hexandrum against carbon tetra chloride induced hepatotoxicity in rats. Biomed Environ Sci. 2013;26:209–21. doi: 10.3967/0895-3988.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Al-Said MS, Mothana RA, Al-Yahya MA, Al-Blowi AS, Al-Sohaibani M, Ahmed AF, et al. Edible oils for liver protection: hepatoprotective potentiality of Moringa oleifera seed oil against chemical-induced hepatitis in rats. J Food Sci. 2012;77:124–30. doi: 10.1111/j.1750-3841.2012.02698.x. [DOI] [PubMed] [Google Scholar]
- 11.Chawla P, Chawla A, Vasudeva N, Sharma SK. A review of chemistry and biological activities of the genus Aerva – a desert plant. Acta Pol Pharm. 2012;69:171–7. [PubMed] [Google Scholar]
- 12.Samejo MQ, Memon S, Bhanger MI, Khan KM. Chemical compositions of the essential oil of Aerva javanica leaves and stems. Pak J Anal Environ Chem. 2012;13:48–51. [Google Scholar]
- 13.Khan AW, Jan S, Parveen S, Khan RA, Saeed A, Tanveer AJ, et al. Phytochemical analysis and enzyme inhibition assay of Aerva javanica for Ulcer. Chem Cent J. 2012;6(1):76. doi: 10.1186/1752-153X-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed N, Mahmood A, Sadeghi Z, Farman M. Ethnopharmacological importance of medicinal flora from the district of Vehari, Punjab province, Pakistan. J Ethnopharmacol. 2015;168:66–78. doi: 10.1016/j.jep.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Sharif A, Ahmed E, Malik A, Mukhtar-Ul-Hassan, Munawar MA, Farrukh A, et al. Antimicrobial Constituents from Aerva javanica . J Chem Soc Pakistan. 2011;33:439–43. [Google Scholar]
- 16.Mufti FUD, Ullah H, Bangash A, Khan N, Hussain S, Ullah F, et al. Antimicrobial activities of Aerva javanica and Paeonia emodi plants. Pak J Pharm Sci. 2012;25:565–9. [PubMed] [Google Scholar]
- 17.Khader JA, Ahmad S, AbdElsalam NM, Ullah R, Islam M. Antifungal activities of different crude fractions of Aerva javanica . J Pure Appl Microbio. 2012;6:1849–52. [Google Scholar]
- 18.Kumar V, Kaur P, Suttee A. Possible role of alcoholic extract of roots of Aerva javanica in cisplatin and gentamicin-induced nephrotoxicity in rats. Indian J Pharmacol. 2008;40:167. [Google Scholar]
- 19.Mussadiq S, Riaz N, Saleem M, Ashraf M, Ismail T, Jabbar A. New acylated flavonoid glycosides from flowers of Aerva javanica . J Asian Nat Prod Res. 2013;15:708–16. doi: 10.1080/10286020.2013.795553. [DOI] [PubMed] [Google Scholar]
- 20.Samejo MQ, Memon S, Bhanger MI, Khan KM. Comparison of chemical composition of Aerva javanica seed essential oils obtained by different extraction methods. Pak J Pharm Sci. 2013;26:757–60. [PubMed] [Google Scholar]
- 21.Parveen R, Baboota S, Ali J, Ahuja A, Vasudev SS, Ahmad S. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch Pharmacol Res. 2011;34:767–74. doi: 10.1007/s12272-011-0510-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen IS, Chen YC, Chou CH, Chuang RF, Sheen LY, Chiu CH. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J Sci Food Agric. 2012;92:1441–7. doi: 10.1002/jsfa.4723. [DOI] [PubMed] [Google Scholar]
- 23.Li CC, Hsiang CY, Wu SL, Ho TY. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-kappaB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxicol. 2012;50:1568–75. doi: 10.1016/j.fct.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 25.King EJ, Armstrong AR. Calcium, phosphorus and phosphate. In: Varley H, editor. Practical clinical biochemistry. New Delhi: CBS; 1988. [Google Scholar]
- 26.Fiala S, Fiala AE, Dixon B. Glutamyl transpeptidase in transplantable, chemically induced rat hepatomas and “spontaneous” mouse hepatomas. J Natl Cancer Inst. 1972;48:1393–401. [PubMed] [Google Scholar]
- 27.Stiehl A. (Hyperbilirubinemia in liver diseases) Fortschr Med. 1982;100:842–5. [PubMed] [Google Scholar]
- 28.Demacker PN, Hijmans AG, Vos-Janssen HE, van't Laar A, Jansen AP. A study of the use of polyethylene glycol in estimating cholesterol in high-density lipoprotein. Clin Chem. 1980;26:1775–9. [PubMed] [Google Scholar]
- 29.Foster LB, Dunn RT. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin Chem. 1973;19:338–40. [PubMed] [Google Scholar]
- 30.Burstein M, Scholnick HR. Turbidimetric estimation of chylomicrons and very low density lipoproteins in human sera after precipitation by sodium lauryl sulfate. Biomedicine. 1973;19:16–19. [PubMed] [Google Scholar]
- 31.Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118:29–32. [Google Scholar]
- 32.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 33.Culling CF. Handbook of histopathological and histochemical techniques. 3rd ed. London: Butterworth; 1974. [Google Scholar]
- 34.Lee Y-J, Kim D-B, Lee J, Cho J-H, Kim B, Choi H-S, et al. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18:12937–50. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller HE. A simplified method for the evaluation of antioxidants. J Am Oil Chem Soc. 1971;48:91–2. [Google Scholar]
- 36.Sarker SD, Latif Z, Gray AI. Natural products isolation. 2nd ed. Totowa, NJ: Humana Press; 2006. [Google Scholar]
- 37.Kar A. Pharmacognosy and pharmacobiotechnology. 2nd ed. New Delhi: New Age International; 2007. [Google Scholar]
- 38.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1:98–106. [Google Scholar]
- 39.Rota C, Chignell CF, Mason RP. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic Biol Med. 1999;27:873–81. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 40.Bala A, Haldar PK, Kar B, Naskar S, Mazumder UK. Carbon tetrachloride: a hepatotoxin causes oxidative stress in murine peritoneal macrophage and peripheral blood lymphocyte cells. Immunopharmacol Immunotoxicol. 2012;34:157–62. doi: 10.3109/08923973.2011.590498. [DOI] [PubMed] [Google Scholar]
- 41.Kus I, Ogeturk M, Oner H, Sahin S, Yekeler H, Sarsilmaz M. Protective effects of melatonin against carbon tetrachloride-induced hepatotoxicity in rats: a light microscopic and biochemical study. Cell Biochem Funct. 2005;23:169–74. doi: 10.1002/cbf.1136. [DOI] [PubMed] [Google Scholar]
- 42.Tirkey N, Pilkhwal S, Kuhad A, Chopra K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:2. doi: 10.1186/1471-2210-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78:713–18. doi: 10.1016/j.lfs.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 44.Suhail M, Suhail S, Gupta BK, Bharat V. Malondialdehyde and antioxidant enzymes in maternal and cord blood, and their correlation in normotensive and preeclamptic women. J Clin Med Res. 2009;1:150–7. doi: 10.4021/jocmr2009.07.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272–7. doi: 10.1016/s0367-326x(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 46.Navarro VJ, Senior JR. Current concepts – drug-related hepatotoxicity. New Engl J Med. 2006;354:731–9. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 47.Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–52. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–22. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.AlAjmi MF, Alam P, Siddiqui NA, Basudan OA, Hussain A. Quantitative analysis of biomarker rutin in different species of genus Ficus by validated NP and RP-HPTLC methods. Pak J Pharm Sci. 2015;28:2213–20. [PubMed] [Google Scholar]
- 50.Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 2014;37:783–8. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 51.Janbaz KH, Saeed SA, Gilani AH. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557–63. doi: 10.1016/s0367-326x(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 52.Domitrović R, Jakovac H, Vasiljev Marchesi V, Vladimir-Knežević S, Cvijanović O, Tadić Z, et al. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol Sin. 2012;33:1260–70. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prigge ST, Boyington JC, Faig M, Doctor KS, Gaffney BJ, Amzel LM. Structure and mechanism of lipoxygenases. Biochimie. 1997;79:629–36. doi: 10.1016/s0300-9084(97)83495-5. [DOI] [PubMed] [Google Scholar]
- 54.Saleem M, Musaddiq S, Riaz N, Zubair M, Ashraf M, Nasar R, et al. Ecdysteroids from the flowers of Aerva javanica . Steroids. 2013;78:1098–102. doi: 10.1016/j.steroids.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Santarpia L, Grandone I, Contaldo F, Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle. 2013;4:31–9. doi: 10.1007/s13539-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]