Our bodies collectively turnover about 200–300 billion cells every day. Such turnover is an integral part of embryonic and postnatal development, as well as routine tissue homeostasis. This process involves induction of programmed cell death in specific cells within the tissues, and the specific recognition and removal of dying cells by a clearance crew composed of the professional, non-professional, and specialized phagocytes. In the past few years, remarkable progress has been made in uncovering many features of apoptotic cell clearance. Some of these new observations challenge the way we view dying cells themselves, and how healthy cells interact with and respond to dying cells. Here, we focus on the homeostatic removal of apoptotic cells in tissues.
Among the different forms of cell death, caspase-dependent apoptosis is thought to account for the majority of homeostatic cellular turnover1. Apoptosis is characterized by cell rounding and shrinking, chromatin condensation, and the formation of plasma membrane blebs or apoptotic bodies2. Apoptotic cell death helps to eliminate cells that are old or no longer needed, without causing damage to the surrounding tissues or initiating an immune response. As part of routine homeostasis, different tissues turnover varying numbers of apoptotic cells, with some tissues undergoing an impressive rate of renewal: hematopoiesis produces billions of cells daily, many with short lifespans (such as neutrophils); epithelial cells of the gastrointestinal tract, which cover an area equivalent in size to a tennis court, are turned over every 4–5 days; in the thymus and the bone marrow, millions of thymocytes and immature B cells, respectively, are eliminated during maturation; in the brain, adult neurogenesis produces thousands of new neurons daily, but only a few survive; and in the testes, spermatogenesis produces millions of germ cells, of which many undergo apoptosis. In addition, there are increased homeostatic turnovers under certain conditions, such as during involution of the mammary gland post-lactation and weaning3. In some situations, pieces of cells (rather than whole cells) are phagocytosed, for example during neuronal pruning. Finally, there are situations where the number of apoptotic cells increases beyond the normal rate within a given tissue, such as during an infection or acute tissue injury.
In these contexts, disposal of apoptotic cells needs to be performed quickly and without eliciting inflammation in the local tissue milieu2, 4. Under homeostatic conditions, the tissue resident phagocytes mediate the corpse removal. In cases of increased cell death, due to infections (epithelial cell apoptosis during lung infections) or sustained ‘sterile’ inflammation (atherosclerotic plaques), corpse clearance is mediated both by resident phagocytes and those recruited from the circulation. Failures in clearing apoptotic cells at early stages of death and their progression to a secondary necrotic state can induce tissue inflammation due to the release of cellular contents or exposure of otherwise sequestered intracellular moieties2. The critical decision of whether or not to initiate an immune response to the dying cell is made by the cell clearance machinery, in response to molecules released by and/or exposed on the dying cells. The phagocyte ultimately responds by actively suppressing or eliciting inflammation2, 4.
Phagocyte types and the tissue contexts
Homeostatic corpse removal within a tissue is determined by the composition of the local ‘clearance crew’. Phagocytes that ingest apoptotic cells have been previously divided into ‘professional’ and ‘non-professional’ phagocytes. Based on existing evidence we suggest a third category, ‘specialized’ phagocytes’ (Fig. 1).
Fig. 1.
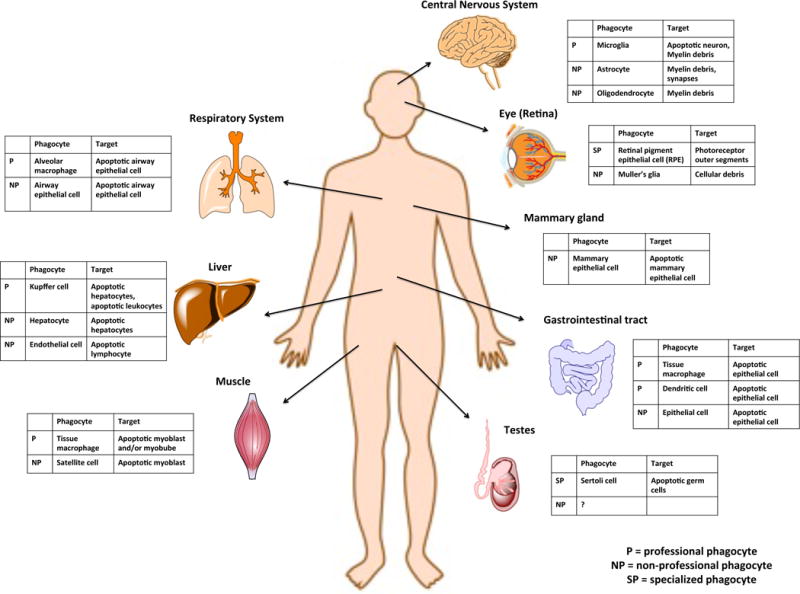
Homeostatic clearance of apoptotic cells via different phagocytes. In many tissues of the body, clearance of apoptotic cells is performed by the professional phagocytes (P), which include the tissue resident macrophages and immature dendritic cells. Many non-hematopoietic cells also have phagocytic functions in ex vivo or in vitro systems. These non-professional phagocytes (NP) include epithelial cells, hepatocytes and endothelial cells of the liver11, astrocytes, oligodendrocytes and neuronal progenitor cells of the central nervous system108–111, or the Muller’s glia of the eye112. Satellite cells of the skeletal muscle have also been reported to engulf apoptotic myoblasts37. Specialized phagocytes (SP) are multifunctional cells that engulf apoptotic cells and include retinal pigment epithelial cell (RPE)113, and Sertoli cells in the testes16.
Professional phagocytes include macrophages and immature dendritic cells. Macrophages have long been known as professional engulfers of apoptotic cells due to their high capacity for engulfment in vitro and in vivo5, 6. Although early studies used macrophages of different sources (native, thioglycollate-elicited, bone marrow derived etc.), our understanding of macrophage types has substantially grown in recent years7, 8. Elegant series of studies now suggest that embryonic yolk-sac derived stem cells colonize most tissues and contribute to the resident macrophage pool9, 10. This self-renewing population differentiates into specific types of tissue resident macrophages, such as peritoneal macrophages, Kupffer cells in the liver, alveolar macrophages in the lung, and microglia in the brain. These resident macrophage-like cells clear dying cells and debris: Kupffer cells clear aged red blood cells11, while microglia clear dying neurons and prune mature neurons12. Besides resident phagocytes, circulating monocytes can also be recruited during infection or injury. Recruited phagocytes can cooperate (or compete) with the resident phagocytes, and thereby influence the immune response13.
Non-professional phagocytes, such as epithelial cells and fibroblasts, have recently gained appreciation for their ability to clear apoptotic cells under routine homeostatic conditions (Fig. 1). Although termed ‘non-professional’ due to their lower efficiency of phagocytosis compared to professional phagocytes, non-professional phagocytes play a major role in tissues where macrophages are scarce or when macrophage access to apoptotic cells is not readily achieved – such as in the alveoli of the lung or the intestinal epithelium. The importance of non-professional phagocytes in corpse clearance has been revealed in several contexts. In macrophage-deficient animals, apoptotic cells generated during development continue to be cleared, albeit with lower efficiency14. Similarly, airway epithelial cells engulf dying apoptotic airway epithelial cells, requiring the small GTPase Rac1 (which functions downstream of several engulfment receptors)15. Epithelial cell-specific deletion of Rac1 results in increased susceptibility to allergen-induced airway inflammation and decreased production of anti-inflammatory mediators15. Similarly, intestinal epithelial cells can also engulf their neighbors in vivo, contributing to the regulation of inflammatory sequelae (Lee et al., unpublished observations). Moreover, during involution of the mammary tissue post-lactation, the epithelial cells of the mammary gland (rather than macrophages) function as primary engulfers3. Because epithelial cells vastly outnumber professional phagocytes and are likely the first to contact a dying adjacent epithelial cell, engulfment by neighboring cells might help maintain the tissue barrier while providing the benefits of anti-inflammatory cytokine production15.
Specialized phagocytes are hybrid, multi-functional phagocytes that are increasingly recognized for their importance in specific tissue contexts. The best examples are Sertoli cells of the testes and retinal pigment epithelial cells (RPE) of the eye (Fig. 1). Sertoli cells, which are non-hematopoietic and post-mitotic, line the epithelium of seminiferous tubules and make up the blood-testes barrier. Sertoli cells clear millions of apoptotic germ cells that arise during spermatogenesis. Their hybrid function is exemplified by the fact that a single Sertoli cell is often in contact with 30–40 germ cells in various stages of differentiation. In addition to serving as nurse cells for the developing spermatocytes, the Sertoli cells phagocytose those germ cells that display improper meiosis or other developmental abnormalities, and disruption of either apoptosis or engulfment can affect spermatogenesis16, 17. Another example of specialized phagocytes are the RPE cells. RPE are long-lived cells that play a critical role in the homeostatic photoreceptor outer segment removal that occurs daily in a circadian fashion (with RPE uptake triggered by light onset)18. Each RPE is estimated to engulf thousands of outer segment discs over its lifetime. Failures in RPE-mediated removal of outer segments can severely affect the integrity of retinal layers and contribute to a predisposition for adverse conditions, such as retinitis pigmentosa18.
Accessing and identifying apoptotic cells
Based on studies by a number of groups, engulfment of apoptotic cells includes distinguishable steps (Fig. 2). First, the dying cell releases ‘find-me’ signals to attract and/or activate the phagocytes. The phagocytes then distinguish the apoptotic cell from healthy living cells via specific engulfment receptors, which recognize ‘eat-me’ signals on the dying cell. Next, the phagocyte undergoes extensive cytoskeletal rearrangement to internalize corpses that are often the same size (e.g. an epithelial cell eating its neighbor). The final step is the processing of the ingested cargo and elicitation of specific phagocyte responses, primarily the secretion of anti-inflammatory mediators that help dampen the local immune response.
Fig. 2.
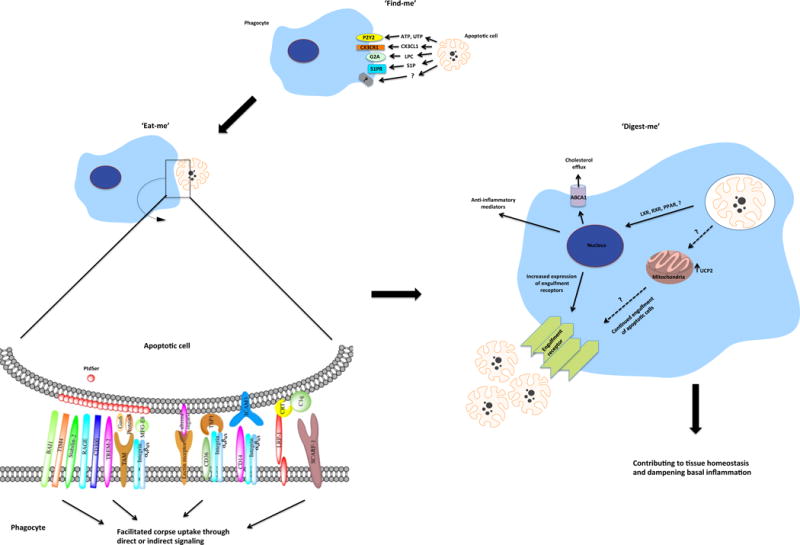
Steps during phagocytosis of apoptotic cells. When a cell initiates the apoptotic program, it releases soluble ‘find-me’ signals that attract phagocytes. The apoptotic cell is distinguished from the nearby living cell via the exposure of ‘eat-me’ signals, the most prominent of which is the phosphatidylserine (PtdSer). Eat-me signals are recognized by different engulfment receptors on the phagocytes, resulting in signaling events that facilitate the apoptotic corpse uptake. Engulfment also elicits the transcriptional up-regulation of the cholesterol efflux transporter ABCA1, and increased expression of engulfment receptors. Within the mitochondria, the levels of the uncoupling protein UCP2 are increased, enabling the continued uptake of apoptotic corpses. Anti-inflammatory mediators are expressed and secreted, contributing to tissue homeostasis and inhibition of local inflammation. PtdSer, phosphatidylserine; P2Y2, purinergic P2 receptor Y2; CX3CR1, CX3C chemokine receptor-1; G2A, G protein-coupled receptor G2A; S1PR, sphingosine-1-phosphate receptor; ATP, adenosine triphosphate; UTP, uridine triphosphate; CX3CL1, chemokine (C-X3-C motif) ligand-1, also known as Fractalkine; LPC, lysophosphatydilcholine; S1P, sphingosine-1-phosphate; LXR, liver X receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator activated receptor; BAI1, brain angiogenesis inhibitor-1; TIM4, T cell immunoglobulin and mucin domain containing molecule-4; RAGE, receptor for advanced glycation end products; TREM-2, triggering receptor expressed on myeloid cells-2; TAM, Tyro Axl Mer family receptor; LRP1, low density lipoprotein receptor related protein-1; SCARF-1 is also known as SREC-1, scavenger receptor expressed by endothelial cell-1; Gas6, growth arrest specific-6; MFG-E8, milk fat globule EGF factor-8; TSP1, thrombospondin-1; ICAM3, intracelullar adhesion molecule-3; CRT, calreticulin.
The release of find me signals is a critical first step in many tissues, as it recruits a potentially distant phagocyte to the dying cell. In some tissues this is particularly important, as in the developing thymus; a dying thymocyte is unlikely to be eaten by its neighbor, as lymphocytes generally lack the capacity to engulf apoptotic cells. Therefore, motile resident phagocytes have to be recruited to the proximity of apoptotic thymocytes. This is achieved through the release of find-me signals from the dying cell, including: nucleotides (ATP, UTP), the chemokine fractalkine (CX3CL1), and the lipids lysophosphatidylcholine and sphingosine-1-phosphate19–23. Among these, only nucleotides and a nucleotide receptor on the phagocyte (P2Y2) are linked to the clearance of apoptotic thymocytes in vivo19. It is possible that find-me signals may serve other functions, such as during the removal of dying epithelial cells by a viable neighbor, when recruitment is not required. Since apoptotic epithelial cells also release find-me signals19, perhaps these signals influence/enhance the engulfment capacity of the neighbor(s). For example, CX3CL1 stimulates phagocyte expression of the milk fat globule-EGF factor 8 (MFG-E8), which bridges apoptotic cells to the phagocytes to facilitate engulfment24.
Next is the recognition of specific eat-me signals on apoptotic cells by engulfment receptors on the phagocytes. To date, the best-studied eat-me signal on apoptotic cells is the exposure of the lipid phosphatidylserine (PtdSer), which is evolutionarily conserved from Caenorhabditis elegans to humans25, 26. In living cells, PtdSer is actively restricted to the inner leaflet of the plasma membrane27, and recent elegant studies from the Nagata group have identified apoptosis-mediated as well as calcium-induced modes of PtdSer exposure28–30. In addition to PtdSer, other moieties that are variably exposed on apoptotic cells include a modified form of intracellular adhesion molecule-3 (ICAM-3), oxidized low-density lipoprotein, calreticulin, annexin I, cell surface-bound thrombospondin and complement C1q, as well as changes in the surface protein charge and glycosylation status31. Conversely, viable cells avoid their removal by displaying ‘don’t-eat-me’ signals CD47 and CD31 or by binding to the CD300a receptor on the phagocyte, and suppressing phagocyte functions32–34.
Engulfment receptors linked to homeostatic clearance
Multiple apoptotic cell recognition and engulfment receptors have been identified in inflammatory and/or homeostatic contexts. These receptors come in different flavors, such as scavenger receptor family members, immunoglobulin domain containing proteins, 7-transmembrane proteins, tyrosine kinases, etc.31. Why we have many different types of engulfment receptors and how they provide specificity is still unclear. In some respects, the diversity of engulfment receptors is similar to that of accessory proteins linked to T cell interaction with antigen presenting cells (APC). While the exposed PtdSer could be viewed as loosely analogous in function to the MHC molecule on an APC, the distinction between the phagocyte-apoptotic cell interaction and the T cell-APC interface lies in the lack of an equivalent to the T cell receptor (TCR) on phagocytes. Rather, the role of the TCR seems to be distributed among the different engulfment receptors. What we have learned so far from studies in animals with specific deletions of individual PtdSer receptors is that, while there is redundancy in function, at least in some cases there are specific needs for particular engulfment receptors. Since not all engulfment receptors are expressed on all phagocyte types, the differences in expression between professional and non-professional phagocytes might influence the homeostatic turnover of dying cells. In fact, a diverse set of phenotypes have been reported in mice with alterations in various molecules linked to PtdSer recognition (Table 1).
Table 1.
Phenotypes in mice lacking receptors linked to PtdSer recognition.
| Mouse strain | Homeostasis phenotype | Induced phenotype | Comment | References |
|---|---|---|---|---|
| Direct PtdSer receptors | ||||
|
Adgrb1−/− (BAI1) |
|
|
37, 40, 41 | |
|
Timd4−/− (TIM4) |
|
|
Reports of autoimmune disease in this strain vary. | 48–50, 116. |
| TIM4-Tg | • |
|
51 | |
|
Stab1−/− (Stabilin-1) |
• |
|
Same phenotype observed in mice with the conditional deletion of Stabilin-1 in macrophages or hematopoietic and endothelial compartment. | 117 |
|
Stab2−/− (Stabilin-2) |
• |
|
118 | |
|
Stab1−/−
Stab2−/− −/− (double deficient) |
|
• | 119 | |
|
Ager−/− (RAGE) |
|
|
120–124 | |
|
Cd300lf−/− (CD300f) |
• |
|
125 | |
| Trem2−/− |
|
|
Opposing phenotypes observed in two mouse models of Alzheimer’s disease. | 126–129 |
| Indirect PtdSer receptors | ||||
| Mer-TK |
|
|
56, 57, 130–137 | |
|
Itgavfl/fl Tek-Cre (Integrin subunit αv) |
|
|
138, 139 | |
|
Itgavfl/fl Lyz2-Cre |
|
|
138, 139 | |
| Itgavfl/flNes-Cre |
|
• | 140 | |
|
Itgavfl/fl Gfap-Cre |
|
• | Specific strain of GFAP-Cre was used. | 141 |
|
Itgb5−/− (Integrin subunit β5) |
|
• | 142 | |
| Bridging molecules | ||||
|
Mfge8−/− (MFG-E8) |
|
|
Reports of autoimmune disease in this strain vary. | 50, 143–149 |
| Gas6−/− | • |
|
150–156 | |
| Protein S deficiency in T cells | • |
|
87 | |
| Other receptors | ||||
| Cd36−/− | • |
|
157–160 | |
| Lrp1fl/flLyz2-Cre | • |
|
161–163 | |
| Lrp1fl/flItgax-Cre | • |
|
164 | |
| Scarf1−/− |
|
• | 165 | |
| C1qa−/− |
|
|
166–170 | |
We discuss below three specific receptors that engage phosphatidylserine: TIM-4, BAI1, and MerTK (Fig. 3). TIM-4 can bind PtdSer directly, but does not have a signaling capacity on its own (i.e., a tethering receptor), while BAI1 is a 7-transmembrane protein that can directly engage PtdSer and also relay intracellular signaling to mediate engulfment. MerTK, on the other hand, is a membrane tyrosine kinase that cannot engage PtdSer directly, but uses bridging molecules that bind PtdSer on apoptotic cells. We chose these receptors as they highlight some of the complexities in PtdSer recognition, and are linked to homeostatic cell turnover.
Fig. 3.
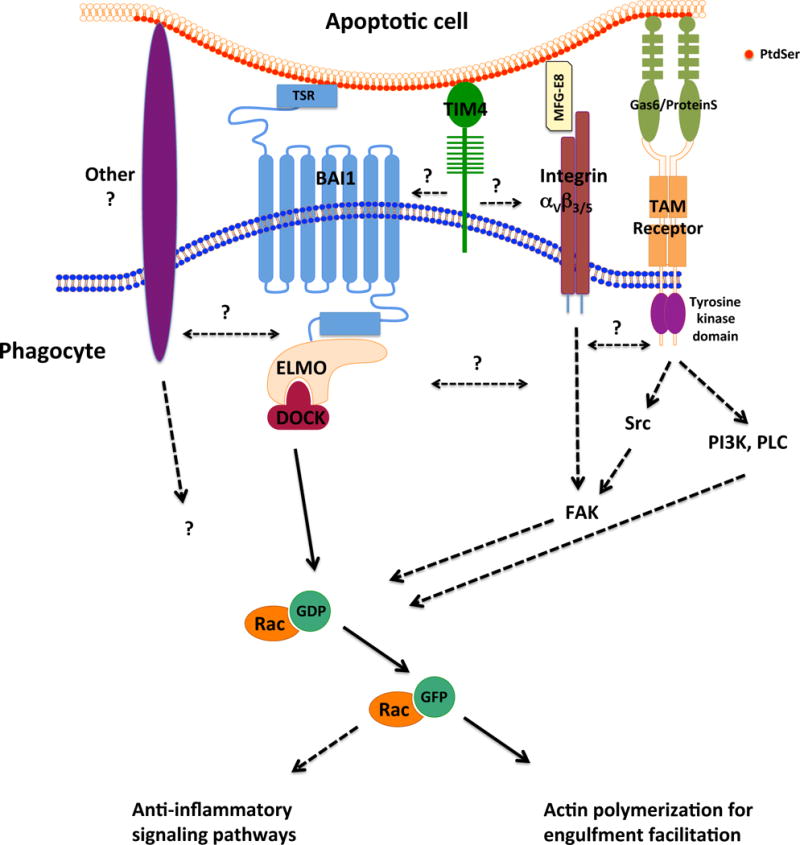
Signaling pathways elicited by three PtdSer recognition receptors. Binding of the apoptotic cell to the phagocyte triggers signaling pathways. BAI1 is a 7-transmembrane receptor that directly binds the PtdSer on the surface of an apoptotic cell, resulting in the recruitment of the Engulfment and cell motility (ELMO)/Downstream of Crk (DOCK) complex, which functions as a guanine exchange factor for the small GTPase Rac39. Rac activation promotes actin cytoskeleton remodeling required for the engulfment of the apoptotic corpse. Integrins αVβ3 or αVβ5 and the Tyro Axl Mer (TAM) family receptors bind apoptotic cells indirectly, via PtdSer-bound bridging molecules MFG-E8, Gas6 or ProteinS, resulting in the activation of the focal adhesion kinase (FAK) and contributing to the activation of Rac114. TAM receptors are tyrosine kinases that also activate cell signaling pathways involving the kinases Src and phosphatidylinositol-3-kinase (PI3K) and phospholipase C (PLC)114, 115. TIM4 functions as a tethering receptor bringing the apoptotic cell in contact with signaling engulfment receptors, and signal through co-receptors. The extent of the connection between the signals elicited by different engulfment receptors awaits further characterization.
BAI1, with the homologs BAI2 and BAI3, belongs to the adhesion subfamily of G-protein-coupled receptors35. Originally identified as an inhibitor of angiogenesis, BAI1 plays a functional role in diverse biological processes, including phagocytosis of apoptotic cells, myoblast fusion, synaptogenesis, and tumor growth36–40. Via its thrombospondin repeats, BAI1 can directly bind PtdSer 39. Upon PtdSer recognition, BAI1 interacts with a cytoplasmic signaling module composed of ELMO1 and Dock180, which function as a guanine exchange factor for Rac1, thereby inducing actin cytoskeletal rearrangements and facilitating the apoptotic cell uptake39.
Although BAI1 deficient mice are grossly normal, several key homeostatic defects are seen. Adult mice with global deletion of BAI1 have smaller skeletal muscle fibers and display delayed healing after muscle injury; since myoblast fusion also appears to involve PtdSer exposure, these results likely reflect an interesting additional function of BAI137. In peritoneal macrophages, binding of apoptotic cells to BAI1 triggers signaling that promotes cholesterol efflux 41, and contributes to the maintenance of lipid homeostasis (see further below). It has also been independently reported that mice lacking BAI1 have deficits in spatial learning and memory. This could be due to BAI1 function in regulating postsynaptic density40. BAI1 expression is particularly high in the brain, testes, and certain hematopoietic compartments39. Although BAI1 mRNA levels in macrophages are lower than those of TIM-4 or MerTK mRNA (unpublished observations), macrophages from BAI1 and TIM-4 deficient mice have comparable deficiencies in the uptake of apoptotic cells41. However, direct comparisons of BAI1 mRNA and protein levels have not been reported to date. BAI1 expression may also be regulated post-transcriptionally, or BAI1 might influence engulfment via mechanisms not requiring high expression.
TIM-4 belongs to a family of cell surface glycoproteins that were originally identified as regulators of T cell function42. The discovery of TIM-4 as a PtdSer recognition receptor was closely followed by the recognition of other members of the TIM family (such as TIM-1 and TIM-3) as PtdSer receptors43, 44. However, unlike BAI1, TIM4 does not activate direct downstream signaling, but rather acts as a tethering receptor45. Although integrins can function cooperatively with TIM-4 for signaling in vitro46, the co-signaling receptor(s) for TIM-4 under endogenous expression conditions is unclear. An elegant study in zebrafish showed that BAI1 and TIM-4 may act at distinct stages of engulfment with possible cooperation between the receptors, where BAI1 contributes to phagosome formation, while TIM-4 contributes to phagosome stabilization47.
In mice, TIM-4 expression is high on tissue resident macrophages, dendritic cells, and particularly peritoneal macrophages44. Macrophages lacking TIM-4 show reduced apoptotic cell engulfment48, 49. Global TIM-4 deficient mice also variably develop signs of autoimmunity48–50, whereas mice with TIM-4 overexpression display reduced secondary immune responses51. These data suggest that homeostatic clearance of apoptotic cells can be influenced by TIM-4, with potential links to immune tolerance. Conditional deletion of Tim-4 in specific cell types is needed for better characterization of its function in immune responses.
Mer tyrosine kinase (MerTK) is a member of the TAM receptor family, which includes Tyro, Axl and Mer receptor tyrosine kinases52. TAM receptors possess immunoglobulin-like domains and fibronectin repeats in the extracellular region and a cytoplasmic tyrosine kinase domain. TAM receptors engage PtdSer on apoptotic cells indirectly, via the soluble ligands Protein S and Gas-652. There are differential requirements for Protein S and Gas-6 in mediating TAM receptor ligation and downstream signaling53, 54. Although MerTK has been reported as a specific marker of macrophages55, it should be noted that many epithelial cells express high levels of MerTK.
TAM receptors are linked to homeostatic clearance of apoptotic cells in several contexts. Single or combined deletion of TAM family members leads to an accumulation of apoptotic germ cells in the testes, with complete lack of mature sperm in mice lacking all three TAM receptors56. Also, mice lacking MerTK show progressive blindness (by 8–12 weeks) due to the deficiency in the circadian RPE-dependent removal of rod outer segments in the retina, thereby revealing the specific and critical requirement for MerTK in the function of retinal epithelial cells57. Moreover, while losing all three TAM receptors does not affect embryonic development, adult mice show decreased clearance of apoptotic cells and develop severe systemic autoimmunity56. This latter phenotype has been linked to TAM receptors function as powerful inhibitors of the immune response58.
Processing the apoptotic cargo
A fascinating but understudied area of apoptotic cell clearance is how phagocytes process the ingested cargo. When a phagocyte engulfs an apoptotic cell, it may double its protein, lipid, and carbohydrate content, yet professional phagocytes manage to rapidly engulf multiple corpses. In tissues that turnover a large number of cells, such as the thymus, the number of macrophages is much lower than that of thymocytes undergoing death. Therefore, a single phagocyte must eat more than one corpse, likely in succession. Several studies suggest that the process of engulfment itself influences the capacity of the phagocyte to engulf additional corpses, linked to increased expression of engulfment receptors via nuclear receptors (LXR, PPARδ, PPARγ and RXR)59–61 (Fig. 2). Continued clearance of apoptotic cells by the phagocyte is also positively regulated by increased expression of UCP2, a mitochondrial uncoupler of oxidative phosphorylation from ATP synthesis62. Whether LXR, PPARδ, PPARγ, RXR and UCP2 expression and induction differ between the professional and non-professional phagocytes under both homeostatic and inflammatory conditions remains to be established.
Among the ingested components degraded in the phagocytic lysosomes, degradation of DNA is of particular importance, as ‘escaped’ DNA can induce breaks in self-tolerance and lead to the rise of autoimmunity4. A key situation where this happens in homeostasis is during erythropoiesis. During the definitive stage of erythropoiesis, DNA from erythroblasts is extruded in structures called pyrenocytes (nuclei surrounded by membrane decorated with phosphatidylserine63). Pyrenocytes are engulfed by neighboring macrophages in a MerTK-dependent fashion64, allowing erythropoiesis to proceed65. The enzyme that degrades DNA in the lysosomes is DNase II65. DNase II is highly expressed in the macrophage, and macrophages lacking DNase II cannot digest the DNA from engulfed apoptotic cells and cannot support erythropoiesis65. In fact, failed DNA digestion leads to the activation of the cyclic cGAS-STING nucleic acid sensing pathway, with production of type I interferon (IFN) and lethal anemia66. Although these mice are rescued from anemia by the added deletion of the IFN-type I receptor, they develop arthritis from excessive tumor necrosis factor (TNF) production, suggesting that undigested DNA from apoptotic cells can induce inflammatory disease67.
Certain components of the ingested apoptotic cell, such as cholesterol, can also be disposed of in other ways. In macrophages, the ATP-binding cassette (ABC) transporters ABCA1 and ABCG1 help efflux intracellular cholesterol to the lipid-rich high-density lipoprotein (HDL), which is then taken up by the liver and excreted in the bile68. Impairments in cholesterol efflux are linked to dyslipidemia and atherosclerosis69. When macrophages engage apoptotic cells, they rapidly increase their ABCA1 expression and cholesterol efflux in a PtdSer dependent manner70. Surprisingly, this early induction of ABCA1 does not require the canonical LXR-mediated pathway (although LXR can be relevant after prolonged exposure of apoptotic cells41). Instead, the BAI1-ELMO1-Dock180-Rac1 signaling module mediates ABCA1 upregulation and cholesterol efflux41. Furthermore, in atherosclerosis-prone mice on a high-fat diet, deletion of BAI1 results in lower serum concentrations of HDL, a risk factor for cardiovascular disease, whereas BAI1 overexpression results in higher ratios of serum HDL to cholesterol and LDL41, suggesting that BAI1 regulates normal lipidemia.
Cell clearance and anti-inflammatory responses
Corpse clearance commences at the earliest stages of apoptosis, prior to the loss of the plasma membrane integrity, to avoid the release of cellular contents. During homeostatic conditions, this occurs rather efficiently, and there is hardly any inflammatory cell recruitment even in tissues with high cellular turnover. However, once the plasma membrane integrity is lost due to the secondary necrosis of late stage apoptotic cells, the released cellular contents can engage receptors for damage-associated molecular patterns (DAMPs) and contribute to immune responses to self-antigens71. The mechanisms of clearing late apoptotic and necrotic cells include opsonization with lectins, properdin, pentraxins, thrombospondin and heparan sulfate proteoglycans. Interestingly, many of the opsonins that facilitate clearance of these cells also facilitate pathogen clearance72. Perhaps the concurrent recognition of the late stage dying cell and the infectious pathogen contributes to faster recovery from infectious injury and resolution of inflammation. Treatment with recombinant human MFG-E8 reduced disease in two mouse models of colitis73, suggesting that enhancing clearance of all PtdSer exposing cells can be of benefit in inflammation. Although delayed or impaired clearance of dying cells (Table 1) can aggravate inflammatory disease, administration of early stage apoptotic cells helps reduce disease severity in inflammation models, likely via elicitation of anti-inflammatory mediators74. This suggests that the benefit versus inflammatory potential of apoptotic cells is in a delicate balance, and likely critical in designing apoptotic cell-based therapies for inflammatory diseases.
Should homeostasis be breached by tissue inflammation with infiltrating cells, the dying cells can include bystander cells and short-lived immune cells (such as neutrophils) that need to be removed during resolution of inflammation. Besides apoptosis, other forms of cell death may also be involved, including primary and secondary necrosis, pyroptosis and necroptosis75. Neutrophils recruited to the sites of bacterial infection can also die via neutrophil extracellular trap formation (NETosis)76, with release of nuclear chromatin and histones to facilitate trapping and killing of bacteria. Due to the release of cellular contents, NETosis is generally thought to incite inflammation, though certain types of NETs can contribute to its resolution77. Necroptosis is a non-apoptotic cell death triggered by TNF (a cytokine abundantly present at the sites of inflammation), or by other stimuli when apoptosis is blocked75. The clearance of necroptotic cells is not yet fully defined. In fact, fascinating but unexplored topics are the relative contribution of different forms of cell death to maintaining homeostasis in any given tissue, how the cells that die by different mechanisms within the same tissue are removed (by the same phagocytes?), and how decisions are made about the phagocyte responses.
Rethinking apoptosis and PtdSer exposure
Apoptosis is closely linked to regenerative processes, as a dying cell can stimulate proliferation in the surrounding viable cells through ‘apoptosis-induced compensatory proliferation’78. This is observed even in the simple metazoan Hydra, where a caspase-dependent apoptotic response caused by injury induces proliferation of the surrounding cells79 (Fig. 4a). Similarly, apoptosis is a requirement for regenerative processes in Xenopus80, planaria (flatworm)81, newt82, and even the mammalian liver83.
Fig. 4.
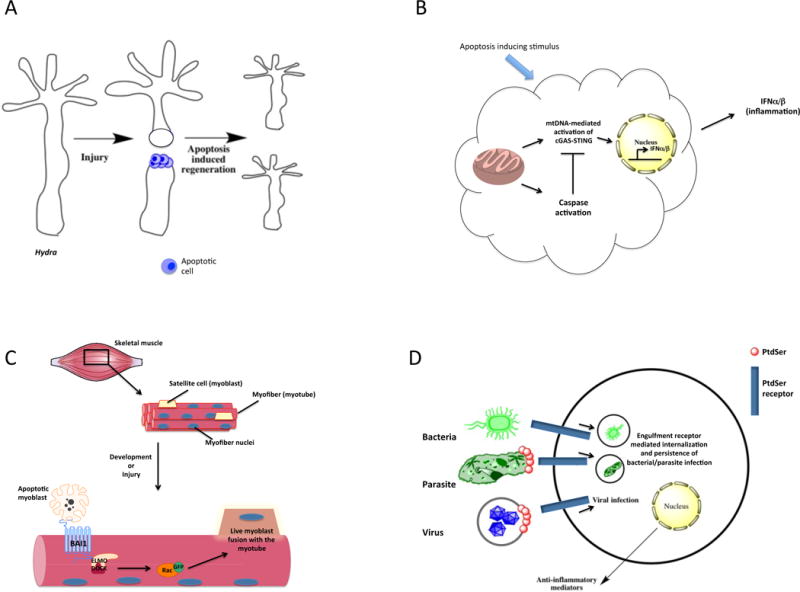
Additional and non-obvious functions of apoptotic cells. (a) Regeneration: In the metazoan Hydra, tissue injury can lead to apoptosis of the cells, which stimulates regenerative processes in the nearby viable tissues via a process called ‘apoptosis-induced compensatory proliferation’. Apoptosis is required for the re-growth of the new Hydra head. (b) Caspase-dependent inhibition of interferon production: In the context of a viral infection, apoptosis leads to the activation of caspases that is linked to inhibiting interferon-α/β (IFN-α/β) production induced by the mitochondrial DNA (mtDNA)-mediated activation of the cGAS-STING pathway. (c) Myoblast fusion: During muscle development and regeneration after muscle injury, apoptosis of myoblasts triggers BAI1 signaling through the ELMO-DOCK complex, leading to Rac activation. This pathway contributes to the fusion of healthy myoblasts with the nascent myotube and promotes muscle development and regeneration. (d) Pathogen exploitation of engulfment receptors: Bacteria, parasites and even viruses have evolved to utilize the apoptotic cell engulfment receptors for the cellular entry and induction of anti-inflammatory signaling in the host cell (phagocyte), aiding in the establishment and persistence of infection.
Interestingly, caspases that are activated during cell death can also regulate subsequent induction of inflammation84. When apoptotic caspases are missing, viral infection causes Bax and Bak-dependent mitochondrial membrane permeabilization, leading to release of mitochondrial DNA, activation of the cGAS-STING pathway via cytosolic DNA recognition, and type I IFN induction (Fig. 4b)84. This suggests that the caspase-dependent death that occurs during most homeostatic conditions could have evolved to dampen local inflammation that may have been adapted by viruses that induce cell lysis.
PtdSer can also be transiently exposed on viable cells. Since such transient PtdSer exposure does not lead to engulfment, PtdSer exposure alone may not be sufficient for stimulating phagocytosis. It is likely that ‘eat-me’ signals in addition to PtdSer, perhaps in combination with the lack of ‘don’t-eat-me’ markers, might be required to ‘confirm’ the impending cell death to the phagocyte85. In T cells, exposure of PtdSer is triggered by TCR stimulation or the ATP receptor P2X786. PtdSer on activated T cells contributes to the downregulation of immune responses by engaging Protein S and triggering TAM receptor mediated anti-inflammatory signaling in antigen presenting cells87. Therefore, PtdSer exposure in this context acts as a rheostat of the immune response, instead of an ‘eat-me’ signal. Transient PtdSer exposure is also observed upon activation of neutrophils and mast cells88, 89. The distinction between apoptotic versus non-apoptotic PtdSer exposure is that the latter is reversible and generally lasts only minutes or even seconds. Exposure of PtdSer was noted during myoblast fusion into skeletal muscle myotubes in vitro90 and subsequently, fusion-inducing cues have been shown to cause death of some myoblasts, and the caspase-dependent PtdSer-exposure is required for the fusion to occur37. Furthermore, the PtdSer receptor BAI1 and its homolog BAI3 act as promoters of myoblast fusion, as mice deficient in BAI1 and BAI3 develop smaller myofibers and show delayed healing after muscle injury37, 91 (Fig. 4c).
PtdSer exposure is also exploited by several microorganisms due to the anti-inflammatory nature of PtdSer-dependent apoptotic cell clearance (Fig. 4d). This was first reported in Leishmania, which exposes PtdSer on the cell surface during the amastigote stage of the life cycle92. PtdSer promotes internalization of amastigotes by the macrophage while also inhibiting the immune response via induction of transforming growth factor-β (TGF-β). Similar mechanisms of evasion have been reported in Toxoplasma gondii93 and Trypanosoma cruzi94. Enveloped viruses also use PtdSer for cellular entry in a process termed ‘apoptotic mimicry’95. The list of viruses that utilize this mechanism is growing rapidly, including HIV96, Vaccinia95, Ebola97, 98, Dengue99, and Pichinde viruses100. Remarkably, even non-enveloped viruses, conventionally thought to require cell lysis for viral transmission, have been suggested to use PtdSer decorated vesicles for packaging of multiple virions for transfer into the new host cell101. Similarly, many PtdSer receptors are linked to viral entry100. Finally, certain PtdSer receptors can also bind bacteria and fungi, including BAI1102, TREM-2103, Stabilin-2104, CD36 and SCARF-1105. Thus, rethinking the role of PtdSer receptors both in the context of apoptotic cell clearance as well as non-apoptotic homeostatic functions and pathogen encounters is warranted.
Impending challenges
In terms of how apoptotic cell clearance regulates homeostasis in tissues, a number of interesting questions remain to be addressed. The first challenge is understanding the role of specific receptors. It is unclear whether there is preference in utilizing particular engulfment receptors or clearance mechanisms to achieve the distinction between homeostatic from inflammatory apoptotic cell turnover. The second challenge is defining the anti-inflammatory responses. The difference between phagocytosis of apoptotic cells versus other targets (such as bacteria or other pathogens) is that routine apoptotic cell uptake is generally not immunogenic; furthermore, it elicits the production of mediators that actively suppress inflammation in the local tissue milieu. However, our understanding of the phagocyte molecular events leading to specific downstream consequences is just beginning to be defined41, 106. Engagement of apoptotic cells is well known to induce TGF-β, which is linked to the differentiation of immunosuppressive regulatory T cells (Treg cells). Whether routine apoptotic cell clearance plays a role in generating Treg cells specific for self-antigens not expressed in the thymus remains to be explored. The third major challenge is in understanding the ‘labor distribution’ between professional and non-professional phagocytes. An intriguing question is whether there is crosstalk between professional and non-professional phagocytes under homeostatic conditions, and whether this might influence the phagocytic capacity of either. Furthermore, in many inflammatory conditions, there are different phagocytes present (resident macrophages, non-professional phagocytes, and recruited phagocytes). It is not known whether professional phagocytes redirect non-professional phagocytes, (such as epithelial cells), to shift their efforts toward proliferation or matrix production for tissue recovery. Moreover, non-professional phagocytes can also produce anti-inflammatory cytokines15, but there may be differences in the spectrum of factors produced and their contribution to the maintenance of the local anti-inflammatory state. Such knowledge could be useful for therapeutic targeting and accelerating tissue recovery after injury. The fourth challenge is deciphering the ‘metabolomics’ of apoptotic cargo processing. We know relatively little about how the target-derived metabolites are processed and used by the phagocyte, or in the phagocyte neighborhood. Release of some of these metabolites may also provide a means for communication between cells in a tissue. In this context, the tumor cell secretion of lactate regulates macrophage phenotypes in a tumor environment107; perhaps similar strategies exist whereby a non-professional phagocyte engulfing an apoptotic cell secretes metabolites that regulate the activation status of macrophages in the local environment. A comprehensive determination of the metabolomics of engulfment could be of relevance to human diseases such as obesity and diabetes. Thus, better defining homeostatic clearance of apoptotic cells could have important implications in our understanding of basic physiology, immune tolerance, and responses to infection.
Acknowledgments
We thank the members of the Ravichandran laboratory, as well as colleagues in the field for their helpful comments and discussions. This work was supported in part by grants from the National Institutes of Health (NIGMS GM064709, HD074981, GM107848, HL120840, and MH096484).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nature reviews Immunology. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monks J, et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell death and differentiation. 2005;12:107–114. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors. Immunological reviews. 2007;220:237–250. doi: 10.1111/j.1600-065X.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 5.Metchnikoff E. In: Lectures on the Comparative Pathology of Inflammation. Starling FA, Starling EH, translators. Vol. 1968. Dover; New York: 1891. [Google Scholar]
- 6.van Furth R, et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bulletin of the World Health Organization. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 7.Lavin Y, Merad M. Macrophages: gatekeepers of tissue integrity. Cancer immunology research. 2013;1:201–209. doi: 10.1158/2326-6066.CIR-13-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeffel G, et al. C-myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dini L, Pagliara P, Carla EC. Phagocytosis of apoptotic cells by liver: a morphological study. Microscopy research and technique. 2002;57:530–540. doi: 10.1002/jemt.10107. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain research. 2014 doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Uderhardt S, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36:834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Wood W, et al. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–5252. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- 15.Juncadella IJ, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott MR, et al. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2010;467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lysiak JJ, Turner SD, Turner TT. Molecular pathway of germ cell apoptosis following ischemia/reperfusion of the rat testis. Biology of reproduction. 2000;63:1465–1472. doi: 10.1095/biolreprod63.5.1465. [DOI] [PubMed] [Google Scholar]
- 18.Burstyn-Cohen T, et al. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76:1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gude DR, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauber K, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 22.Peter C, Wesselborg S, Herrmann M, Lauber K. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis: an international journal on programmed cell death. 2010;15:1007–1028. doi: 10.1007/s10495-010-0472-1. [DOI] [PubMed] [Google Scholar]
- 23.Truman LA, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 24.Miksa M, Amin D, Wu R, Ravikumar TS, Wang P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol Med. 2007;13:553–560. doi: 10.2119/2007-00019.Miksa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darland-Ransom M, et al. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 26.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 27.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annual review of physiology. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341:403–406. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 29.Segawa K, et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 31.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Molecular cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 32.Brown S, et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 33.Simhadri VR, et al. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119:2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annual review of immunology. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 35.Hamann J, et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacological reviews. 2015;67:338–367. doi: 10.1124/pr.114.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duman JG, et al. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:6964–6978. doi: 10.1523/JNEUROSCI.3978-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochreiter-Hufford AE, et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur B, Brat DJ, Devi NS, Van Meir EG. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene. 2005;24:3632–3642. doi: 10.1038/sj.onc.1208317. [DOI] [PubMed] [Google Scholar]
- 39.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 40.Zhu D, et al. BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. The Journal of clinical investigation. 2015;125:1497–1508. doi: 10.1172/JCI74603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fond AM, Lee CS, Schulman IG, Kiss RS, Ravichandran KS. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. The Journal of clinical investigation. 2015;125:2748–2758. doi: 10.1172/JCI80300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunological reviews. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Current biology: CB. 2009;19:346–351. doi: 10.1016/j.cub.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 46.Flannagan RS, Canton J, Furuya W, Glogauer M, Grinstein S. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Molecular biology of the cell. 2014;25:1511–1522. doi: 10.1091/mbc.E13-04-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazaheri F, et al. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nature communications. 2014;5:4046. doi: 10.1038/ncomms5046. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Manzanet R, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong K, et al. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyanishi M, Segawa K, Nagata S. Synergistic effect of Tim4 and MFG-E8 null mutations on the development of autoimmunity. International immunology. 2012;24:551–559. doi: 10.1093/intimm/dxs064. [DOI] [PubMed] [Google Scholar]
- 51.Albacker LA, et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J Immunol. 2010;185:6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemke G. Biology of the TAM receptors. Cold Spring Harbor perspectives in biology. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lew ED, et al. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. eLife. 2014;3 doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nature immunology. 2014;15:920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 57.Duncan JL, et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Investigative ophthalmology & visual science. 2003;44:826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- 58.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 59.Mukundan L, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nature medicine. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.N AG, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roszer T, et al. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J Immunol. 2011;186:621–631. doi: 10.4049/jimmunol.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park D, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–224. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida H, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–758. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 64.Toda S, Segawa K, Nagata S. MerTK-mediated engulfment of pyrenocytes by central macrophages in erythroblastic islands. Blood. 2014;123:3963–3971. doi: 10.1182/blood-2014-01-547976. [DOI] [PubMed] [Google Scholar]
- 65.Kawane K, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292:1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nature immunology. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 67.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 68.Marcel YL, Ouimet M, Wang MD. Regulation of cholesterol efflux from macrophages. Current opinion in lipidology. 2008;19:455–461. doi: 10.1097/MOL.0b013e32830f4a1d. [DOI] [PubMed] [Google Scholar]
- 69.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiological reviews. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 70.Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Current biology: CB. 2006;16:2252–2258. doi: 10.1016/j.cub.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 71.Janko C, et al. CRP/anti-CRP antibodies assembly on the surfaces of cell remnants switches their phagocytic clearance toward inflammation. Frontiers in immunology. 2011;2:70. doi: 10.3389/fimmu.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell death and differentiation. 2010;17:381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Brenner M, Yang WL, Wang P. Recombinant human MFG-E8 ameliorates colon damage in DSS- and TNBS-induced colitis in mice. Laboratory investigation; a journal of technical methods and pathology. 2015;95:480–490. doi: 10.1038/labinvest.2015.32. [DOI] [PubMed] [Google Scholar]
- 74.Gatza E, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 76.Remijsen Q, et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell death and differentiation. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schauer C, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nature medicine. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 78.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends in cell biology. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chera S, et al. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Developmental cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Tseng AS, Adams DS, Qiu D, Koustubhan P, Levin M. Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Developmental biology. 2007;301:62–69. doi: 10.1016/j.ydbio.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vlaskalin T, Wong CJ, Tsilfidis C. Growth and apoptosis during larval forelimb development and adult forelimb regeneration in the newt (Notophthalmus viridescens) Development genes and evolution. 2004;214:423–431. doi: 10.1007/s00427-004-0417-1. [DOI] [PubMed] [Google Scholar]
- 83.Li F, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Science signaling. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. The Journal of experimental medicine. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elliott JI, et al. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nature cell biology. 2005;7:808–816. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 87.Carrera Silva EA, et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013;39:160–170. doi: 10.1016/j.immuni.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin S, et al. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. International archives of allergy and immunology. 2000;123:249–258. doi: 10.1159/000024451. [DOI] [PubMed] [Google Scholar]
- 89.Frasch SC, et al. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. The Journal of biological chemistry. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 90.van den Eijnde SM, et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. Journal of cell science. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 91.Hamoud N, Tran V, Croteau LP, Kania A, Cote JF. G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3745–3750. doi: 10.1073/pnas.1313886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wanderley JL, Moreira ME, Benjamin A, Bonomo AC, Barcinski MA. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol. 2006;176:1834–1839. doi: 10.4049/jimmunol.176.3.1834. [DOI] [PubMed] [Google Scholar]
- 93.Seabra SH, de Souza W, Damatta RA. Toxoplasma gondii exposes phosphatidylserine inducing a TGF-beta1 autocrine effect orchestrating macrophage evasion. Biochemical and biophysical research communications. 2004;324:744–752. doi: 10.1016/j.bbrc.2004.09.114. [DOI] [PubMed] [Google Scholar]
- 94.Damatta RA, et al. Trypanosoma cruzi exposes phosphatidylserine as an evasion mechanism. FEMS microbiology letters. 2007;266:29–33. doi: 10.1111/j.1574-6968.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 95.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 96.Callahan MK, et al. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- 97.Brindley MA, et al. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology. 2011;415:83–94. doi: 10.1016/j.virol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hunt CL, Kolokoltsov AA, Davey RA, Maury W. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. Journal of virology. 2011;85:334–347. doi: 10.1128/JVI.01278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meertens L, et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell host & microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morizono K, Chen IS. Role of phosphatidylserine receptors in enveloped virus infection. Journal of virology. 2014;88:4275–4290. doi: 10.1128/JVI.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen YH, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Das S, et al. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.N’Diaye EN, et al. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. The Journal of cell biology. 2009;184:215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adachi H, Tsujimoto M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. The Journal of biological chemistry. 2002;277:34264–34270. doi: 10.1074/jbc.M204277200. [DOI] [PubMed] [Google Scholar]
- 105.Means TK, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. The Journal of experimental medicine. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henson PM. Dampening inflammation. Nature immunology. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 107.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaultier A, et al. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. Journal of cell science. 2009;122:1155–1162. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes & development. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu Z, et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nature cell biology. 2011;13:1076–1083. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garcia M, Vecino E. Role of Muller glia in neuroprotection and regeneration in the retina. Histology and histopathology. 2003;18:1205–1218. doi: 10.14670/HH-18.1205. [DOI] [PubMed] [Google Scholar]
- 113.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. Journal of cell science. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- 115.Todt JC, Hu B, Curtis JL. The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. Journal of leukocyte biology. 2004;75:705–713. doi: 10.1189/jlb.0903439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ji H, et al. T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury. Hepatology. 2014;60:2052–2064. doi: 10.1002/hep.27334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karikoski M, et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:6452–6464. doi: 10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- 118.Hirose Y, et al. Inhibition of Stabilin-2 elevates circulating hyaluronic acid levels and prevents tumor metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4263–4268. doi: 10.1073/pnas.1117560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schledzewski K, et al. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. The Journal of clinical investigation. 2011;121:703–714. doi: 10.1172/JCI44740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Englert JM, et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. The American journal of pathology. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Englert JM, et al. Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. International journal of clinical and experimental pathology. 2011;4:241–254. [PMC free article] [PubMed] [Google Scholar]
- 122.He M, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. American journal of physiology. Lung cellular and molecular physiology. 2007;293:L1427–1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 123.He M, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO reports. 2011;12:358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liliensiek B, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. The Journal of clinical investigation. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tian L, et al. p85alpha recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nature communications. 2014;5:3146. doi: 10.1038/ncomms4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cantoni C, et al. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta neuropathologica. 2015;129:429–447. doi: 10.1007/s00401-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jay TR, et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. The Journal of experimental medicine. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Poliani PL, et al. TREM2 sustains microglial expansion during aging and response to demyelination. The Journal of clinical investigation. 2015;125:2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bosurgi L, et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13091–13096. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 132.D’Cruz PM, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human molecular genetics. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 133.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 134.Neher JJ, et al. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4098–4107. doi: 10.1073/pnas.1308679110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prasad D, et al. TAM receptor function in the retinal pigment epithelium. Molecular and cellular neurosciences. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 136.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 137.Weinger JG, et al. Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. Journal of neuroinflammation. 2011;8:49. doi: 10.1186/1742-2094-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. The Journal of clinical investigation. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lacy-Hulbert A, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCarty JH, et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 141.McCarty JH, et al. Genetic ablation of alphav integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. The American journal of pathology. 2008;172:1740–1747. doi: 10.2353/ajpath.2008.070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nandrot EF, Finnemann SC. Lack of alphavbeta5 integrin receptor or its ligand MFG-E8: distinct effects on retinal function. Ophthalmic research. 2008;40:120–123. doi: 10.1159/000119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aziz M, Matsuda A, Yang WL, Jacob A, Wang P. Milk fat globule-epidermal growth factor-factor 8 attenuates neutrophil infiltration in acute lung injury via modulation of CXCR2. J Immunol. 2012;189:393–402. doi: 10.4049/jimmunol.1200262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fricker M, et al. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:2657–2666. doi: 10.1523/JNEUROSCI.4837-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 146.Kusunoki R, et al. Role of milk fat globule-epidermal growth factor 8 in colonic inflammation and carcinogenesis. Journal of gastroenterology. 2015 doi: 10.1007/s00535-014-1036-x. [DOI] [PubMed] [Google Scholar]
- 147.Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Frontiers in pharmacology. 2012;3:27. doi: 10.3389/fphar.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng Y, Elkon KB. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. The Journal of clinical investigation. 2011;121:2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ait-Oufella H, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 150.Akitake-Kawano R, et al. Inhibitory role of Gas6 in intestinal tumorigenesis. Carcinogenesis. 2013;34:1567–1574. doi: 10.1093/carcin/bgt069. [DOI] [PubMed] [Google Scholar]
- 151.Angelillo-Scherrer A, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nature medicine. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 152.Binder MD, et al. Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:5195–5206. doi: 10.1523/JNEUROSCI.1180-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Binder MD, et al. Gas6 increases myelination by oligodendrocytes and its deficiency delays recovery following cuprizone-induced demyelination. PloS one. 2011;6:e17727. doi: 10.1371/journal.pone.0017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burnier L, et al. Gas6 deficiency in recipient mice of allogeneic transplantation alleviates hepatic graft-versus-host disease. Blood. 2010;115:3390–3397. doi: 10.1182/blood-2009-02-206920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Llacuna L, et al. Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology. 2010;52:1371–1379. doi: 10.1002/hep.23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yanagita M, et al. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. The Journal of clinical investigation. 2002;110:239–246. doi: 10.1172/JCI14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cai L, Wang Z, Ji A, Meyer JM, van der Westhuyzen DR. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PloS one. 2012;7:e36785. doi: 10.1371/journal.pone.0036785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Greenberg ME, et al. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. The Journal of experimental medicine. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kennedy DJ, et al. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1481–1487. doi: 10.1161/ATVBAHA.109.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parks BW, et al. CD36, but not G2A, modulates efferocytosis, inflammation, and fibrosis following bleomycin-induced lung injury. Journal of lipid research. 2013;54:1114–1123. doi: 10.1194/jlr.M035352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circulation research. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 162.Yancey PG, et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:787–795. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yancey PG, et al. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation. 2011;124:454–464. doi: 10.1161/CIRCULATIONAHA.111.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Subramanian M, et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. The Journal of clinical investigation. 2014;124:1296–1308. doi: 10.1172/JCI72051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramirez-Ortiz ZG, et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nature immunology. 2013;14:917–926. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bhatia VK, et al. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. The American journal of pathology. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bossi F, et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4209–4214. doi: 10.1073/pnas.1311968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature genetics. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 169.Chu Y, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]


