Abstract
BACKGROUND
Humans with familial lecithin:cholesterol acyltransferase (LCAT) deficiency (FLD) have extremely low or undetectable HDL-C levels and by early adulthood develop many manifestations of the disorder, including corneal opacities, anemia, and renal disease.
OBJECTIVE
To determine if infusions of recombinant human LCAT (rhLCAT) could reverse the anemia, halt progression of renal disease and normalize HDL in FLD.
METHODS
rhLCAT (ACP-501) was infused i.v. over 1 hour on 3 occasions in a dose optimization phase (0.3, 3.0, and 9.0 mg/kg), then 3.0 or 9.0 mg/kg every 1–2 weeks for 7 months in a maintenance phase. Plasma lipoproteins, lipids, LCAT levels, and several measures of renal function and other clinical labs were monitored.
RESULTS
LCAT concentration peaked at the end of each infusion and decreased to near baseline over 7 days. Renal function generally stabilized or improved and the anemia improved. After infusion, HDL-C rapidly increased, peaking near normal in 8–12 hours; analysis of HDL particles by various methods all revealed rapid sequential disappearance of preβ-HDL and small α-4 HDL and appearance of normal α-HDL. LDL-C increased more slowly than HDL-C. Of note, triglyceride routinely decreased after meals following infusion, in contrast to the usual post-prandial increase in the absence of rhLCAT infusion.
CONCLUSIONS
rhLCAT infusions were well tolerated in this first-in-human study in FLD; the anemia improved, as did most parameters related to renal function in spite of advanced disease. Plasma lipids transiently normalized, and there was rapid sequential conversion of small preβ-HDL particles to mature spherical α-HDL particles.
Keywords: Cholesterol, HDL, triglyceride, renal disease, lecithin cholesterol acyltransferase, lecithin cholesterol acyltransferase deficiency, LCAT, recombinant enzyme replacement, Lipoprotein-X
INTRODUCTION
Lecithin:cholesterol acyltransferase (LCAT) is a plasma enzyme that catalyzes the production of cholesteryl esters (CE) from free cholesterol (FC) and phosphatidylcholine (lecithin) 1. In humans, about 90% of CE in plasma is synthesized by LCAT mainly in HDL 2. It is believed that newly formed CE accumulate in the core of HDL particles, resulting in the maturation of HDL particles from small discoidal particles to mature, spherical α-HDL 3. In humans, the resulting CE in mature HDL are then directly removed by the liver (minor route) or transferred to apolipoprotein B-containing lipoproteins by cholesteryl ester transfer protein (CETP) (major route) and cleared via the classical hepatic low-density lipoprotein (LDL) receptor pathway 2, originally described by Glomset as reverse cholesterol transport (RCT) 4.
Inherited mutations in the gene for LCAT result in two autosomal recessive forms of LCAT deficiency. Patients with a total loss of LCAT activity are classified as having familial LCAT deficiency (FLD) and have a marked decrease in HDL-C levels (<10 mg/dL), plasma CE <25% of total cholesterol (TC) (normal > 70%), mild to severe hypertriglyceridemia, lipoprotein-X (Lp-X) in plasma, corneal opacities, normochromic normocytic anemia and progressive renal disease 5–8. FLD patients often develop proteinuria as young adults and then go on to develop nephrotic syndrome and end stage renal disease (ESRD) typically in their 40’s and 50’s 1. There is no effective treatment except for dialysis or renal transplantation, and the disease can rapidly reoccur in the transplanted kidney 9–11. Renal disease may develop secondary to the appearance of Lp-X, which is a vesicular-like abnormal lipoprotein particle rich in phospholipid (PL) and FC that accumulates in the kidney 12. Patients with fish-eye disease (FED) have a partial LCAT deficiency with some residual LCAT activity 1, 8. These patients are relatively asymptomatic with no Lp-X or renal disease but have reduced HDL-C and corneal opacities.
FLD patients have an abnormal distribution of HDL subfractions; the majority of their plasma apoA-I is found in small, disc-shaped, poorly lipidated preβ-HDL particles and α-4 HDL particles containing PL and FC 13. Interestingly, patients with LCAT deficiency do not have a markedly increased risk for cardiovascular disease in most studies 1, 14, likely because they also have low levels of LDL-C due to the decreased formation of CE on HDL, which are normally transferred from HDL to LDL by CETP.
Recently, recombinant human LCAT (rhLCAT; ACP-501) was shown to be safe in a phase I study of subjects with stable cardiovascular disease 15 (ClinicalTrials.gov NCT01554800) and is being developed as a potential therapy for acute coronary syndrome. In this report, we describe the first-in-human use of enzyme replacement therapy (ERT) with rhLCAT in a patient with FLD and its effect on lipoprotein metabolism, and hematologic and renal function.
METHODS
Study Design
This single-center study was approved by the National Heart, Lung and Blood Institute, Institute Review Board, prior to patient recruitment. The subject provided informed consent prior to participation in the study. The study was conducted after FDA review under an Investigational New Drug 117100 as an Expanded Access Protocol. This is a first-in-human study of ACP-501 (rhLCAT) in a subject with FLD.
The subject was administered 1 hour i.v. infusions of rhLCAT (ACP-501) during a dose escalation optimization phase (0.9, 3.0 and 9.0 mg/kg over 22 days) (Online Figure 1), followed by a maintenance phase of 10 infusions of each of the 2 higher doses weekly or bi-weekly over 7 months (Online Table 1).
A detailed Methods section is available in the Online Data Supplement.
Statistics
Summary statistics were reported as percent change or fold change from prestudy levels or preinfusion baseline levels.
RESULTS
Demographics of Subject
A 52-year-old male with FLD and ESRD previously described 16 was enrolled in an expanded access use protocol (IND 117,100) to determine whether dialysis could be avoided or delayed. Over the 31 months prior to inclusion, the patient’s renal function rapidly declined (creatinine increasing from 2.5 to 5.6 mg/dL), necessitating the placement of a fistula in his arm in anticipation of dialysis within weeks. Baseline labs included: BUN 159 mg/dL, creatinine 5.6 mg/dL, eGFR 13 mLs/min/1.73m2, 24-hour urine protein 2,307 mg, hemoglobin (HGB) 8.2 g/dL, hematocrit (HCT) 24.7%, TC 80 mg/dL, LDL-C 46 mg/dL, HDL-C <5 mg/dL and triglyceride (TG) 147 mg/dL. See full clinical history in Online Supplement.
Summary of Safety of rhLCAT
Over the 8-month course of rhLCAT (ACP-501) therapy (Online Figure 1), the patient received a total of 23 infusions that were well tolerated by the patient. There were no infusion site reactions or infusion toxicities. Other than favorable changes in creatinine, BUN, cystatin C, HGB and HCT, as summarized below, there were no other clinically meaningful shifts in clinical laboratory parameters or physical exam during the study. There were three AEs (atrial fibrillation, a mild viral syndrome, and elective hemodialysis at the completion of the study) that were not attributed to ACP-501.
Atrial fibrillation, which occurred 72 hours after receiving the third dose of rhLCAT, was classified as an SAE. The patient had a long history of atrial fibrillation, since the age of 27. Atrial fibrillation has not been previously reported to be associated with FLD, and hence given the patient’s prior history this event was viewed to be unrelated to the patient’s underlying lipid disorder or to the rhLCAT treatment. The patient presented with palpitations typical of his previous atrial fibrillation following a vigorous walk. He had a heart rate of 115 bpm and was stable and failed to convert with i.v. diltiazem as he had in the past. He had a controlled pulse rate throughout from 90–130. The patient was treated electively by cardioversion and amiodarone treatment and remained in normal sinus rhythm for the remainder of the study. Amiodarone is known to increase blood creatinine levels resulting in a slight increase in the creatinine, but the BUN and cystatin C remained stable for the remainder of the study 17.
The subject presented with a viral syndrome consisting of chills and fatigue that started on day 65, the day before dose 10 infusion. He developed a low grade fever prior to the infusion and became febrile to 39.9°C before normalizing in 3 da ys. This was associated with a drop in the HDL-C, apoA-I, CE, and LDL-C and worsening of his 24 hour urine protein, which took about 4 weeks for his labs to recover.
The infusions appeared to stabilize renal function and delayed imminent dialysis by 8 months. After supplies of rhLCAT (ACP-501) became limited, the subject elected to begin hemodialysis, as per the recommendation of his nephrologist.
Pharmacokinetics of rhLCAT
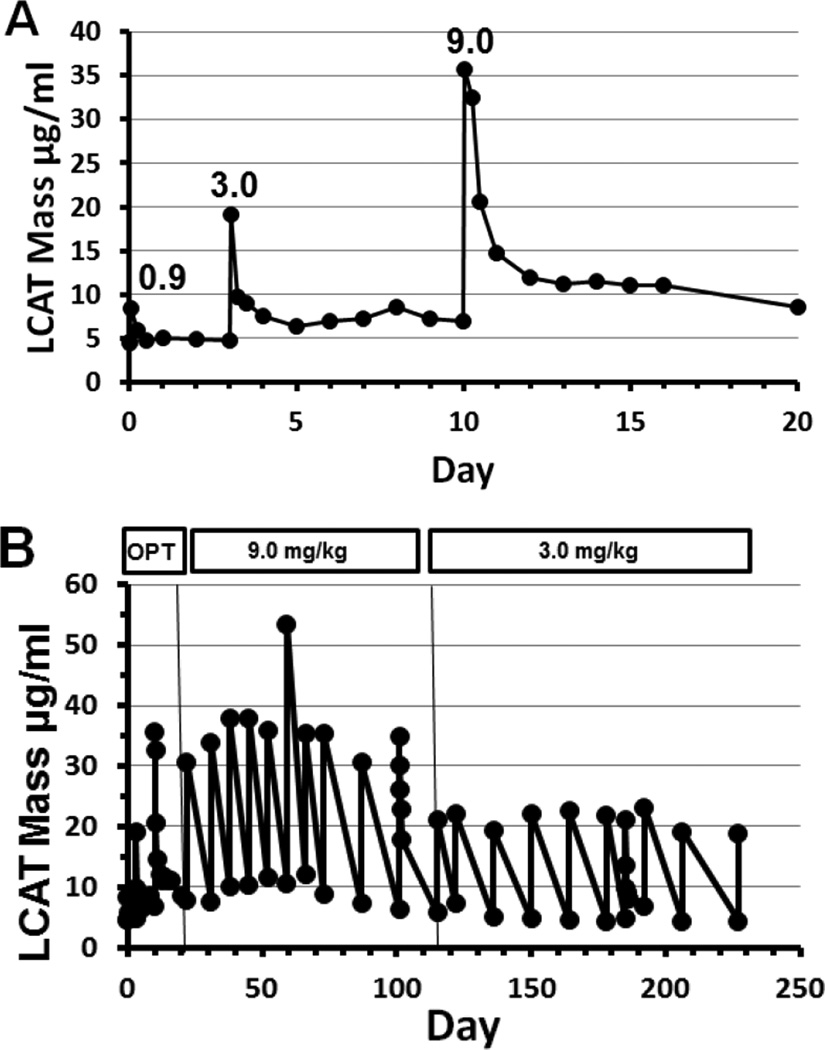
Peak concentrations of rhLCAT were observed at the completion of the 1 hour infusion (Figure 1A). Peak LCAT mass increased with increasing dose, during the optimization phase and maintenance phase. The peak LCAT mass remained relatively constant over time for the 9.0 mg/kg and for the 3.0 mg/kg doses during the maintenance phase (Figure 1B).
Figure 1. LCAT mass during dose optimization and maintenance phases.
(A) Optimization phase. LCAT mass was determined at 0, 1, 6, 12 and 24 hours and then daily following optimization phase doses of 0.9, 3.0, or 9.0 mg/kg administered i.v. over 1 hour. Peak concentrations of LCAT were observed at the end of the 1 hour infusion. LCAT mass increased with increasing dose. (B) Optimization (OPT) and maintenance phases. During maintenance phase, LCAT mass was determined just before and at the end of each infusion. On dose 13 (9.0 mg/kg) and dose 20 (3.0 mg/kg), LCAT mass was also determined at 6, 12, and 24 hours. Peak LCAT mass increased as the dose increased and was relatively constant with each dose.
Effect of rhLCAT on Lipid Parameters
Lipid parameters for the optimization phase are shown in Figure 2 and lipid parameters over the duration of the entire study are shown in Figures 3 and 4.
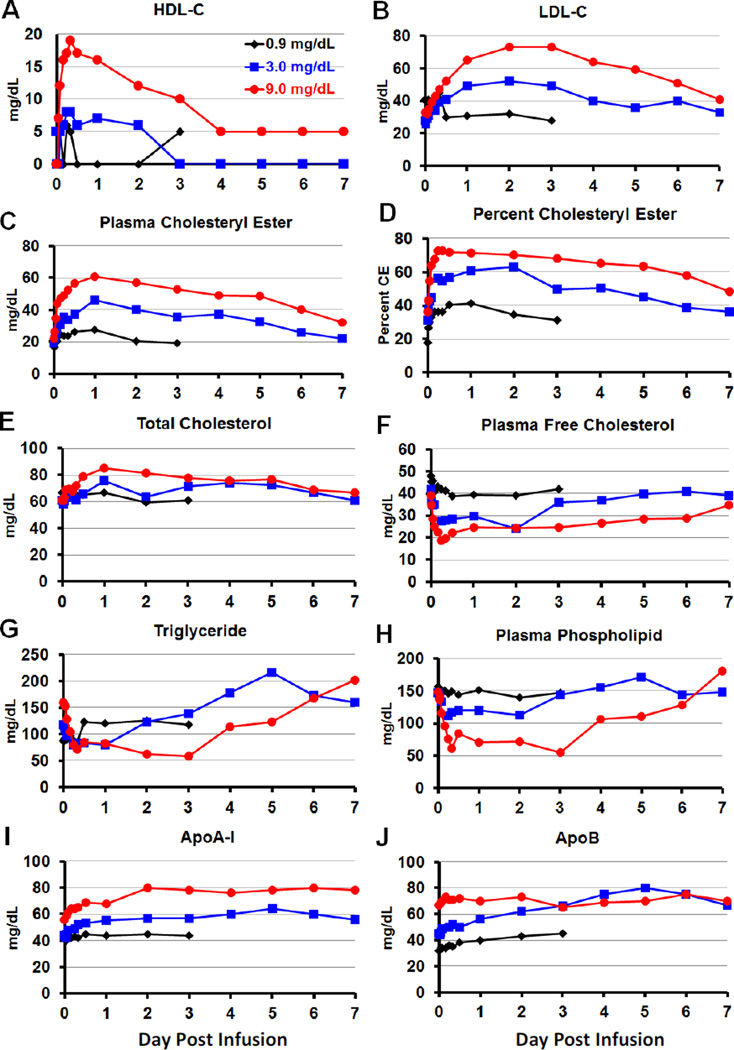
Figure 2. Optimization phase - lipoproteins and lipids.
Dose optimization phase included the 0.9 mg/kg dose (black) observed for 3 days, then a 3.0 mg/kg dose (blue) observed for 7 days, and then a 9.0 mg/kg dose (red) observed for 7 days. The rhLCAT infusion was given i.v. over 1 hour starting at time 0. Samples were taken at 0, 0.5, 1, 2, 4, 6, 8, 12 and 24 hours and then a fasting daily sample for up to 7 days. Atrial fibrillation occurred 3 days after the 9.0 mg/kg dose. The subject fasted for 12 hours before and during the infusion, and then resumed his regular diet. (A) HDL-C, (B) LDL-C, (C) Plasma CE, (D) Percent CE, (E) Plasma TC, (F) Plasma FC, (G) TG, (H) Plasma PL, (I) ApoA-I, and (J) ApoB.
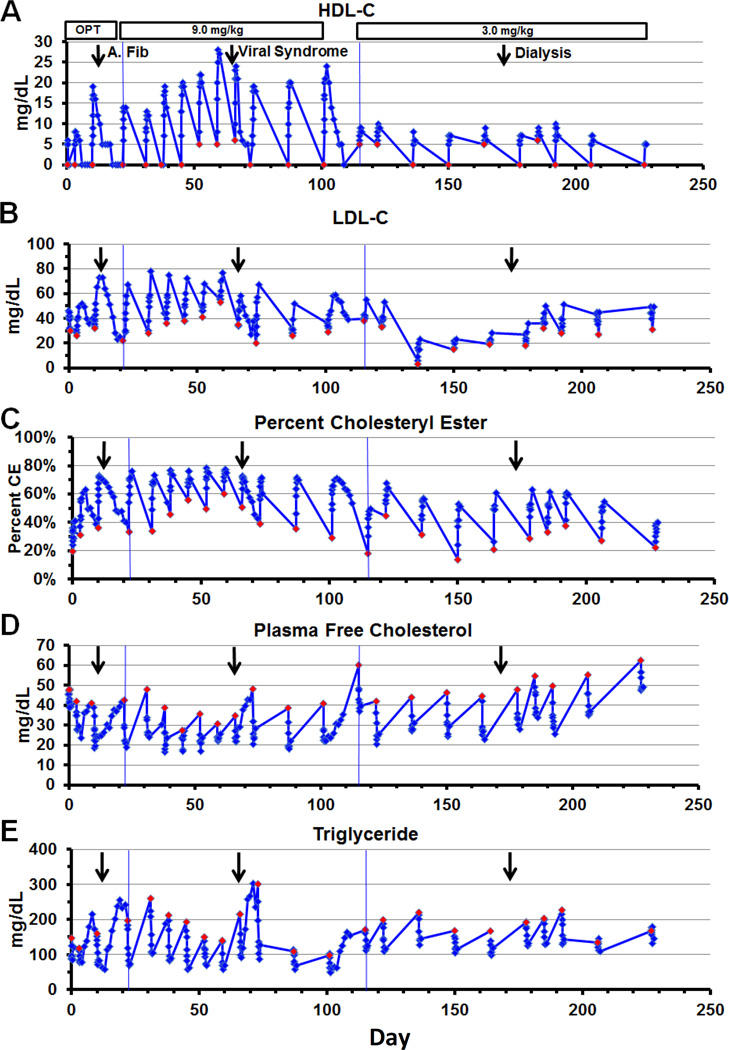
Figure 3. Optimization (OPT) and maintenance phases - lipoproteins and lipids.
Three doses (0.9, 3.0 and 9.0 mg/kg) were given between 0 and 21 days, during the optimization phase. Ten doses at 9.0 mg/kg maintenance phase were given usually weekly between day 22 and 114 and 10 doses at 3.0 mg/kg were given usually biweekly between day 115 and 228. The infusion was given i.v. over 1 hour and started at time 0. Blood was taken at 0, 1, 2, 4, 6, 8, 12 and 24 hours post infusion. Blood was taken daily for 1 week following the viral syndrome (dose 10) and following the last 9.0 mg/kg dose (dose 13). Atrial fibrillation occurred 3 days after dose 3 on day 13 (arrow). A viral syndrome started the day before dose 10 on day 65 (arrow). Hemodialysis started 1 week before dose 19 (arrow). Red diamonds represent time of infusion. The subject fasted for 12 hours before and during the infusion, and then resumed his regular diet. (A) HDL-C, (B) LDL-C, (C) Percent CE, (D) Plasma FC, and (E) TG.
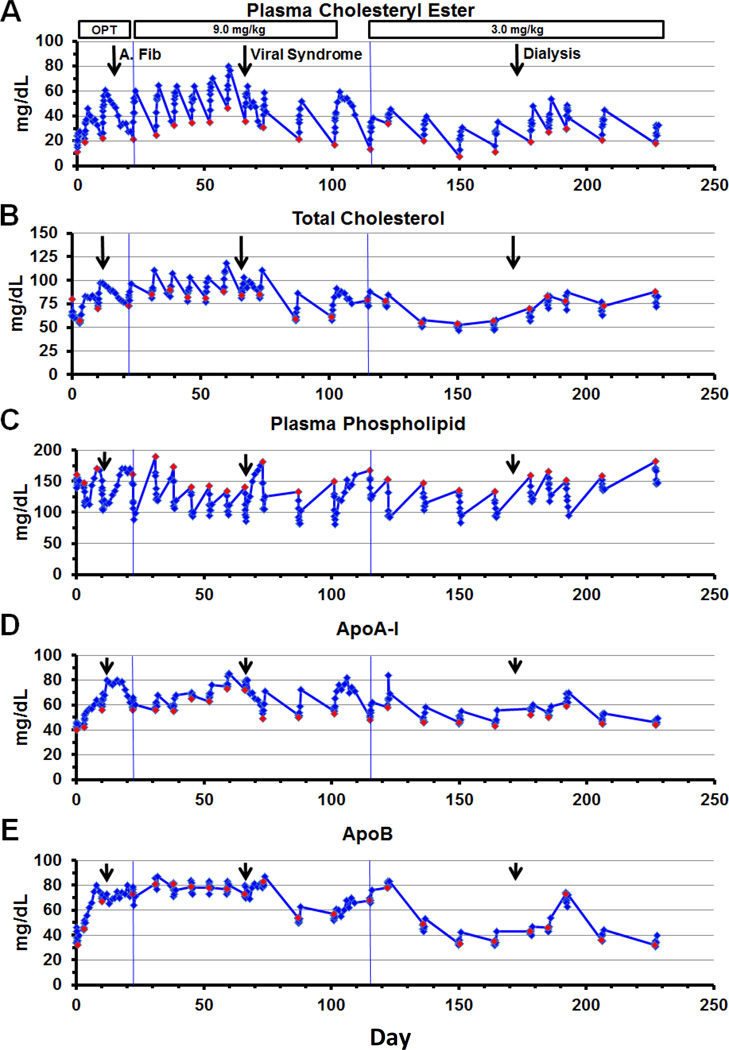
Figure 4.
Optimization (OPT) and maintenance phases - lipids and apolipoproteins
Three doses (0.9, 3.0 and 9.0 mg/kg) were given between 0 and 21 days, during the optimization phase. Ten doses at 9.0 mg/kg maintenance phase were given usually weekly between day 22 and 114 and 10 doses at 3.0 mg/kg were given usually biweekly between day 115 and 228. The infusion was given i.v. over 1 hour and started at time 0. Blood was taken at 0, 1, 2, 4, 6, 8, 12 and 24 hours post infusion. Blood was taken daily for 1 week following the viral syndrome (dose 10) and following the last 9.0 mg/kg dose (dose 13). Atrial fibrillation occurred 3 days after dose 3 on day 13 (arrow). A viral syndrome started the day before dose 10 on day 65 (arrow). Hemodialysis started 1 week before dose 19 (arrow). Red diamonds represent time of infusion. (A) Plasma CE, (B) TC, (C) Plasma PL, (D) ApoA-I, and (E) ApoB.
HDL-C was <5 mg/dL at screening and increased immediately after rhLCAT infusion. HDL-C peaked 8–12 hours after infusion in the optimization phase after the 3.0 and 9.0 mg/kg doses, with a minimal response at 0.9 mg/kg (Figure 2A). HDL-C peaked later at 12–24 hours after most infusions in the maintenance phase and weekly infusions resulted in a higher peak HDL-C than biweekly (Figure 3A). The peak HDL-C on the 9.0 mg/kg dose increased after repeated weekly doses (dose 5–9), with measureable HDL-C 7 days post infusion (dose 7–9) prior to the viral syndrome on day 65.
LDL-C was very low (46 mg/dL) at screening, which is characteristic of FLD. In contrast to HDL-C, the rise in LDL-C after rhLCAT infusion was slower and peaked later. LDL-C increased after a 2–4 hour delay before reaching peak levels by 2–3 days (Figure 2B and 5E). LDL-C increased in a dose dependent manner and remained above the preinfusion baseline 7 days after the 3.0 and 9.0 mg/kg doses in the optimization phase (Figure 2B). Weekly infusions resulted in higher peak LDL-C and higher day 7 levels than biweekly in the maintenance phase (Figure 3B). LDL-C reached near normal levels of 70 mg/dL at peak and peak levels plateaued on the 9.0 mg/kg dose after repeated weekly infusions prior to the viral syndrome.
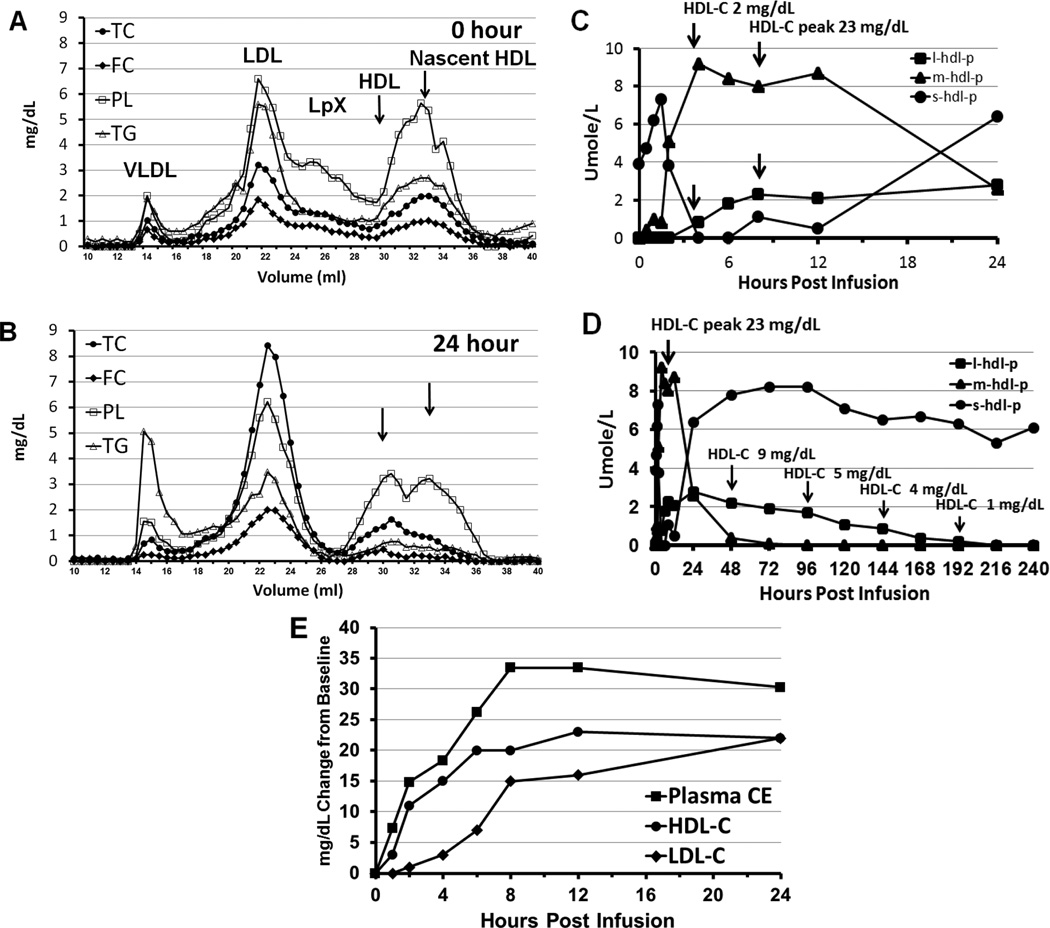
Figure 5. Effect of rhLCAT infusion on lipid and lipoprotein levels.
FPLC analysis of plasma lipids before (A) and 24 hours after (B) after the first 9.0 mg/kg rhLCAT infusion (optimization phase). (A) FPLC at 0 hour just before infusion. Lp-X like particles were identified as phospholipid-rich particles across broad size range. LDL-C was over 50% FC. Small nascent HDL predominates with near absence of HDL. (B) FPLC at 24 hours. Small nascent HDL particles were converted to larger CE-rich HDL-sized particles. The increase in TC to near normal levels was due to the formation of LDL-CE and HDL-CE since FC decreased. Baseline low LDL-C was increased to near normal CE-rich LDL levels 24 hours post infusion. NMR analysis of small (s), medium (m), and large (l) HDL over 24 hours (C) and 240 hours (D) after the first 9.0 mg/kg infusion. Small HDL was the only particle present at baseline, consistent with the α-4 HDL observed on the 1D and 2D gels. (C) The rhLCAT infusion was given i.v. over 1 hour starting at time 0. Small HDL, the substrate of rhLCAT, increased for 2 hours but then disappeared through 12 hours likely due to rapid precursor turnover. Medium HDL lipoproteins was absent at time 0 and after a brief delay peaked at 4 hours, the same time that large HDL appeared and HDL-C was measureable at 2 mg/dL. HDL-C peaked at 23 mg/dL at 8 hours concomitantly as large HDL peaked. (D) Small HDL reappeared by 24 hours correlating with α-4 HDL on 1D and 2D gels. Over the next 192 hours, HDL-C paralleled the slow decline and disappearance of large HDL. (E) Effect of rhLCAT infusion on HDL-C, LDL-C and plasma CE: mg/dL change from time 0. Increased plasma CE and HDL-C appeared within 1 hour and initially increased at the same rate. LDL-C increased after a 2 hour delay. From 4–8 hours, plasma cholesteryl ester continued to increase, approaching the sum of HDL-C and LDL-C. Data shown for dose 9 (9.0 mg/kg).
Plasma CE increased immediately in a dose dependent manner initially paralleling HDL-C appearance but peaked later at 24 hours and then remained above the preinfusion baseline in all 3 doses in the optimization phase (Figure 2C). Peak CE increased over 2-fold in the 3.0 mg/kg dose and almost 3-fold in the 9.0 mg/kg dose. After the 0.9 mg/kg dose, peak plasma CE increased about 1.5-fold despite only a minimal change in HDL-C. During the maintenance phase, peak CE increased more than 3-fold after repeated weekly doses on the 9.0 mg/kg but less on the biweekly 3.0 mg/kg doses (Figure 4A). Peak and nadir levels were always higher than baseline, during the weekly 9.0 mg/kg rhLCAT infusion.
The % CE peak occurred rapidly after infusion, reaching normal levels of about 70%. During the optimization phase, the time to peak % CE occurred rapidly (8–24 hours) and plateaued for 2 days in a dose dependent manner (Figure 2D). The % CE plateaued at 73% CE in the 9.0 mg/kg dose compared to the 19% CE prestudy level. The peak % CE levels increased to 70% or more after all infusions of the weekly 9.0 mg/kg dose, during the maintenance phase maintaining near normal % CE levels through 7 days (Figure 3C). Peak and nadir % CE remained above 50% on the 9.0 mg/kg dose during doses 6–10 prior to the viral syndrome. Biweekly infusion of 3.0 mg/kg resulted in a lower % CE peak of about 50% and nadir of about 30% but still successive infusions resulted in a 1.5 to 3-fold increase in % CE.
ApoA-I increased in a dose dependent manner over 2 days then plateaued thru 7 days as opposed to the lipid changes in the optimization phase (Figure 2I). ApoA-I increased by about 45% and 95% compared to the prestudy levels after the 3.0 mg/kg and 9.0 mg/kg doses, respectively. In the maintenance phase, apoA-I rapidly increased in parallel with the appearance of HDL-C and increased in a dose dependent manner (Figure 4D). ApoA-I increased by more than 50% from baseline in the 9.0 mg/kg dose, during weekly infusions versus the biweekly infusion and tended to plateau with successive doses. ApoA-I decreased during the viral syndrome.
ApoB levels approached normal levels and then plateaued (9.0 mg/kg dose) similar to the pattern of apoA-I as opposed to the lipid changes. During the optimization phase, apoB also gradually increased in a dose dependent manner after the 0.9 mg/kg and 3.0 mg/kg doses but remained elevated at the 9.0 mg/kg dose (Figure 2J). ApoB rapidly increased in a dose dependent manner in the 9.0 mg/kg and 3.0 mg/kg dose in the maintenance phase (Figure 4E). ApoB plateaued at normal levels during the 9.0 mg/kg dose and remained stable with successive doses. ApoB levels were disturbed following the viral syndrome at day 65 and transiently increased after the start of dialysis at dose 19 on day 178.
The overall effect of infusion of rhLCAT on lipid parameters was to convert a dyslipidemic profile characteristic of FLD to a nearly normal lipoprotein profile. TC increased similar to LDL-C and CE but of smaller magnitude due both to an increase in CE and a decrease in FC. During the optimization phase, TC increased in a dose dependent manner and the peak TC increased 39% from prestudy levels in the 9.0 mg/kg dose (Figure 2E); this increase was less than the increase in CE because FC decreased. TC increased in a dose dependent manner in the maintenance phase and the peak TC plateaued after repeated doses at 9.0 mg/kg and remained below 120 mg/dL (Figure 4B).
Concomitantly, FC rapidly decreased in a dose dependent manner and remained below the preinfusion baseline after all 3 doses in the optimization phase (Figure 2F). Nadir FC was 49% below the preinfusion baseline and 60% below the prestudy level after the 9.0 mg/kg dose. FC rapidly decreased to about 50% of baseline by 12–24 hours, and the nadir was consistently 20 mg/dL after all 9.0 mg/kg infusions in the maintenance phase (Figure 3D).
Plasma PL decreased rapidly, in a dose dependent manner, by 24% after the 3.0 mg/kg and 63% after the 9.0 mg/kg dose compared to prestudy levels in the optimization phase (Figure 2H). PL remained decreased for 3 days and 6 days after the 3.0 and 9.0 mg/kg doses, respectively. The PL trough decreased about 33% and plateaued after repeated doses at 9.0 mg/kg in the maintenance phase (Figure 4C).
TG increased postprandially in the patient in the absence of rhLCAT infusion, as expected. The subject fasted for 12 hours before and during all infusions and then had his regular diet; TG remained unchanged for over 12 hours after the 0.9 mg/kg dose in the optimization phase. Of major interest, TG rapidly decreased postprandially by 30% and 63% after the 3.0 and 9.0 mg/kg doses, respectively, and did not return to baseline for 3–5 days (Figure 2G). Similarly, TG rapidly and routinely decreased postprandially by about 65% after every 9.0 mg/kg dose and by 40% after every 3.0 mg/kg dose in the maintenance phase, and did not increase to baseline for 3–5 days (Figure 3E).
Effect of rhLCAT on Lipoprotein Particles
Lipoprotein particle composition and changes in HDL subpopulation distribution following rhLCAT infusion were sequentially evaluated by a variety of methods, including FPLC (Figure 5A and 5B), NMR (Figure 5C and 5D), native 1D gel electrophoresis (Figure 6) and native-native 2D gel analysis of apoA-I containing HDL subpopulations (Figure 7). Formation of normal sized α-HDL particles from small preβ-HDL particles and small discoidal α-4 HDL following rhLCAT infusion was evident by all methods used (Figure 5–7). Small nascent HDL particles were converted to larger CE-rich HDL-sized particles (Figure 5A and 5B). The increase in TC was due to the formation of normal levels of LDL-CE and HDL-CE since FC decreased. Small preβ-HDL particles and FC-rich α-4 HDL, the major substrate of rhLCAT, rapidly disappeared during the 24 hours following infusion, the time of maximal LCAT mass and CE formation. Changes in the distribution of apoA-I on HDL subfractions (Figure 6B and 7) paralleled the appearance of CE on α-HDL (Figure 5A and 5B). α-3 HDL was immediately generated after rhLCAT infusion (Figure 6 and 7). Larger α-2 HDL appeared at 4–6 hours and α-1 HDL appeared at 12–24 hours, coinciding with maximal concentrations of HDL-C.
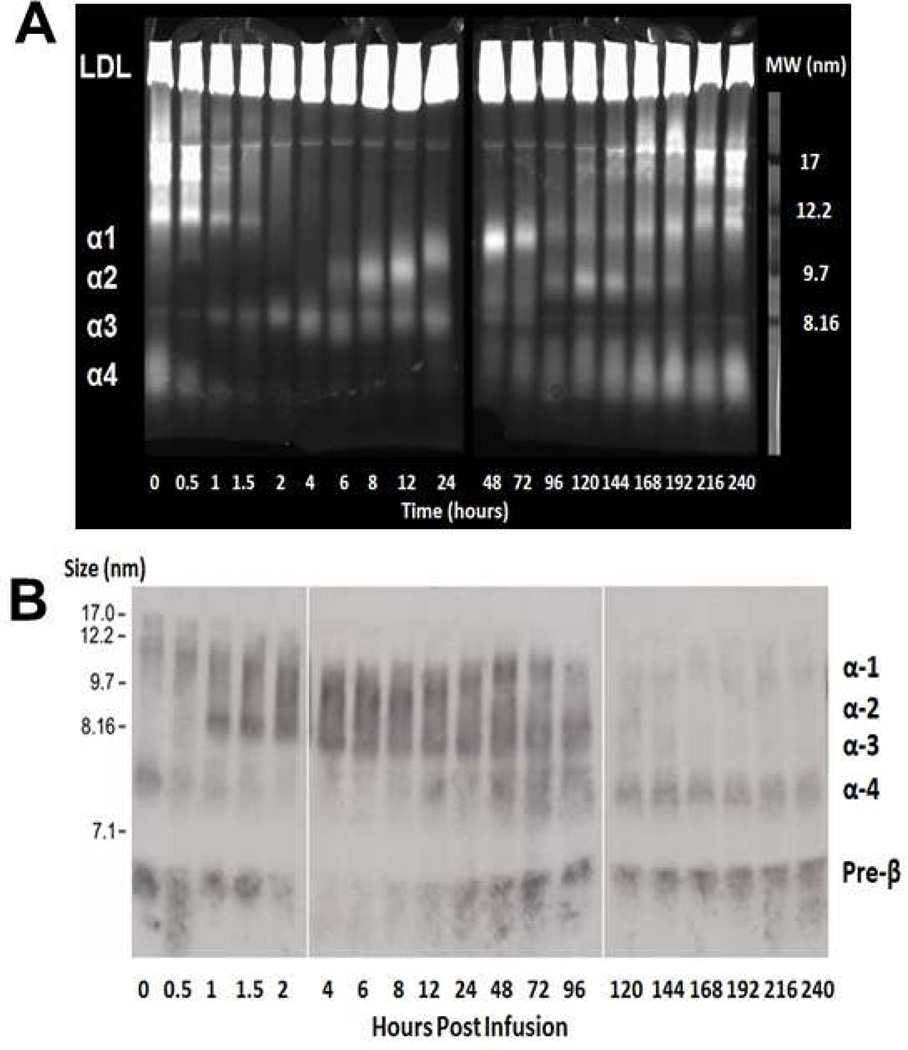
Figure 6. HDL subpopulations analysis by 1D gel electrophoresis following the first 9.0 mg/kg dose.
(A) Plasma was subjected to electrophoresis on a 4–12% native acrylamideTris-Glycine gel stained with filipin, which stains free cholesterol as well as phospholipids. At baseline, there was only α-4 HDL FC and PL and a complete absence of large α-HDL. α-4 HDL, the substrate for rhLCAT, rapidly decreased at the end of the infusion at peak rhLCAT levels and reappeared after 24–48 hours. Larger sized α-HDL, the product of rhLCAT, was sequentially generated with α-3 HDL immediately appearing during the infusion. α-2 HDL appeared at 6 hours followed by α-1 HDL at 24 hours. Larger α-HDL slowly disappeared after 192 hours. 17 nm Lp-X particles rich in FC/PL were abundant at 0 hours, disappeared by 2 hours, gradually reappeared at 120 hours and returned to near baseline by 240 hours. (B) ApoA-I Western blot analysis of plasma subjected to electrophoresis on a 4–28% TBE gel. At baseline, there was only apoA-I on preβ-HDL and α-4 HDL. α-4 HDL, the substrate for rhLCAT, rapidly decreased at the end of the infusion at peak rhLCAT levels and reappeared after 24–48 hours, paralleling the appearance of CE and disappearance of FC. Larger sized α-HDL, the product of rhLCAT, was sequentially generated with α-3 HDL immediately appearing during the infusion. ApoA-I appeared on α-2 HDL by 1.5 hours followed quickly by α-1 HDL. ApoA-I on α-2 HDL and α-1 HDL disappeared after 120–144 hours post infusion and returned to the preinfusion preβ-HDL and α-4 HDL.
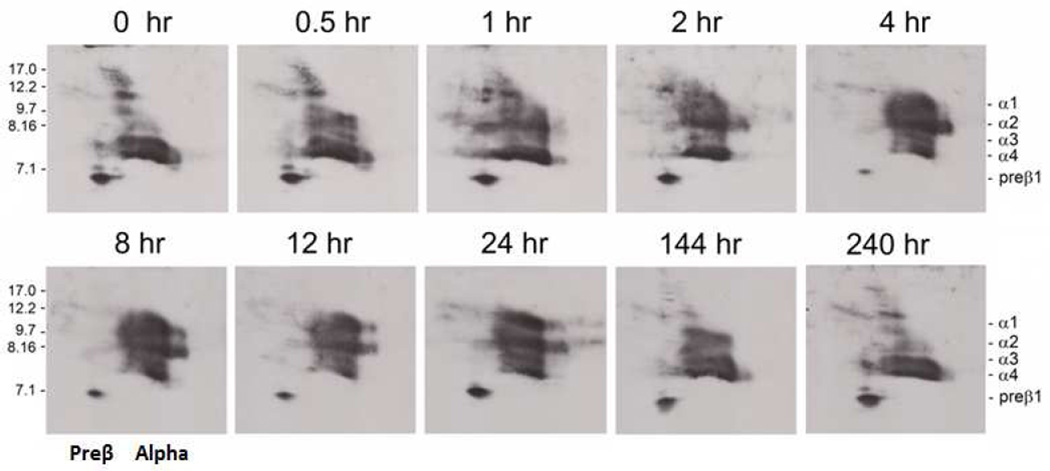
Figure 7. Native-native 2D gel analysis of apoA-I-containing HDL subpopulations.
Fasting plasma at various times between 0 and 240 hours following the first 9.0 mg/kg infusion of rhLCAT was run on native-native 2D gels. ApoA-I was detected by immunoblotting with an anti-apoA-I antibody. Molecular size markers in nm are shown. Small apoA-I discoidal preβ1 HDL and small discoidal α-4 HDL along with a constellation of unusually large apoA-I containing particles between the preβ and α-migrating particles were present at 0 hour. During the 1 hour infusion, larger α-3 and α-2 HDL began to appear and the unusually large apoA-I particles shifted towards the alpha region of the gel. By 4 hours, a normal HDL subpopulation pattern had emerged and this persisted through 12 hours. After 12 hours, preβ1 HDL increased; α-1 and α-2 HDL particles slowly diminished and by 240 hours the HDL subpopulation pattern had returned to the 0 hour pattern.
HDL particles detected by NMR (Figure 5C and 5D) correlated with the appearance and disappearance of HDL particles isolated on 1D and 2D gels (Figure 6 and 7) and HDL-C levels. Small HDL, the substrate of rhLCAT, detected by NMR at baseline paralleled α-4 HDL, which initially disappeared and then reappeared after 12–24 hours (Figure 5C, 5D, and 6A). Large HDL appeared at 4 hours when HDL-C was first measureable at 2 mg/dL. Large HDL peaked at 8 hours concomitantly as HDL-C peaked at 23 mg/dL (Figure 5C and 5D) and the disappearance of large HDL, the product of rhLCAT, paralleled the slow decline of HDL-C and α-1 HDL over 192 hours (Figure 6 and 7).
Lp-X disappearance was indirectly assayed by changes in FPLC analysis of the PL-rich particles (Figure 5A and 5B) and by plasma PL concentration (Figure 2H and 4C). Lp-X like particles were identified by FPLC as PL-rich particles across broad size ranges at baseline (Figure 5A) and were absent at 24 hours corresponding with the rapid decrease of plasma PL. Additionally, large (~17 nm) FC/PL-rich particles abundant at time 0 (Figure 6A), corresponding in size to the Lp-X particles (Figure 5A), began to disappear immediately after LCAT infusion and were gone 2 hours after LCAT infusion. They gradually reappeared at 120 hours and were back to near baseline levels at 240 hours.
Changes in Renal Parameters on rhLCAT
Renal parameters, including serum creatinine and BUN, estimated glomerular filtration rate (eGFR), cystatin C, spot urine protein and 24-hour urine protein, were collected throughout the study (Table 1). During the optimization phase, 24-hour urine protein improved overall (2,307 to 1,843 mg/24h). Twenty-four hour urine protein was somewhat variable but initially remained stable (2,498 mg/24h) compared to baseline at the 9.0 mg/kg rhLCAT dose, which was usually administered once a week (Table 1). Prior to receiving dose 10, the subject had developed a viral-like symptom (chills and fatigue). Over the next few days, the patient became febrile and the 24-hour proteinuria transiently worsened for several weeks. This was associated with a drop in the baseline HDL-C, apoA-I, CE, and LDL-C. Thereafter, his proteinuria appeared to improve with treatment and continued to improve (1,347 mg/24h) even when the patient was started on the 3.0 mg/kg dose, which was usually administered at biweekly intervals. Random spot urine protein largely paralleled the 24-hour urine protein excretion but varied considerably, depending upon the total urine volume.
Table 1.
Renal and Hematology Parameters Over Time
| Time point | Dose (mg/kg) |
Creatinine (mg/dL) |
BUN (mg/dL) |
eGFR (mLs/min /1.73m2) |
Cystatin-C (mg/L) |
Spot Urine Protein |
24h Urine protein (mg/24h) |
HGB (g/dL) |
HCT (%) |
|---|---|---|---|---|---|---|---|---|---|
| Screening | n/a | 5.56 | 159 | 13 | 4.16 | 75 | 2307 | 8.2 | 24.7 |
| Optimization Phase 0.9, 3.0 and 9.0 mg/kg | |||||||||
| Dose 1, 24hr | 0.9 | 4.94 | 139 | 15 | 3.24 | 64 | 2672 | 8.1 | 25.0 |
| Dose 2, 24hr | 3.0 | 4.73 | 114 | 16 | 3.43 | 2091 | 8.5 | 26.7 | |
| Dose 3, 24hr | 9.0 | 4.88 | 109 | 15 | 3.30 | 2098 | 8.8 | 27.3 | |
| Dose 3, 240hr | 9.0 | 5.51 * | 97 | 13 | 3.24 | 1843 | 8.4 | 25.2 | |
| Maintenance Phase 9.0 mg/kg | |||||||||
| Dose 4, 0hr | 9.0 | 5.52 | 94 | 13 | 2.96 | 1843 | 8.7 | 25.7 | |
| Dose 4, 24hr | 9.0 | 5.51 | 89 | 11 | 76 | ||||
| Dose 5, 0hr | 9.0 | 6.45 | 102 | 9 | 3.11 | 80 | 1923 | 9.5 | 29.2 |
| Dose 5, 24hr | 9.0 | 6.28 | 104 | 9 | |||||
| Dose 6, 0hr | 9.0 | 5.89 | 110 | 10 | 3.55 | 72 | 1948 | 10.1 | 30.6 |
| Dose 6, 24hr | 9.0 | 5.90 | 110 | 10 | |||||
| Dose 7, 0hr | 9.0 | 6.27 | 118 | 9 | 3.47 | 77 | 2229 | 10.2 | 30.9 |
| Dose 7, 24hr | 9.0 | 5.68 | 112 | 11 | |||||
| Dose 8, 0hr | 9.0 | 5.82 | 115 | 10 | 3.30 | 75 | 2498 | 10.4 | 30.7 |
| Dose 8, 24hr | 9.0 | 5.58 | 107 | 11 | |||||
| Dose 9, 0hr | 9.0 | 5.87† | 118 | 10 | 3.09 | 113 | 3434 | 10.2 | 30.3 |
| Dose 9, 24hr | 9.0 | 5.63 | 106 | 11 | |||||
| Dose 10, 0hr | 9.0 | 6.07 | 87 | 10 | 3.00 | 194‡ | 4452‡ | 10.2 | 30.4 |
| Dose 10, 24hr | 9.0 | 6.11 | 89 | 10 | 4887 | ||||
| Dose 11, 0hr | 9.0 | 6.43 | 81 | 9 | 3.32 | 103 | 2904 | 10.1 | 30.3 |
| Dose 11, 24hr | 9.0 | 6.23 | 81 | 9 | |||||
| Dose 12, 0hr | 9.0 | 5.58 | 106 | 11 | 3.22 | 148 | 4923 | 9.7§ | 29.1§ |
| Dose 12, 24hr | 9.0 | 5.53 | 110 | 11 | |||||
| Dose 13, 0hr | 9.0 | 6.81 | 111 | 8 | 2.88 | 156 | 3908 | 8.4§ | 24.5§ |
| Dose 13, 24hr | 9.0 | 6.63 | 108 | 9 | |||||
| Maintenance Phase 3.0 mg/kg | |||||||||
| Dose 14, 0hr | 3.0 | 6.70 | 93 | 9 | 3.07 | 131 | 3543 | 8.3§ | 24.8§ |
| Dose 14, 24hr | 3.0 | 6.54 | 91 | 9 | |||||
| Dose 15, 0hr | 3.0 | 6.42 | 100 | 9 | 3.14 | 109 | 2907 | 8.2 | 24.7 |
| Dose 15, 24hr | 3.0 | 6.30 | 103 | 9 | |||||
| Dose 16, 0hr | 3.0 | 7.18 | 113 | 8 | 2.92 | 75 | 1838 | 8.9 | 27.3 |
| Dose 16, 24hr | 3.0 | 6.93 | 114 | 8 | |||||
| Dose 17, 0hr | 3.0 | 6.88 | 139 | 8 | 3.51 | 92 | 1867 | 9.5 | 28.8 |
| Dose 17, 24hr | 3.0 | 6.61 | 132 | 9 | |||||
| Dose 18, 0hr | 3.0 | 6.03 | 137 | 10 | 3.62 | 70 | 1728 | 9.6 | 28.6 |
| Dose 18, 24hr | 3.0 | 6.08 | 138 | 10 | |||||
| Dose 19, 0hr | 3.0 | 5.02∥ | 58∥ | 12 | 3.66 | 86 | 1559 | 8.4∥ | 25.8∥ |
| Dose 19, 24hr | 3.0 | 5.37 | 64 | 11 | |||||
| Dose 20, 0hr | 3.0 | 5.47 | 45 | 11 | 3.96 | 95 | 1362 | 8.5 | 26.2 |
| Dose 20, 24hr | 3.0 | 6.20 | 51 | 9 | |||||
| Dose 21, 0hr | 3.0 | 5.19 | 46 | 12 | 3.90 | 81 | 1349 | 8.6 | 26.3 |
| Dose 21, 24hr | 3.0 | 5.99 | 51 | 10 | |||||
| Dose 22, 0hr | 3.0 | 6.19 | 42 | 9 | 3.86 | 107 | 1347 | 9.3 | 28.8 |
| Dose 22, 24hr | 3.0 | 6.69 | 51 | 9 | |||||
GFR – estimated glomerular filtration rate;
amiodarone started 72 hours post infusion for atrial fibrillation.
amiodarone dose doubled.
viral infection.
HGB – hemoglobin; HCT – hematocrit;
missed 3 doses (6 weeks) of Aranesp;
dialysis started 1 week prior.
The BUN showed a modest improvement during the dose optimization phase (159 to 97 mg/dL) and during the maintenance phase on both the 9.0 mg/kg and 3.0 mg/kg dose (Table 1). The creatinine slightly improved compared to baseline by approximately 12%, during the dose optimization phase (5.56 to 4.88 mg/dL). However, 72 hours following dose 3, the subject developed atrial fibrillation, requiring the initiation of amiodarone. Amiodarone is well known to decrease creatinine excretion 17 and the patient’s serum creatinine increased by approximately 13% after being placed on the drug. The amiodarone dose was doubled on day 54 prior to dose 9, which further increased the creatinine. Consistent with the rhLCAT treatment slightly improving or at least stabilizing renal function, cystatin C was less affected by amiodarone treatment and decreased by 29% compared to baseline (4.16 to 2.96 mg/L), during the optimization phase. During the dose maintenance phase on the weekly 9.0 mg/kg dose, the patient’s cystatin C remained decreased (2.88 mg/L) but returned to baseline once the biweekly 3.0 mg/kg dose was started (Table 1). The estimated glomerular filtration rate (eGFR) was severely impaired at the start of the study and remained unchanged.
Changes in Hematology Parameters on rhLCAT
The effect of rhLCAT on anemia was determined by hemoglobin (HGB) and hematocrit (HCT) from pre-study through the end of treatment (Table 1). There was a significant upward trend in both parameters through the 9.0 mg/kg dose in the maintenance phase, and the patient noted an improvement in exercise tolerance. Compared to baseline, the HGB increased (8.2 to 10.1 g/dL) by 2 g/dL or an approximate 25% increase over a 4 week period, during this period. The HGB and HCT improved transiently, but returned to baseline during a period of 6 weeks (Dose 12–14) when darbepoetin alfa was not available to the subject. However, the HGB and HCT again returned to near maximum levels over 4 weeks when darbepoetin alfa was restarted when the 3.0 mg/kg dose was administered bi-weekly.
DISCUSSION
We report the first-in-human results of rhLCAT in a patient with FLD. rhLCAT therapy was safe and well tolerated over an 8-month period and produced normal HDL subfractions and near normal HDL-C levels. Several features of rhLCAT make it particularly amenable as an ERT 14, particularly its relatively low plasma concentration and its effectiveness for 1–2 weeks after infusion. Moreover, rhLCAT does not need to be targeted to any specific tissue or organelle, since it catalyzes its reaction in the plasma compartment, thus making it easier to deliver and monitor.
CE, the product of the LCAT reaction, is the lipid parameter that is most closely related to the biochemical effect of rhLCAT (ACP-501). The low levels of CE in FLD result in low HDL-C, thus making this lipoprotein parameter also useful to follow. During the optimization phase, a dose-dependent increase in CE, % CE, and HDL-C and a decrease in FC was observed as would be predicted based on the enzyme reaction mechanism of LCAT. Interestingly, apoA-I and apoB also increased with each dose but quickly plateaued, which is probably a consequence of the longer half-life of the newly formed HDL and LDL particles 18. In the maintenance phase, peak CE and HDL-C levels were sustained from one infusion to the next and were always greater than baseline levels throughout the 9.0 mg/kg maintenance phase and higher levels were achieved with weekly versus biweekly infusions. The % CE in the 9.0 mg/kg dose increased from 19% to a normal level of >70%, peaking at 24 hours, and the % CE nadir increased slightly with successive infusions.
rhLCAT consistently had a major effect on lowering plasma TG levels, suggesting a previously unrecognized role for LCAT in TG metabolism. TG concentrations decreased by more than 40–65% following every infusion and remained low for up to 4–5 days. Normally, TG increases after a meal, but the patient’s TG always decreased postprandially following the rhLCAT infusion. A potential mechanism for the rapid and sustained drop in TG could be the redistribution of apoA-II and apoC-III, inhibitors of lipoprotein lipase, from VLDL to newly formed HDL following rhLCAT.
Results from this study also enabled us to observe for the first time in humans the sequential formation of HDL from small preβ-HDL particles and small discoidal α-4 HDL to the normal formation of larger α-HDL particles. Small phospholipid-rich particles were converted to normal CE-rich HDL sized particles. CE increased in both HDL and LDL and HDL-FC decreased. Interestingly, CE and HDL-C increased in parallel for 6 hours starting immediately after infusion, while there was a 2–4 hour delay in the appearance on LDL-C (Figure 5E). The low LDL-C levels observed at baseline increased to near normal levels by 1–2 days (Figure 2B). This was consistent with the initial formation of CE by LCAT mostly occurring on HDL followed by the transfer of HDL-CE to LDL by CETP 2.
Sequential HDL particle formation was also measured by NMR, and the results closely paralleled the formation of HDL subparticles on 1D and 2D gels. Small HDL paralleled the disappearance and reemergence of α-4 HDL (Figure 5C and 5D). Large HDL appearance and later disappearance coincided with HDL-C concentration. These findings support the use of NMR lipoprotein particle analysis as a method to follow HDL maturation in future studies.
The possibility of preventing or reversing some of the pathologic features of FLD by rhLCAT treatment was supported by the change observed in the subject’s anemia. The mechanism for hemolysis in FLD is possibly due to the lack of exchange of cholesterol between red blood cells and HDL 2. The 4-week delay in the improvement of the hemoglobin was likely due to the time it took for the generation of newly synthesized red blood cells that were then protected by normal HDL formed after rhLCAT infusion.
The cause of renal disease in FLD is not known, but the low levels of CE correlate with the presence of Lp-X, which is implicated in the pathogenesis of the renal disease 19–21. This is supported by the fact that FED patients, which have some residual LCAT activity and have normal % CE 8, do not form Lp-X and do not develop renal disease. We had no direct measurement of Lp-X but have indirect evidence supporting its disappearance after rhLCAT. We observed an immediate 50% decrease in phospholipid that was sustained over 7 days, when CE was also increased, along with the rapid disappearance of abnormal PL particles spread across the FPLC consistent with the disappearance of PL-rich Lp-X particles. Importantly, the sustained increased % CE above baseline, particularly on the 9.0 mg/kg dose, suggests that sufficient CE levels may be formed from the rhLCAT treatment to prevent Lp-X formation.
The main objective of this study was to determine if the renal disease in FLD can be stabilized or even reversed by rhLCAT (ACP-501) therapy. At presentation, the patient already had advanced renal disease, and it was uncertain whether the loss in renal function would be reversible. As shown in Table 1, the rhLCAT treatment appeared to slightly improve the subject’s renal function or at least stabilized it, which up until the time of the rhLCAT treatment was rapidly worsening. This will have to be carefully assessed in future clinical trials, but it may be necessary to start rhLCAT therapy at an earlier stage in the disease process to be more effective. Fortunately, FLD patients typically first present with proteinuria, and it can take as long as 20–30 years before end stage renal disease develops 1. Hence, if the diagnosis is made early enough, the development of significant renal disease could possibly be prevented by rhLCAT treatment.
In summary, the beneficial changes in clinical, biochemical and lipoprotein parameters in the first report of a FLD patient treated by ERT are encouraging and support continued development of rhLCAT therapy.
Supplementary Material
Acknowledgements
We thank nurses on the NIH Clinical Center 5NW care unit, the NIH investigational pharmacy, and the patient for participating in this study. We want to thank Dr. E. Schaefer at Tufts University for advice and referring the patient. The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does it imply endorsement by the U.S. government. AstraZeneca PLC and its global biological arm MedImmune, LLC, acquired AlphaCore Pharma, LLC.
Source of Funding
The Intramural Research Program of the Cardiovascular and Pulmonary Branch, National Heart, Lung, and Blood Institute of the National Institutes of Health reviewed and supported the study. AlphaCore Pharma, LLC supplied the ACP-501.
Nonstandard Abbreviations and Acronyms
- LCAT
lecithin:cholesterol acyltransferase
- RCT
reverse cholesterol transport
- ACP-501
recombinant human LCAT
- rHDL
reconstituted high-density lipoprotein
- rhLCAT
recombinant human LCAT
- CETP
cholesteryl ester transfer protein
- FLD
Familial LCAT Deficiency
- CE
cholesteryl esters
- TC
total cholesterol
- ApoB
apolipoprotein B
- ApoA-I
apolipoprotein A-I
- HGB
hemoglobin
- HCT
hematocrit
- ERT
enzyme replacement therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Rebecca Bakker-Arkema, Bruce J. Auerbach, Brian R. Krause, and Reynold Homan were employees of AlphaCore Pharma, LLC (now owned by AstraZeneca PLC). Rebecca Bakker-Arkema is currently employed by MedImmune, LLC, Gaithersburg, MD.
REFERENCES
- 1.Santamarina-Fojo S, Hoeg JM, Assmann G, Brewer HB. Lecithin Cholesterol Acyltransferase Deficiency and Fish Eye Disease. In: Scriver CR, Beaudet D, Valle D, Sly WS, editors. The Metabolic & Molecular Basis of Inherited Disease. New York, NY: McGraw-Hill; 2001. pp. 2817–2833. [Google Scholar]
- 2.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. Journal of Lipid Research. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Barter PJ. Hugh sinclair lecture: the regulation and remodelling of HDL by plasma factors. Atherosclerosis Supplements. 2002;3:39–47. doi: 10.1016/s1567-5688(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 4.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. Journal of Lipid Research. 1968;9:155–167. [PubMed] [Google Scholar]
- 5.Norum K, Gjone E, Glomset JA. Familial Lecithin:cholesterol Acyltransferase Deficiency, Including Fish Eye Disease. In: Scriver CR, Beaudet AL, Sly WS, editors. The Metabolic Basis of Inherited Disease. New York, NY: McGraw-Hill; 1989. pp. 1181–1194. [Google Scholar]
- 6.Cogan DG, Kruth HS, Datilis MB, Martin N. Corneal opacity in LCAT disease. Cornea. 1992;11:595–599. doi: 10.1097/00003226-199211000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Palmiero PM, Sbeity Z, Liebmann J, Ritch R. In vivo imaging of the cornea in a patient with lecithin-cholesterol acyltransferase deficiency. Cornea. 2009;28:1061–1064. doi: 10.1097/ICO.0b013e31819839ae. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222:299–306. doi: 10.1016/j.atherosclerosis.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Weber CL, Frohlich J, Wang J, Hegele RA, Chan-Yan C. Stability of lipids on peritoneal dialysis in a patient with familial LCAT deficiency. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:2084–2088. doi: 10.1093/ndt/gfm233. [DOI] [PubMed] [Google Scholar]
- 10.Flatmark AL, Hovig T, Myhre E, Gjone E. Renal transplantation in patients with familial lecithin: cholesterol-acetyltransferase deficiency. Transplantation Proceedings. 1977;9:1665–1671. [PubMed] [Google Scholar]
- 11.Panescu V, Grignon Y, Hestin D, Rostoker G, Frimat L, Renoult E, Gamberoni J, Grignon G, Kessler M. Recurrence of lecithin cholesterol acyltransferase deficiency after kidney transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1997;12:2430–2432. doi: 10.1093/ndt/12.11.2430. [DOI] [PubMed] [Google Scholar]
- 12.Forte T, Nichols A, Glomset J, Norum K. The ultrastructure of plasma lipoproteins in lecithin:cholesterol acyltransferase deficiency. Scandinavian Journal of Clinical and Laboratory Investigation Supplementum. 1974;137:121–132. [PubMed] [Google Scholar]
- 13.Asztalos BF, Schaefer EJ, Horvath KV, Yamashita S, Miller M, Franceschini G, Calabresi L. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. Journal of Lipid Research. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Rousset X, Shamburek R, Vaisman B, Amar M, Remaley AT. Lecithin cholesterol acyltransferase: an anti- or pro-atherogenic factor? Current Atherosclerosis Reports. 2011;13:249–256. doi: 10.1007/s11883-011-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamburek RD, Bakker-Arkema R, Shamburek AM, Freeman LA, Amar M, Auerbach B, Krause BR, Homan R, Adelman SJ, Collins HL, Sampson M, Wolska A, Remaley A. Safety and Tolerability of ACP-501, a Recombinant Human Lecithin:Cholesterol Acyltransferase, in a Phase 1 Single-Dose Escalation Study. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.306223. pii: CIRCRESAHA.115.306223. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roshan B, Ganda OP, Desilva R, Ganim RB, Ward E, Haessler SD, Polisecki EY, Asztalos BF, Schaefer EJ. Homozygous lecithin:cholesterol acyltransferase (LCAT) deficiency due to a new loss of function mutation and review of the literature. Journal of Clinical Lipidology. 2011;5:493–499. doi: 10.1016/j.jacl.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak PT, Sharma AD, Carruthers SG. Creatinine elevation in patients receiving amiodarone correlates with serum amiodarone concentration. British Journal of Clinical Pharmacology. 1993;36:125–127. doi: 10.1111/j.1365-2125.1993.tb04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiwaki M, Ikewaki K, Bader G, Nazih H, Hannuksela M, Remaley AT, Shamburek RD, Brewer HB., Jr Human lecithin:cholesterol acyltransferase deficiency: in vivo kinetics of low-density lipoprotein and lipoprotein-X. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1370–1375. doi: 10.1161/01.ATV.0000217910.90210.99. [DOI] [PubMed] [Google Scholar]
- 19.Borysiewicz LK, Soutar AK, Evans DJ, Thompson GR, Rees AJ. Renal failure in familial lecithin: cholesterol acyltransferase deficiency. The Quarterly Journal of Medicine. 1982;51:411–426. [PubMed] [Google Scholar]
- 20.Hovig T, Gjone E. Familial plasma lecithin: cholesterol acyltransferase (LCAT) deficiency. Ultrastructural aspects of a new syndrome with particular reference to lesions in the kidneys and the spleen. Acta Pathologica et Microbiologica Scandinavica Section A, Pathology. 1973;81:681–697. [PubMed] [Google Scholar]
- 21.Imbasciati E, Paties C, Scarpioni L, Mihatsch MJ. Renal lesions in familial lecithin-cholesterol acyltransferase deficiency. Ultrastructural heterogeneity of glomerular changes. American Journal of Nephrology. 1986;6:66–70. doi: 10.1159/000167056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.