Abstract
Aims:
AMPD1 c.34C > T (rs17602729) polymorphism results in AMPD1 deficiency. We examined the association of AMPD1 deficiency and variability of hemodynamic response to regadenoson.
Subjects & methods:
Genotyping for c.34C>T was performed in 267 patients undergoing regadenoson cardiac stress testing.
Results:
Carriers of c.34C >T variant exhibited higher relative changes in systolic blood pressure (SBP) compared with wild-type subjects ([%] SBP change to peak: 12 ± 25 vs 5 ± 13%; p = 0.01) ([%] SBP change to nadir: -3 ± 15 vs -7 ± 11%; p = 0.04). Change in heart rate was similar between groups, but side effects were more common in carriers of the variant (+LR = 4.2; p = 0.04).
Conclusion:
AMPD1 deficiency may be involved in the modulation of regadenoson’s systemic effects.
Keywords: : adenosine, genetic, myocardial perfusion imaging, regadenoson
AMPD1 c.34C>T (rs17602729) is a polymorphism present in 12–18% of Caucasians and 19% of African–Americans [1,2]. This variant results in the substitution of cytosine for thymidine, leading to a premature stop codon and substantially diminished enzymatic function [2,3]. AMPD1 deficiency leads to reduced clearance of adenosine monophosphate (AMP) and increased production of adenosine in skeletal muscles. The heterozygous population exhibits partial enzymatic deficiency. Although carriers of this variant are frequently asymptomatic and phenotypically similar to wild types (CC), carrier status, both homozygous (TT) and heterozygous (CT) has been associated with myalgias or weakness after prolonged exercise [4] and increased blood flow response to sprint exercise [5].
Adenosine has been the principle agent used in pharmacological cardiac stress tests due to its effects on coronary vasodilation. By stimulating adenosine A2A receptors on arteriolar vascular smooth muscle cells, adenosine induces coronary arterial vasodilation and myocardial hyperemia, the desired cardiac state for myocardial perfusion imaging (MPI). The nonselective activation of A1, A2B and A3 receptors by adenosine, however, can lead to adverse effects including nausea, chest pain, dyspnea and less commonly, bronchospasm and heart blocks [6,7]. In 2008, regadenoson, an adenosine analogue and selective A2A adenosine receptor agonist was approved by the US FDA [8]. Regadenoson has since rapidly replaced adenosine during MPI because of desirable factors including a longer half-life, uniform dosing and ease of administration as a rapid bolus. In contrast to adenosine, regadenoson achieves myocardial hyperemia quicker and maintains it longer, attributes that make it optimal for radionuclide MPI [9]. Adenosine causes a sympathetic increase in the heart rate (HR), and systolic blood pressure (SBP), along with a drop in the diastolic blood pressure [10]. Factors that exacerbate or mitigate this physiologic response have been poorly studied.
The effects of genetic polymorphisms on variation in response to in vivo administration of regadenoson have not been studied. We hypothesized that carriers of the AMPD1 c.34C>T variant allele linked to AMPD1 deficiency exhibit an altered hemodynamic response to A2A receptor stimulation by regadenoson.
Subjects & methods
Patients
The study protocol was approved by the Indiana University institutional review board. Written informed consent was obtained from all participants.
Subjects who were scheduled for resting regadenoson nuclear stress testing were eligible to be enrolled in this study. Subjects who underwent a combination of exercise and nuclear pharmacologic stress testing with regadenoson were excluded from analysis. Data on demographics, medical history, family history, risk factors and dietary history were collected.
Genotyping
Genomic DNA was extracted by using the Qiagen Flexigene DNA Kit #51206 following the protocol for isolation of DNA from 100–500 µl buffy coat (Germantown, USA). AMPD1 c.34C>T (rs17602729) was analyzed using an open array genotyping platform (Life Technologies, NY, USA) according to the manufacturer’s instructions. Alleles of interest were amplified by using sequence-specific primers as well as two allele-specific TaqMan® probes (Applied Biosystems, CA, USA). Allelic discrimination was used to determine individual genotypes.
Regadenoson administration
A standard dose of 0.4 mg of regadenoson was administered through a peripheral intravenous line over a period of 10 s, followed by a saline flush of 5 ml over another 10 s.
Study measurements
Hemodynamic parameters, including SBP and HR, were measured prior to regadenoson infusion, then at 1 min intervals over a period of 5 min thereafter. Self-reported side effects were recorded. Primary outcomes were [1] change in HR [2], change in SBP to peak (both absolute and relative [%]) and [3] change in SBP to nadir (both absolute and relative [%]) postregadenoson administration. Change in HR was defined as the difference between the peak postadministration HR and the HR prior to regadenoson administration (baseline HR). Absolute change in SBP to peak was defined as the difference between the peak postadministration SBP and the baseline SBP. Likewise, absolute change in SBP to nadir was defined as the difference between the nadir postadministration SBP and the baseline SBP. Percentage [%] change was calculated by dividing the absolute change by the baseline SBP and multiplying the result by a 100. Secondary outcomes included the incidence of side effects reported by patients (nausea, abdominal pain, chest pain, dyspnea, dizziness, flushing and headache).
Statistical analysis
Statistical analyses were performed by using SPSS software, version 21.0 (IBM, IL, USA). Statistical significance was defined as p-value of less than 0.05. All statistical tests were two-sided, and values are represented as the mean ± standard deviation, unless otherwise indicated. Unpaired two-sided Student’s t-test was used to compare normally distributed continuous variables. Categorical variables were compared by using the chi-square test. AMPD1 genotypes (CC and CT + TT) were included in forward stepwise multivariate linear regression analysis, along with clinical variables associated with p < 0.1 in univariate analysis.
Results
The study population consisted of 267 individuals who underwent regadenoson stress testing, 55% of whom were females. The mean age was 58 years, and the majority was Caucasian [72.0%]. Baseline patient characteristics are summarized in Table 1. Two patients were homozygous for the AMPD1 T variant allele, 40 were heterozygous and 225 were wild type. Distribution of genotypes was consistent with Hardy–Weinberg equilibrium (p > 0.05). Carriers of the T allele, including homozygous and heterozygous individuals, were analyzed as a single group, and compared with the wild-type group, unless otherwise indicated.
Table 1. . Baseline characteristics.
| Characteristics | Total, (n = 267) | Wild-type (CC), (n = 225) | Carrier (CT + TT), (n = 42) | Heterozygous (CT), (n = 40) | Homozygous (TT), (n = 2) | p-value (CC vs CT + TT) | p-value (CC vs CT vs TT) |
|---|---|---|---|---|---|---|---|
| Mean age (years) |
58.4 |
58.6 |
57.2 |
57 |
61.1 |
0.54 |
0.39 |
| Mean BMI (kg/m2) |
35 |
35 |
34.9 |
35.2 |
28.2 |
0.36 |
0.15 |
| Males |
120/267 (44.9%) |
103/225 (45.8%) |
17/42 (40.5%) |
16/40 (40%) |
1/2 (50%) |
0.53 |
0.79 |
| Females |
147/267 (55.1%) |
122/225 (54.2%) |
25/42 (59.5%) |
24/40 (60%) |
1/2 (50%) |
0.53 |
0.79 |
|
Race | |||||||
| Caucasian |
192/267 (72.0%) |
156/225 (69.3%) |
36/42 (85.7%) |
34/40 (85%) |
2/2 (100%) |
0.03 |
0.09 |
| African–American |
71/267 (26.6%) |
67/225 (39.8%) |
4/42 (9.5%) |
4/40 (10%) |
0/2 (0%) |
0.01 |
0.02 |
| Others |
4/267 (1.5%) |
2/225 (1.0%) |
2/42 (4.8%) |
2/40 (5%) |
0/2 (0%) |
0.06 |
0.44 |
| Coffee drinker |
211/265 (79.6%) |
178/225 (79.1%) |
33/42 (78.6%) |
31/40 (77.5%) |
2/2 (100%) |
0.88 |
0.77 |
| Smoking |
87/265 (32.8%) |
71/225 (31.6%) |
16/42 (38.1%) |
16/40 (40%) |
0/2 (0%) |
0.36 |
0.32 |
| Hyperlipidemia |
182/267 (68.2%) |
150/225 (66.7%) |
32/42 (76.2%) |
30/40 (75%) |
2/2 (100%) |
0.23 |
0.36 |
| Coronary artery disease |
82/267 (30.7%) |
65/225 (28.9%) |
17/42 (40.5%) |
15/40 (37.5%) |
2/2 (100%) |
0.14 |
0.06 |
| Prior PCI |
58/267 (21.7%) |
46/225 (20.4%) |
12/42 (28.6%) |
12/40 (30%) |
0/2 (0%) |
0.24 |
0.3 |
| Prior CABG |
25/267 (9.4%) |
19/225 (8.4%) |
6/42 (14.3%) |
5/40 (12.5%) |
1/2 (50%) |
0.23 |
0.1 |
| Hypertension |
215/267 (80.5%) |
180/225 (80.0%) |
35/42 (83.3%) |
33/40 (82.5%) |
2/2 (100%) |
0.62 |
0.73 |
| Congestive heart failure |
29/267 (10.9%) |
24/225 (10.7%) |
5/42 (11.9%) |
5/40 (12.5%) |
0/2 (0%) |
0.81 |
0.83 |
| Diabetes mellitus |
111/267 (41.6%) |
93/225 (41.3%) |
18/42 (42.9%) |
17/40 (42.5%) |
1/2 (50%) |
0.86 |
0.96 |
| Chronic kidney disease |
10/267 (3.7%) |
9/225 (4.0%) |
1/42 (2.4%) |
1/40 (2.5%) |
0/2 (0%) |
0.61 |
0.87 |
| Peripheral vascular disease |
37/267 (13.9%) |
32/225 (14.2%) |
5/42 (11.9%) |
4/40 (10%) |
1/2 (50%) |
0.69 |
0.29 |
| Stroke |
27/267 (10.1%) |
26/225 (11.6%) |
1/42 (2.4%) |
1/40 (2.5%) |
0/2 (0%) |
0.07 |
0.19 |
| Beta blocker |
126/267 (47.2%) |
104/225 (46.2%) |
22/42 (52.4%) |
20/40 (50%) |
2/2 (100%) |
0.61 |
0.57 |
| Calcium channel blocker |
74/267 (27.7%) |
64/225 (28.4%) |
10/42 (23.8%) |
10/40 (25%) |
0/2 (0%) |
0.54 |
0.62 |
| ACE inhibitor | 126/267 (47.2%) | 110/225 (48.9%) | 16/42 (38.1%) | 15/40 (37.5%) | 1/2 (50%) | 0.41 | 0.76 |
ACE: Angiotensin-converting enzyme; CABG: Coronary arteries bypass grafting; PCI: Percutaneous coronary intervention.
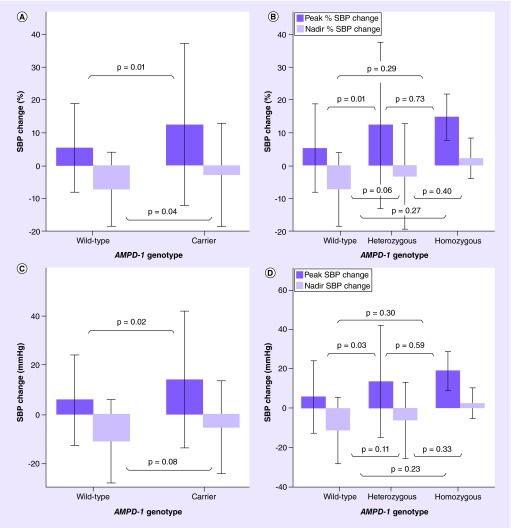
There was no significant difference in baseline, peak and nadir SBP, and baseline and peak HR in the CT + TT group when compared with the CC group (Figure 1). The relative rise in SBP (% SBP change to peak) was significantly higher in the carrier group as compared with the wild-type group (12 ± 25 vs 5 ± 13%; p = 0.01) (Figure 2A), as was the absolute rise in SBP (SBP change to peak: 14 ± 28 vs 6 ± 18 mm; p = 0.02) (Figure 2C). There was no significant difference in the absolute drop in SBP (SBP change to nadir: -11 ± 17 vs -5 ± 18 mm; p = 0.08) between both groups (Figure 2C), but the relative decrease in SBP was significantly different (% SBP change to nadir: -7 ± 11 vs -3 ± 15%; p = 0.04) (Figure 2A). HR change did not differ significantly (31 ± 14 vs 30 ± 14 bpm; p = 0.6). In a multivariate linear regression analysis, age, gender, hyperlipidemia and smoking significantly affected the association between [%] SBP change to peak and AMPD1 genotype (Table 2). The same variables, with the exception of smoking, affected the absolute SBP change to peak (Table 2), while both relative and absolute SBP change to nadir were only affected by age (Table 2). After accounting for confounding variables, T allele carriers remained significantly associated with a more elevated [%] change in SBP to peak (p = 0.009) (Table 2) and with a lesser decrease in [%] SBP change to nadir (p = 0.044) (Table 2). The absolute SBP change to peak continued to be significant (p = 0.012) (Table 2), while the absolute SBP change to nadir remained nonsignificant (p = 0.061) after multivariate adjustment (Table 2). Multivariate linear regression analysis of HR change and AMPD1 genotype demonstrated significant association of previous percutaneous coronary intervention (PCI), systolic heart failure, age and gender with HR change (Table 2). After adjustment of significant covariates, AMPD1 c.34C>T polymorphism was not significantly associated with mean HR change (p = 0.554).
Figure 1. . Systolic blood pressure and heart rate response to regadenoson.
(A) SBP at baseline, peak and nadir in wild-type and carrier groups. (B) SBP at baseline, peak and nadir in wild-type, heterozygous and homozygous groups. (C) Baseline HR and peak HR in wild-type and carrier groups. (D) Baseline HR and peak HR in wild-type, heterozygous and homozygous groups.
HR: Heart rate; SBP: Systolic blood pressure.
Figure 2. . Absolute and percentage Systolic blood pressure change in response to regadenoson.
(A) Percentage SBP change to peak and nadir in wild-type and carrier groups. (B) Percentage SBP change to peak and nadir in wild-type, heterozygous and homozygous groups. (C) Absolute SBP change to peak and nadir in wild-type and carrier groups. (D) Absolute SBP change to peak and nadir in wild-type, heterozygous and homozygous groups.
SBP: Systolic blood pressure.
Table 2. . Multivariate analyses of systolic blood pressure and heart rate.
| Hemodynamic response to regadenoson | Variable | Effect size | CI | p-value |
|---|---|---|---|---|
| Percentage SBP change to peak | AMPD1 (CT + TT) | 7.02 | (1.78–12.26) | 0.009 |
| Age | -0.26 | (-0.44 to -0.07) | 0.007 | |
| Male gender | -5.39 | (-9.13 to -1.64) | 0.005 | |
| Hyperlipidemia | -4.44 | (-8.61 to -0.27) | 0.037 | |
| |
Smoking |
4.041 |
(0.01–8.08) |
0.05 |
| Absolute SBP change to peak | AMPD1 (CT + TT) | 8.7 | (1.95–15.46) | 0.012 |
| Age | -0.32 | (-0.55 to -0.08) | 0.018 | |
| Male gender | -6.05 | (-10.88 to -1.26) | 0.014 | |
| |
Hyperlipidemia |
-6.58 |
(-11.92 to -1.24) |
0.016 |
| Percentage SBP change to nadir | AMPD1 (CT + TT) | 4.18 | (0.11–8.24) | 0.044 |
| |
Age |
-0.21 |
(-0.35 to -0.07) |
0.004 |
| Absolute SBP change to nadir | AMPD1 (CT + TT) | 5.58 | (-0.27–11.42) | 0.061 |
| |
Age |
-0.29 |
(-0.49 to -0.09) |
0.005 |
| HR change | AMPD1 (CT + TT) | 1.31 | (-3.04–5.65) | 0.554 |
| Age | -0.21 | (-0.36 to -0.05) | 0.008 | |
| Male gender | -4.15 | (-7.32 to -0.98) | 0.011 | |
| Systolic heart failure | -7.48 | (-12.49 to -2.48) | 0.004 | |
| Prior PCI | -5.35 | (-9.21 to -1.49) | 0.007 |
HR: Heart rate; PCI: Percutaneous coronary intervention; SBP: Systolic blood pressure.
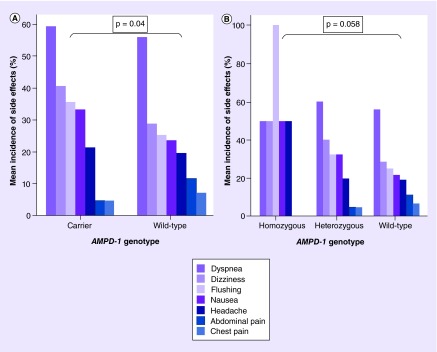
Secondary outcomes measured included the incidence of side effects after regadenoson administration. Side effects reported by patients included nausea, abdominal pain, chest pain, dyspnea, dizziness, flushing and headache. Subjects were categorized in two groups: [1] no side effects reported and [2] one or more side effects reported. The incidence of side effects was significantly increased in carriers (CT + TT) as compared with the wild-type group (CC) (39/42 [93%] vs 182/225 [81%]; likelihood ratio [+] = 4.2; p = 0.04; odds ratio = 3.1; 95% CI: 0.91–10.4) (Figure 3). There was an increased incidence of all side effects except chest and abdominal pain in the carrier group. The incidence of side effects increased with every additional T allele, exhibiting a gene-dose effect (Figure 3).
Figure 3. . Incidence of side effects following regadenoson administration.
(A) Incidence of side effects following regadenoson administration in carrier and wild-type groups. (B) Incidence of side effects following regadenoson administration in homozygous, heterozygous and wild-type groups.
Discussion
AMPD1 mediates the transformation of AMP into inosine monophosphate in the cytosol of skeletal muscle cells. Catalysis by AMPD1 plays an important role in the purine salvage nucleotide cycle and is a crucial step in the regeneration of energy during states of ischemia. In response to hypoxic states, tissue cells become dependent on oxidative phosphorylation in an attempt to meet increasing energy demands. This results in an increased production of AMP, which is in turn dephosphorylated by 5’-nucleotidase to form adenosine [2]. Adenosine has both direct and indirect downstream effects. By inducing smooth muscle relaxation and coronary arterial vasodilation via A2A receptor binding, adenosine improves blood flow and oxygen delivery [2,11]. Stimulation of A1 receptor by adenosine induces negative inotropy and chronotropy, thereby minimizing oxygen demand [2,9]. In addition to these direct vasomotor effects, adenosine stimulates carotid-body chemoreceptors and sympathetic afferent nerves resulting in systemic vasoconstriction. This complex mechanism leads to an overall increase in blood pressure and HR [10].
AMPD1 c.34C>T is a common genetic polymorphism, found in 12–18% of Caucasians, 2% of whom are homozygous [1,2] and leads to functional AMPD1 deficiency. Lack of AMPD1 in skeletal muscles reduces the ability to regenerate ATP stores, and may therefore result in decreased exercise capacity and symptoms of muscle fatigue [12,13]. Forearm blood flow in response to transient ischemia is increased significantly in T variant allele carriers when measured using venous occlusion plethysmography [14]. Femoral artery blood flow measured by ultrasonography is also increased in subjects with AMPD1 deficiency during cycling sprint exercise with more rapid recovery postexercise, but lower peak power, as compared with normal subjects, an effect that has been attributed to an AMPD1-dependent increase in adenosine formation during exercise [5]. Sabina et al. reported a 16-fold increase in postexercise adenosine levels in muscle biopsies obtained from subjects with AMPD1 deficiency compared with a twofold increase in controls [12]. Consistent with these findings, lower prevalence of AMPD1 T variant allele has been documented in top-level endurance athletes as compared with controls [1,15].
In this study, we examine the effects of regadenoson on SBP and HR among patients that carry the T variant allele in the context of pharmacologic cardiac stress testing. We found that in response to regadenoson, carriers (CT + TT) exhibited a higher relative rise in SBP at its peak and a smaller relative drop in SBP at its lowest measurement. However, HR changes did not differ between both groups (31 ± 14 vs 30 ± 14 bpm; p = 0.6). Since absolute changes in SBP are affected by baseline measurements of SBP, the use of relative [%] SBP change allows adjustment for the differences in the baseline measurements. It is therefore likely a more accurate reflection of the magnitude of change in SBP, whether to peak or to nadir. Among T allele carriers, we observed a significant difference in SBP change in response to regadenoson, with higher relative rise in SBP and a smaller relative drop in SBP. In view of the complex effects of adenosine and its analogues, multiple mechanisms may explain this observation. Activation of A2A receptors triggers a state of hyperemia in the myocardium and induces sympathetic excitation [9]. AMPD1 is present in both skeletal muscle cells [16], as well as myocardium [17], and could therefore influence the A2A-mediated response during MPI. Kalsi et al. have demonstrated decreased AMPD activity in human myocardium harvested at time of left ventricular assist device implantation or heart transplant in T allele carriers with advanced heart failure as compared with wild-type controls [18]. Klinger et al. described two potential mechanisms involved in the vasodilatory effect of adenosine on endothelial cells. The binding of adenosine to A2A receptors on endothelial cells may trigger an internal signaling pathway that activates nitric oxide synthase, resulting in increased vasodilation. Adenosine is also thought to have an additional direct vasodilatory effect on arterial smooth muscle cells [11]. Sympathetic activation causes peripheral vasoconstriction, triggering augmented local endogenous adenosine production in response to a state of increased demand. In addition, stimulation of A2A receptors in atrial myocytes results in ryanodine receptor phosphorylation, and release of Ca2+ from sarcoplasmic reticulum, possibly contributing to the rise in HR consistently observed with regadenoson administration [19,20].
AMPD1 deficiency leads to accumulation of intracellular AMP during hypoxia. Increase in AMP/ATP ratio has been demonstrated to result in activation of AMP-activated protein kinase during hypoxia and result in pulmonary vasoconstriction [21]. While not previously studied, alteration of AMP-activated protein kinase activity could occur in AMPD1-deficient subjects, and possibly influence the vasoactive response to A2A stimulation in vascular smooth muscle cells. Activation of AMP-activated protein kinase has been shown to inhibit nitric oxide-mediated aortic vascular smooth muscle cell relaxation [22]. Variability in peripheral vasoconstriction may in part explain the increase in SBP without significant difference in HR response among AMPD1-deficient subjects.
The HR response was not significantly different across the different AMPD1 genotypes, possibly because the resulting tachycardia is predominantly influenced by ryanodine receptor activation in the sinus nodal tissue.
The majority of adenosine’s side effects are thought to be secondary to its vasodilatory properties on vessels of the skin, brain and abdominal viscera, leading to flushing, nausea, headache and abdominal pain respectively [23]. In our study, we demonstrate a higher rate and higher likelihood for the occurrence of side effects from regadenoson administration in T allele variant carriers as compared with wild-type individuals. Interestingly, the order of side effect incidence was identical in both groups, with dyspnea, dizziness and flushing (in descending order) being the most common (Figure 3). Increased incidence of adenosine-specific side effects with in vivo administration of exogenous adenosine analogues in AMPD1-deficient patients may be the result of adenosine accumulation and activation of non-A2A adenosine receptors.
With regards to the FDA warning issued in November 2013 concerning the increased incidence of fatal myocardial infarction in patients receiving regadenoson or adenosine for cardiac nuclear stress tests, our study population, whether carriers of the c.34C>T mutation or wild type for that polymorphism, had no occurrences of adverse myocardial infarction. Continuous monitoring of electrocardiograms during regadenoson stress testing did not show any instances of AV nodal blockade in all the subjects tested.
Limitations of our study include a small number of homozygous individuals for c.34C>T and various co-morbidities that could account for unadjusted confounding in our analysis. The disparity in the racial groups (Caucasian and African–American) between carriers and noncarriers of the T allele is likely related to their prevalence in our geographic location, and the prevalence of the carrier state in general. Additionally, race was included in the multivariate analysis and did not significantly affect hemodynamic response (both SBP and HR). Further studies will be required before conclusions can be drawn whether pretest determination of AMPD1 genotype would be helpful prior to performance of regadenoson stress testing, in specific further analysis of whether altered response to regadenoson influences the sensitivity or specificity of pharmacologic MPI in diagnosis of coronary ischemia.
Conclusion
Regadenoson has become the predominantly used agent in pharmacologic MPI, yet its interplay with native adenosine metabolism is incompletely understood and merits further investigation. Our study suggests that carriers of the AMPD1 c.34C>T variant exhibit an exaggerated response to regadenoson and demonstrate an increased likelihood of adverse side effects. Further studies are needed to elucidate the association of AMPD1 variants and the phenotypic response to regadenoson stress testing, as well as sensitivity and specificity of MPI.
Executive summary.
Common genetic polymorphisms may play a role in the hemodynamic response to regadenoson during cardiac stress testing.
AMPD1 deficiency resulted in an altered systemic response to regadenoson, with the involved subjects exhibiting higher SBP after regadenoson administration.
Carriers of the AMPD1 T variant allele were more likely to develop side effects after regadenoson administration.
Footnotes
Financial & competing interests disclosure
This publication was made possible in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part by grant number (U54–RR025761; Anantha Shekhar, PI) from the NIH, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. DNA was extracted by the Specimen Storage Facility of the Indiana Clinical and Translational Sciences Institute which is supported, in part, by a Clinical and Translational Sciences Award (grant no. UL1TR001108. Anantha Shekhar, PI) and Clinical and Translational Sciences Institute Specimen Storage Facility construction was funded in part by grant CO6–RR020128–01 (RS Fife, PI, K Cornetta, Co-I). The project was also supported by the Indiana University Health Values Grant, the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative and the Methodist Research Institute Showalter Grant for Cardiovascular Research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Rubio JC, Martin MA, Rabadan M, et al. Frequency of the C34T mutation of the AMPD1 gene in world-class endurance athletes: does this mutation impair performance? J. Appl. Physiol. (1985) 2005;98(6):2108–2112. doi: 10.1152/japplphysiol.01371.2004. [DOI] [PubMed] [Google Scholar]
- 2.Binkley PF, Auseon A, Cooke G. A polymorphism of the gene encoding AMPD1: clinical impact and proposed mechanisms in congestive heart failure. Congest. Heart Fail. 2004;10(6):274–278. doi: 10.1111/j.1527-5299.2004.02017.x. quiz 9–80. [DOI] [PubMed] [Google Scholar]
- 3.Morisaki T, Gross M, Morisaki H, Pongratz D, Zöllner N, Holmes EW. Molecular basis of AMP deaminase deficiency in skeletal muscle. Proc. Natl Acad. Sci. USA. 1992;89(14):6457–6461. doi: 10.1073/pnas.89.14.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabina RL. Myoadenylate deaminase deficiency. A common inherited defect with heterogeneous clinical presentation. Neurol. Clin. 2000;18(1):185–194. doi: 10.1016/s0733-8619(05)70184-5. [DOI] [PubMed] [Google Scholar]
- 5.Norman B, Nygren AT, Nowak J, Sabina RL. The effect of AMPD1 genotype on blood flow response to sprint exercise. Eur. J. Appl. Physiol. 2008;103(2):173–180. doi: 10.1007/s00421-008-0683-0. [DOI] [PubMed] [Google Scholar]
- 6.Buhr C, Gossl M, Erbel R, Eggebrecht H. Regadenoson in the detection of coronary artery disease. Vasc. Health Risk Manag. 2008;4(2):337–340. doi: 10.2147/vhrm.s1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston DL, Daley JR, Hodge DO, Hopfenspirger MR, Gibbons RJ. Hemodynamic responses and adverse effects associated with adenosine and dipyridamole pharmacologic stress testing: a comparison in 2,000 patients. Mayo Clin. Proc. 1995;70(4):331–336. doi: 10.4065/70.4.331. [DOI] [PubMed] [Google Scholar]
- 8.de Lera Ruiz M, Lim YH, Zheng J. Adenosine A receptor as a drug discovery target. J. Med. Chem. 2013;57(9):3623–3650. doi: 10.1021/jm4011669. [DOI] [PubMed] [Google Scholar]
- 9.Al Jaroudi W, Iskandrian AE. Regadenoson: a new myocardial stress agent. J. Am. Coll. Cardiol. 2009;54(13):1123–1130. doi: 10.1016/j.jacc.2009.04.089. [DOI] [PubMed] [Google Scholar]
- 10.Riksen NP, Rongen GA, Yellon D, Smits P. Human in vivo research on the vascular effects of adenosine. Eur. J. Pharmacol. 2008;585(2–3):220–227. doi: 10.1016/j.ejphar.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 11.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal. 2002;14(2):99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 12.Sabina RL, Swain JL, Olanow CW, et al. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J. Clin. Invest. 1984;73(3):720–730. doi: 10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomaes T, Thomis M, Onkelinx S, et al. A genetic predisposition score for muscular endophenotypes predicts the increase in aerobic power after training: the CAREGENE study. BMC Genet. 2011;12(84):1–10. doi: 10.1186/1471-2156-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hand BD, Roth SM, Roltsch MH, et al. AMPD1 gene polymorphism and the vasodilatory response to ischemia. Life Sci. 2006;79(15):1413–1418. doi: 10.1016/j.lfs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Cieszczyk P, Eider J, Ostanek M, et al. Is the C34T polymorphism of the AMPD1 gene associated with athlete performance in rowing? Int. J. Sports Med. 2011;32(12):987–991. doi: 10.1055/s-0031-1283186. [DOI] [PubMed] [Google Scholar]
- 16.Dudley GA, Terjung RL. Influence of acidosis on AMP deaminase activity in contracting fast-twitch muscle. Am. J. Physiol. 1985;248(1 Pt 1):C43–C50. doi: 10.1152/ajpcell.1985.248.1.C43. [DOI] [PubMed] [Google Scholar]
- 17.Rubio R, Berne RM. Release of adenosine by the normal myocardium in dogs and its relationship to the regulation of coronary resistance. Circ. Res. 1969;25(4):407–415. doi: 10.1161/01.res.25.4.407. [DOI] [PubMed] [Google Scholar]
- 18.Kalsi KK, Yuen AH, Rybakowska IM, et al. Decreased cardiac activity of AMP deaminase in subjects with the AMPD1 mutation – a potential mechanism of protection in heart failure. Cardiovasc. Res. 2003;59(3):678–684. doi: 10.1016/s0008-6363(03)00497-8. [DOI] [PubMed] [Google Scholar]
- 19.Hove-Madsen L, Prat-Vidal C, Llach A, et al. Adenosine A2A receptors are expressed in human atrial myocytes and modulate spontaneous sarcoplasmic reticulum calcium release. Cardiovasc. Res. 2006;72(2):292–302. doi: 10.1016/j.cardiores.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Llach A, Molina CE, Prat-Vidal C, et al. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur. Heart J. 2011;32(6):721–729. doi: 10.1093/eurheartj/ehq464. [DOI] [PubMed] [Google Scholar]
- 21.Robertson TP, Mustard KJ, Lewis TH, et al. AMP-activated protein kinase and hypoxic pulmonary vasoconstriction. Eur. J. Pharmacol. 2008;595(1–3):39–43. doi: 10.1016/j.ejphar.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis B, Rahman A, Arner A. AMP-activated kinase relaxes agonist induced contractions in the mouse aorta via effects on PKC signaling and inhibits NO-induced relaxation. Eur. J. Pharmacol. 2012;695(1–3):88–95. doi: 10.1016/j.ejphar.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Abreu A, Mahmarian JJ, Nishimura S, Boyce TM, Verani MS. Tolerance and safety of pharmacologic coronary vasodilation with adenosine in association with thallium–201 scintigraphy in patients with suspected coronary artery disease. J. Am. Coll. Cardiol. 1991;18(3):730–735. doi: 10.1016/0735-1097(91)90796-c. [DOI] [PubMed] [Google Scholar]