Abstract
Most human immunodeficiency virus (HIV)–infected individuals experience increases in peripheral CD4+ T cell counts with suppressive antiretroviral therapy (ART) that achieves plasma HIV RNA levels that are less than the limit of detection. However, some individuals experience decreasing CD4+ T cell counts despite suppression of plasma viremia. We evaluated 4 patients with a history of CD4+ T cell decline despite successfully suppressive ART, from a median of 719 cells/mm3 (range, 360–1141 cells/mm3) to 227 cells/mm3 (range, 174–311 cells/mm3) over a period of 18–24 months; 3 of the patients were receiving tenofovir and didanosine, which may have contributed to this decrease. There was no evidence of HIV replication, nor of antiretroviral drug resistance in the blood or lymphoid tissue, or increased proliferation or decreased thymic production of naive CD4+ T cells. All 4 patients had significant fibrosis of the T cell zone of lymphoid tissue, which appeared to be an important factor in the failure to reconstitute T cells.
CD4+ T cell lymphopenia is a hallmark of human immunodeficiency virus type 1 (HIV-1) infection [1]. In the absence of combination antiretroviral therapy (ART), the vast majority of HIV-infected persons experience an inexorable and progressive decrease in the CD4+ T lymphocyte counts, leading to opportunistic infections and death [2]. The precise mechanisms involved in T cell depletion in the absence of ART are largely unknown. Because there is significant replication of HIV, it has been suggested that direct viral cytopathogenicity accounts for at least some HIV-associated CD4+ T cell loss [3]. Evidence also suggests a role for indirect effects of HIV infection on the immune system, such as persistent immune activation leading to apoptosis of uninfected cells, as well as decreased thymic output of naive CD4+ T cells [2, 4–7].
The use of combination ART generally results in suppression of HIV replication, increases in peripheral CD4+ T cell counts, and decreased morbidity and mortality due to HIV/AIDS in countries with widespread access to such therapy [8]. However, there are anecdotal reports of HIV-infected patients who maintain levels of plasma HIV RNA that are less than the limit of detection, with or without [9] ART, but who nonetheless experience sustained declines in peripheral CD4+ T cell counts. T cell depletion despite successful suppression of plasma viremia seems paradoxical in such patients.
It is difficult to delineate the pathogenesis of declining CD4+ T cell numbers despite suppression of plasma HIV RNA. There may be plasma HIV levels below the detection limits of most assays, or there may be significant HIV replication in reservoirs, such as lymphoid tissue (LT), that does not spill over into the plasma [4–5]. Such replication could be associated with high CD4+ T cell turnover [6] and cell death, decreased production [2] of naive CD4+ T cells, or changes in LT architecture that impair normal T cell homeostasis [7]. We evaluated the possible contribution of those pathogenic mechanisms in 4 patients with a history of declining CD4+ T cell counts despite suppression of plasma HIV RNA below the limit of detection during 3- or 4-drug ART.
MATERIALS AND METHODS
Patients
We evaluated 4 patients with chronic HIV infection who were receiving 3- or 4-drug ART and had a history of CD4+ T cell decline from a median of 719 cells/mm3 (range, 360–1141 cells/mm3) to 227 cells/mm3 (range, 174–311 cells/mm3) over an 18–24-month period, despite suppression of plasma viremia to <400 copies/mL and <50 copies/mL at enrollment. Levels of plasma HIV RNA, peripheral CD4+ T cells, and routine safety laboratory markers were evaluated. Over time, ART regimens were changed commensurate with standards of clinical care. Lymph node biopsies for the 4 patients were performed ≥18 months (18, 156, 84, and 21 months), after initiation of ART. Relevant patient characteristics are summarized in table 1.
Table 1.
Patient characteristics 18-24 months before enrollment.
| Patient | Plasma HIV RNA history, copies/mLa | Plasma HIV RNA at enrollment, copies/mL | CD4+ T cell count,bcells/mm3 |
CD4+ T cell count at enrollment, cells/mm3 | CD4+ T cell count at enrollment, % | ART regimen at enrollment, (months on ART regimen] | ART regimen at change | Sex; age, years | |
|---|---|---|---|---|---|---|---|---|---|
| Highest count | Nadir count | ||||||||
| 1 | BLD | <50 | 1141 | 311 | 311 | 20 | ddl, TDF, 3TC (18) | TDF, 3TC, ABC, ATZ, RTV | F; 51 |
| 2 | BLD | <50 | 746 | 272 | 272 | 24 | ddl, TDF, 3TC (24) | EFV, AZT, 3TC | M; 66 |
| 3 | BLD | <50 | 692 | 174 | 219 | 19 | ddl, ABC, NVP (29) | ddl, ABC, NVP, ATZ | F; 39 |
| 4 | BLD | <50 | 360 | 182 | 193 | 16 | ddl, TDF, EFV, d4T (7) | ddl, TDF, EFV | M; 34 |
| All patients, median | ... | ... | 719 | 227 | 246 | ... | ... | ... | ... |
NOTE. ABC, abacavir; ART, combination antiretroviral therapy; ATZ, atazanavir; AZT, zidovudine; BLD, below the limit of detection for the assay; d4T, stavudine; ddl, didanosine; EFV, efavirenz; HIV, human immunodeficiency virus; NVP, nevirapine; RTV, ritonavir, TDF, tenofovir; 3TC, lamivudine.
These assays were performed in the 18-24 months before enrollment and represent values of <50 or <400 copies/mL, depending on the assay.
The highest and nadir CD4+ T cell counts were obtained during an 18-24-month period before enrollment.
Measurement of HIV levels in the peripheral blood
Lymphocyte subsets were analyzed. Levels of plasma HIV RNA (copies/mL) and cell-associated HIV RNA (copies per 106 peripheral blood mononuclear cells [PBMCs]) were quantified by branched-chain DNA assay (Bayer Versant HIV-1 3.0) with a detection limit of <50 copies/mL and the following modifications. Cells were washed 3 times in PBS, and 106 cells were lysed with 120 μL of lysis reagent. The lysate was incubated at 63°C for 2 h. The assay proceeded according to the manufacturer's instructions using 100 μL of lysate. Ultrasensitive assays of plasma HIV RNA were performed; 1 mL of plasma was pelleted by ultracentrifugation and HIV-1 RNA was extracted and quantified using a previously described real-time polymerase chain reaction (PCR) assay, with a limit of quantitation of 1 copy HIV-1 RNA/mL of plasma [10] and adaptations for the use of 1 mL of plasma rather than 7 mL [11]. Additionally, DNA was isolated from CD8+ T cell–depleted PBMCs (Puregene, Gentra Systems). Proviral HIV DNA was quantified by HIV long terminal repeat PCR, as described elsewhere [12], with adaptations for real-time PCR technology.
Measurement of HIV levels in LT
Inguinal lymph nodes were surgically excised with patients under local anesthesia, as described elsewhere [13–14]. Lymph node mononuclear cells (LNMCs) were extracted, and a portion of each lymph node was immediately fixed for in situ hybridization (ISH) and immunohistochemistry (IHC). All patients signed informed consents to participate in this protocol, which was approved by the Internal Review Board of the National Institute of Allergy and Infectious Diseases.
LNMC HIV DNA proviral copy numbers and cell-associated HIV RNA levels were determined as described for PBMCs. ISH was performed using standard procedures to detect HIV RNA, as described elsewhere [14–15]. IHC was performed with primary antibodies for HIV gag p24 antigen (molecular clone Kal-1) and a staining kit (Dako). Secondary antibodies were revealed by means of tyramide signal amplification (PerkinElmer). The presence of Ki-67 in LNMCs was evaluated by IHC using the molecular clone MIB-1 (Dako), as described elsewhere [14–15].
Quantitative image analysis of collagen deposition in the T cell zone (TZ) of LT
Analysis of collagen fibers in lymph node tissue to determine fibrosis has been described elsewhere [7]. In brief, collagen fibers were treated with a trichrome stain. Multiple images were acquired from the TZ of each lymph node. These images were analyzed using Fovea Pro (Reindeer Graphics) and Photoshop CS, version 7.0 (Adobe), software to determine the percentage area of LT that contained collagen fibers. In 2 patients (patients 1 and 2), tissue specimens were not available for trichrome analysis. However, fibrosis in the hematoxylin-eosin (HE)–stained sections was quantified using techniques similar to those for trichrome analysis. To evaluate the performance of HE staining for fibrosis, quantitative image analyses were compared for trichrome and HE staining in patients 3 and 4, with variation between techniques of <0.8% in patient 3 and 1.7% in patient 4.
Antiretroviral drug resistance mutations in peripheral blood and LT
Qualitative genotypic analysis of drug resistance mutations with oligonucleotide ligation assay was performed on proviral HIV DNA from PBMCs and LNMCs, as described elsewhere [16–17]. Replication-competent cells in the peripheral blood were evaluated for mutations associated with antiretroviral drugs by TRUGENE HIV-1 genotyping (Visible Genetics) after CD8+ T cell–depleted PBMCs were stimulated for up to 21 days in culture with anti-CD3 antibody.
Production of CD4+ T cells by the thymus
To assess thymic output of naive T cells, real-time PCR of T cell receptor excision circles (TRECs) was performed, as described elsewhere [18].
Statistical methods
Pre- and post-ART regression slopes were compared in a form of regression known as change point analysis; specifically, CD4 (month) = , where z = 1 if month >0 and 0 otherwise. P values are 2-sided. Testing for autocorrelation was done with the Durbin-Watson statistic. Adjustment for serial correlation was required in patient 4. Given the small sample size and commensurate limitations of repeated measures with unequal time points, the effect of a change in ART regimen was analyzed for each subject.
RESULTS
HIV burden in peripheral blood and LT
Reports indicate that certain patients have detectable plasma HIV RNA levels below the limit of detection with standard clinical assays [10, 19]. After enrollment, all 4 patients had <50 copies HIV RNA/mL plasma at 2 measurements a median of 2 months apart. Therefore, HIV RNA was quantified at enrollment by an ultrasensitive assay with a detection limit of <1 copy/mL plasma. Results ranged from <1 to 5 copies/mL (table 2). PBMC-associated HIV RNA levels were <50 copies/106 PBMCs, and proviral HIV DNA levels ranged from 6 to 292 copies/106 CD8+ T cell–depleted PBMCs (table 2). Comparable levels for each assay have been reported in aviremic patients without CD4+ T cell loss [20–23].
Table 2.
Patient laboratory evaluations.
| Patient | Ultrasensitive plasma HIV RNA, copies/mL | Peripheral caRNA level, copies/106 PBMCs | Lymph node caRNA level, copies/106 LNMCs | Peripheral pvDNA level, copies/106 CD8-depleted PBMCs | Lymph node pvDNA level, copies/106 LNMCs | Resistance demonstrated by OLA | Resistance demonstrated by TRUGENE |
|---|---|---|---|---|---|---|---|
| 1 | 5 | <50 | ND | 48 | NA | None | ND |
| 2 | <1 | <50 | <50 | 6 | NA | Low peripheral levels of M184V | ND |
| 3 | 1 | <50 | <50 | 81 | 800 | None | None |
| 4 | <1 | <50 | <50 | 292 | 60 | None | ND |
NOTE. caRNA, cell-associated human immunodeficiency virus (HIV) RNA; LNMCs, lymph node mononuclear cells; NA, not amplifiable with polymerase chain reaction, given several attempts; ND, not determined, insufficient sample to assay; OLA, oligonucleotide ligation assay; PBMCs, peripheral blood mononuclear cells; pvDNA, proviral HIV DNA; TRUGENE, genotypic resistance assay (Visible Genetics).
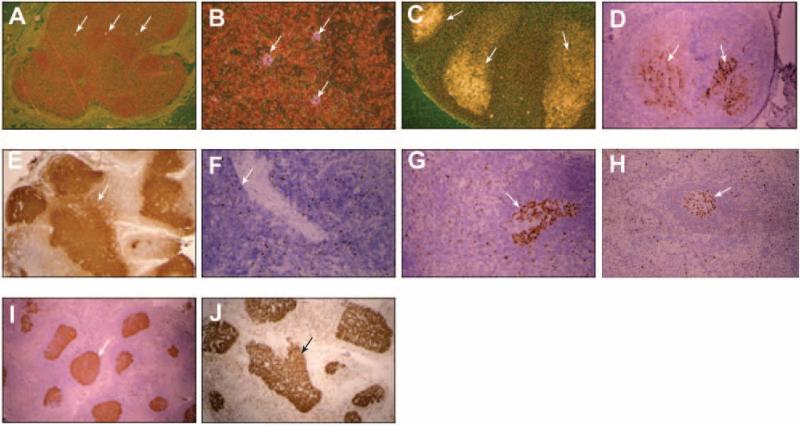
LT is a known reservoir of HIV replication despite undetectable plasma HIV RNA [4–5, 24–29]. Therefore, loss of peripheral CD4+ T cells could be due to significant HIV replication in LT. Low-level viral replication was demonstrated by ISH in patient 2 (figure 1A and 1B), and IHC demonstrated some HIV gag p24 antigen associated with follicular dendritic cells (FDCs) in all 4 patients (figure 1D). Similar levels of HIV RNA [5, 13, 15, 30–34] and p24 [7, 13–15, 27] have been reported in patients receiving ART without CD4+ T cell loss. Notably, p24 antigen may remain associated with FDCs in LT for years without active viral replication [15, 27, 32].
Figure 1.
Results of in situ hybridization (ISH) and immunohistochemistry (IHC). Human immunodeficiency virus (HIV) RNA in lymph node tissue was evaluated by ISH. A and B, In patient 2, HIV staining appears pink in a pattern indicating HIV replication (arrows) (original magnification in A, ×40; original magnification in B, ×160). C, In a positive control from an HIV-infected patient not receiving antiretroviral therapy, HIV staining appears white (original magnification, ×40). HIV gag protein p24 was evaluated in lymph node tissue by IHC and appears brown on staining. Arrows indicate areas of HIV infection. D, Staining revealed the presence of p24 in all 4 patients, with the most extensive p24 observed in patient 4 (original magnification, ×40). Arrows indicate examples of p24 staining. E, A p24-positive control sample from an untreated, HIV-positive patient (original magnification, ×40). Arrows indicate examples of p24 staining. F–I, The presence of the nuclear proliferation marker Ki-67 in lymph node tissue was assessed by IHC; Ki-67 appears brown and was observed in all 4 patients, with the most staining in patient 4 (F–I, patients 1–4, respectively; F and G, original magnification ×64; H and I, original magnification ×40). Arrows indicate examples of Ki67 staining. J, A positive control sample from an HIV-positive patient shows lymph node tissue with extensive Ki-67 staining (original magnification, ×40). Arrows indicate examples of Ki67 staining.
LNMCs were evaluated for cell-associated HIV RNA (levels in patients 2, 3, and 4, <50 copies/106 LNMCs) and proviral HIV DNA (levels in patients 3 and 4, 800 and 60 copies/106 LNMCs, respectively) (table 2). Comparable levels have been reported in aviremic patients without CD4+ T cell loss [17, 22, 31, 35].
Resistance to antiretroviral drugs in peripheral blood and LT
Virus isolated from LNMCs of HIV-infected patients receiving ART has shown evidence of drug resistance–associated mutations [34]. Occult HIV replication not detectable by currently available assays could lead to antiretroviral drug resistance and loss of CD4+ T cells. Therefore, we evaluated the presence of occult drug resistance–associated mutations in LNMCs and PBMCs with the highly sensitive oligonucleotide ligation assay. Low levels of M184V were detected in PBMCs of patient 2; this mutation had been identified in plasma 15 months before enrollment (table 2). In patient 3, replication-competent virus grew from PBMCs in culture, but no drug resistance–associated mutations were detected (table2). There were not enough cells for us to evaluate replication-competent virus in LNMCs from this patient.
Cellular proliferation in LT
Failure to reconstitute peripheral CD4+ T cells during HIV infection has been attributed to continued T cell activation and turnover, leading to cell death [6]. Therefore, we used IHC to evaluate the presence of Ki-67, a cellular proliferation marker, in LNMCs. All patients had low levels of Ki-67 (figure 1F–1I), but comparable levels have been reported in LT of aviremic, HIV-infected patients without CD4+ T cell loss.
Thymic production of naive CD4+ T cells
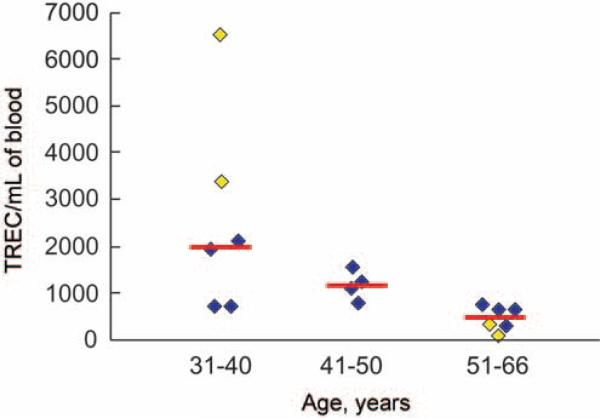
HIV infection can alter thymic production of CD4+ T cells, as evidenced by quantification of TRECs, a by-product of T cell receptor gene rearrangement during thymic T cell maturation [36]. Suppressive ART has been associated with an increase in thymic production, and thus peripheral levels, of CD4+ T cells [37–39]. Therefore, loss of CD4+ T cells despite receipt of suppressive ART may be a result of lowered thymic production. We quantified TRECs in peripheral blood as an indicator of naive T cell production. Results ranged from 51 to 6495 TRECs/mL blood (figure 2), and the number of TRECs correlated inversely with age, as demonstrated elsewhere [36] (figure 2). These results were compared with those in 12 HIV-infected, age-matched control patients receiving suppressive ART without CD4+ T cell loss (figure 2). Although none of the study participants were 41–50 years old, a control group in this age range was included to demonstrate a decline in TREC productivity with age.
Figure 2.
Results of quantitative T cell receptor excision circle (TREC) assays. Patients 3 and 4, who were also the youngest patients (39 and 34 years old, respectively), had the highest TREC values (1902 and 6495 TRECs/mL blood, respectively) (yellow symbols in the 31–40-year age bracket). Patients 1 and 2 (aged 51 and 66 years, respectively), had the lowest TREC values (278 and 51 TRECs/mL blood, respectively) (yellow symbols in the 51–66-year age bracket). Serving as controls were 12 age-matched human immunodeficiency virus (HIV)–infected patients undergoing antiretroviral therapy who did not experience declines in CD4+ T cells (4 in each age group; blue symbols). Median values for the age groups, represented by red lines, were as follows: 31–40 years, 1992 TRECs/mL blood; 41–50 years, 1137 TRECs/mL blood; and 51–66 years, 460 TRECs/mL blood.
TZ architecture in LT
Infection with HIV can damage LT architecture [1, 7, 15, 40], which may adversely affect normal T cell homeostasis [40]. In certain patients, these effects are reversible with ART [41], but in the 4 study patients, persistent damage to LT may have irreversibly impaired T cell production. Histologic analysis of the 4 patients’ LT follicles and paracortical regions revealed marked abnormalities with small, unstimulated, or absent follicles and significant TZ depletion of lymphocytes. In patients 1 and 2, HE staining of LT collagen revealed that 23.7% and 34.2% of the TZ, respectively, was occupied by collagen (figure 3A–3D). In patients 3 and 4, the percentages were 24.6% and 29.0%, respectively (figure 3E–3H). Quantitative image analysis of the TZ demonstrated marked abnormalities of architecture in all participants. These results are consistent with a previous report describing 6 patients with undetectable plasma HIV RNA and no CD4+ T cell loss who had TZ collagen levels of 2.4%–12.3% [7]. A control sample from an HIV-infected patient without a history of CD4+ T cell loss after initiation of ART (394 cells/mm3 at baseline and 789 cells/mm3 after 24 months) had a TZ collagen level of 3.8% (figure 3I).
Figure 3.
Results of quantitative analysis of collagen deposition in the T cell zone (TZ). To evaluate the presence of fibrosis in lymph node tissue, the percentage area of TZ occupied by collagen was determined. For each patient, an average of 18 sections were evaluated, and 2 representative sections are shown (original magnification, ×40 for all images). A–D, Collagen fibers were analyzed using hematoxylin-eosin staining in patients 1 (A and B) and 2 (C and D); these fibers appear pink (arrows). E–H, Collagen fibers were analyzed using the Masson method with trichrome staining in patients 3 (E and F) and 4 (G and H); these fibers appear blue (arrows). I, In an human immunodeficiency virus–infected patient without declines in CD4+ T cell numbers, collagen fibers were stained blue using the Masson method (figure was published previously [7]); collagen fibers were present mainly in the high endothelial venules and, in smaller proportions, in the cellular portion of the TZ (arrow).
The effect of changes in ART
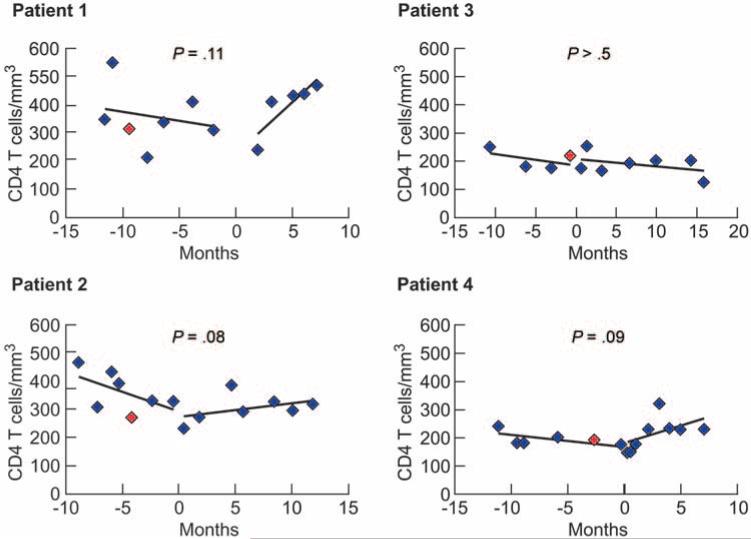
Although there was no evidence of HIV replication, as demonstrated by multiple markers, it is possible that the assays were not sensitive enough to detect occult replication. This was concerning in light of recent reports that certain triple nucleoside reverse-transcription inhibitor (NRTI) regimens (which patients 1, 2, and 3 were receiving) are associated with therapeutic failure [42–43]. Therefore, we examined whether a clinically directed, empirical change in ART would lead to an increase in CD4+ T cells, suggesting that occult HIV replication was contributing to CD4+ T cell loss in the study participants. There was no statistically significant difference in CD4+ T cell levels during the period before and after a change in ART regimen (median interval before regimen change, 11 months [range, 9–12 months]; median interval after change, 10 months [range, 7–16 months]) (figure 4). Although the change in slope after a change in ART regimen was borderline significant in patients 1, 2, and 4, 95% confidence limits for the mean change in slope (−8.1 to 48.4) included 0. The median CD4+ T cell count for all 4 patients was 267 cells/mm3 (range, 174–551 cells/mm3) before and 263 cells/mm3 (range, 126–467 cells/mm3) after the change in ART regimen.
Figure 4.
CD4+ T cell counts after a change in antiretroviral therapy (ART). Counts were evaluated before (median, 11 months) and after (median, 10 months) a change in ART regimen (see separate lines in each panel). A break in the slope indicates the change in ART. P values represent the significance of the change in linear slope after a change in ART. Red symbols indicate the time of enrollment for each patient.
DISCUSSION
In many HIV-infected individuals who receive ART, plasma HIV RNA is suppressed below the limit of detection and CD4+ T cell counts progressively increase [9]. However, certain patients experience a progressive decrease in CD4+ T cell counts despite suppression of plasma HIV RNA. We evaluated the etiology of the latter phenomenon in 4 patients, considering factors that have been proposed as significant in T cell homeostasis in HIV-infected persons. There was no consistent pattern of significant occult HIV replication, increased cellular turnover or decreased thymic production of naive CD4+ T cells in these patients, nor were peripheral CD4+ T cells recovered after a change in ART regimen. The only common findings were collagen deposition and compromised LT architecture, particularly in the TZ which could impair T cell maturation.
LT can remain a site for active viral replication despite undetectable plasma viremia [26–27, 31–32, 35]. We evaluated LT specimens and found no evidence of significant, ongoing HIV replication. Some HIV replication was demonstrated by ISH in patient 2, whose levels were consistent with those found in patients with undetectable viremia and no loss of CD4+ T cells. Our inability to induce replication-competent HIV from PBMCs in 3 of 4 patients and LNMCs in 4 of 4 patients may also suggest significant suppression of HIV replication, although enriching for CD4+ T cells or increasing cell numbers in the cell culture may have produced infectious virus. Finally, a lack of drug resistance–associated mutations in PBMCs or LNMCs suggests limited viral replication, because mutations arise during active replication.
Increased cellular proliferation and decreased thymic production of CD4+ T cells have been implicated in altered T cell homeostasis in patients with detectable HIV RNA; these effects can be reversed with suppressive ART. In all 4 patients, IHC detected FDC-associated HIV p24 antigen (figure 1D), which may contribute to cellular activation. However, because similar levels of p24 antigen have been reported in patients receiving ART without CD4+ T cell loss [7, 13–15, 27] and because p24 antigen can remain associated with FDCs in LT for years without viral replication [15, 27, 32], this factor is probably not significant in the CD4+ T cell loss reported here. Levels of the nuclear proliferation marker Ki-67 in LT were consistent with those reported in aviremic patients without CD4+ T cell loss. Other markers, such as HLA-DR and chemokine (C-C motif) receptor 5, might provide additional insight into cellular activation. However, insufficient material prevented these analyses. In addition, there was no evidence of decreased thymic production of naive T cells compared with that in age-matched controls with suppressed plasma HIV RNA and no decline in CD4+ T cells. Although the study group was too small for statistical analyses, the median TREC blood level for the patient group was 1090 TRECs/mL (range, 51–6495 TRECs/mL), compared with the control group median of 744 TRECs/mL (range, 303–3347 TRECs/mL). The data did not indicate lower levels of TRECs in the study group.
Because of the significant literature on the levels of various markers of HIV replication and cellular proliferation [5, 13–15, 24–27, 30–35, 40], including findings from our own laboratory, and the low levels of these markers in the study patients, a control group for the assays was not used. However, with a median age of 45 years (range, 34–66 years) and TREC measurements that vary with age [36], it was important to have age-matched controls for the evaluation of thymic production.
Although there was no evidence of occult HIV replication, cellular proliferation, or altered thymic production to explain CD4+ T cell loss, all 4 patients showed evidence of substantial TZ fibrosis in LT. Almost 30% of the TZ was replaced by scar tissue in the 4 patients, compared with 3.8% in a control patient and 2.4% and 12.3% in a previous report [7] of patients with undetectable plasma HIV RNA without CD4+ T cell loss. The TZ supports the naive T cell population after migration from the thymus, supplies memory CD4+ T cells, and is therefore an important factor in maintaining a normal T cell population. Recent reports have indicated that greater TZ fibrosis is associated with fewer TZ CD4+ T cells and a poorer response to ART [41]. Interestingly, CD4+ T cells actually decreased in these patients, suggesting that the process of inflammation leading to TZ fibrosis might have been ongoing, even after HIV replication was suppressed. It should be noted that, during HIV infection, inguinal lymph nodes may differ immunopathologically from lymph nodes at other anatomic sites, owing to chronic immune activation. Comparator lymph nodes used as references in other assays were also inguinal.
The change in slopes after a change in ART regimen was nearly significant for 3 of the 4 patients (patients 1, 2, and 4). Given the small number of subjects studied, the results of statistical analysis are inconclusive. Over time or with a larger sample size allowing for collective rather than individual analysis, a significant increase in CD4+ T cell count might be observed after a change in ART regimen. Because there was no evidence of decreased thymic output of naive T cells but clear, consistent evidence of LT fibrosis and damaged architecture, impaired T cell homeostasis at the level of the LT may have been a factor in declining CD4+ T cell counts, despite receipt of suppressive ART in these 4 patients. The reasons for the persistent damage to the LT architecture are unclear but could include very high virus loads during acute infection, persistent prior levels of high HIV replication for prolonged periods, and the relatively advanced age of the patients. The failure to reconstitute peripheral CD4+ T cells despite suppression of plasma HIV RNA is consistent with previous findings from our laboratory and others demonstrating significant destruction of LT with disease progression [7, 40, 44–46].
At enrollment, patients 1, 2, and 4 were receiving a triple-NRTI regimen that included tenofovir and didanosine, and none of the comparator patients in any evaluation were receiving that ART combination. Previous publications have indicated a paradoxical decrease in the CD4+ T cell count in subsets of patients receiving tenofovir plus didanosine, despite virologic suppression, suggesting that this combination may limit recovery of CD4+ T cell counts or actually lead to a decrease in CD4+ T cell counts [47–48]. The concomitant treatment with a third NRTI was reported to be an additional predictor of decreases in the CD4+ T cell count [42–43]. Although the mechanism underlying this phenomenon is unclear, it has been postulated that purine salvage enzymes might be inhibited under these circumstances, thus leading to lymphocyte toxicity and subsequent CD4+ T cell loss. Therefore, the drug regimen in 3 of 4 patients may have been a key factor in the decline in CD4+ T cell counts. However, after a change in ART, there was no significant increase in CD4+ T cell counts in any of the patients, including patients 1 and 2, who did not continue to receive a tenofovir plus didanosine combination. Patients 1, 2, and 4 experienced an upward trend in CD4+ T cell numbers after the change in ART (figure 4), which might become statistically significant over time. However, patient 4 continued to receive tenofovir plus didanosine after a change in ART and experienced a similar upward trend in CD4+ T cell numbers. Therefore, the data indicated that the ART regimen was not the sole factor in the decline of CD4+ T cell counts despite suppressed plasma HIV RNA levels, but we cannot rule out the possibility that it contributed to the decline.
The small sample size in the present study limited our ability to analyze the impact of a change in ART regimens, and 3 of the 4 patients were receiving tenofovir plus didanosine. A longitudinal prospective study could therefore help clarify the role of fibrosis in CD4+ T cell loss in patients who experience a decline in peripheral CD4+ T cell counts despite successfully suppressive ART, because the etiology could be multifactorial. In light of recent findings indicating that gut-associated LT (GALT) is a major source for HIV replication [49–50], evaluation of GALT should be included in future studies involving CD4+ T cell loss in aviremic patients. However, our findings do demonstrate that, in certain patients, declining peripheral CD4+ T cell counts may be associated with severe damage to LT architecture that can be clinically irreversible with currently available interventions.
Acknowledgments
We thank the patients, whose commitments were critical to this study. We also thank Mary Rust for her editorial assistance and John Weddle for his expert graphic design.
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health; National Cancer Institute, National Institutes of Health (contract N01-CO-12400).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 3rd International AIDS Society Conference on HIV Pathogenesis and Treatment, Rio de Janeiro, Brazil, 24–27 July 2005 (abstract WePe8.1.B01).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
References
- 1.Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–22. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 2.Dybul M, Connors M, Fauci AS. Immunology of HIV infection. In: Paul W, editor. Fundamental immunology. 5th ed. Lippincott Williams & Williams; Philadelphia: 2003. pp. 1285–318. [Google Scholar]
- 3.Perelson AS. Modeling viral and immune system dynamics. Nat Rev Immunol. 2002;2:28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- 4.Furtado MR, Callaway DS, Phair JP, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–22. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–13. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 6.Anthony KB, Yoder C, Metcalf JA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–33. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HIV/AIDS surveillance report. Vol 12, part 2. Centers for Disease Control and Prevention, National Center for HIV, STD, and TB Prevention; Atlanta: 2000. [Google Scholar]
- 9.Greenough TC, Sullivan JL, Desrosiers RC. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N Engl J Med. 1999;340:236–7. doi: 10.1056/NEJM199901213400314. [DOI] [PubMed] [Google Scholar]
- 10.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun TW, Carruth L, Finzi D. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 13.Dybul M, Chun TW, Ward DJ, et al. Evaluation of lymph node virus burden in human immunodeficiency virus-infected patients receiving efavirenz-based protease inhibitor—sparing highly active antiretroviral therapy. J Infect Dis. 2000;181:1273–9. doi: 10.1086/315407. [DOI] [PubMed] [Google Scholar]
- 14.Orenstein JM, Bhat N, Yoder C, et al. Rapid activation of lymph nodes and mononuclear cell HIV expression upon interrupting highly active antiretroviral therapy in patients after prolonged viral suppression. AIDS. 2000;14:1709–15. doi: 10.1097/00002030-200008180-00004. [DOI] [PubMed] [Google Scholar]
- 15.Orenstein JM, Feinberg M, Yoder C, et al. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS. 1999;13:2219–29. doi: 10.1097/00002030-199911120-00004. [DOI] [PubMed] [Google Scholar]
- 16.Beck IA, Mahalanabis M, Pepper G, et al. Rapid and sensitive oligonucleotide ligation assay for detection of mutations in human immunodeficiency virus type 1 associated with high-level resistance to protease inhibitors. J Clin Microbiol. 2002;40:1413–9. doi: 10.1128/JCM.40.4.1413-1419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dybul M, Nies-Kraske E, Dewar R, et al. A proof-of-concept study of short-cycle intermittent antiretroviral therapy with a once-daily regimen of didanosine, lamivudine, and efavirenz for the treatment of chronic HIV infection. J Infect Dis. 2004;189:1974–82. doi: 10.1086/386344. [DOI] [PubMed] [Google Scholar]
- 18.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–81. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 19.Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001;286:171–9. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 20.Garrigue I, Pellegrin I, Hoen B, et al. Cell-associated HIV-1-DNA quantitation after highly active antiretroviral therapy-treated primary infection in patients with persistently undetectable plasma HIV-1 RNA. AIDS. 2000;14:2851–5. doi: 10.1097/00002030-200012220-00006. [DOI] [PubMed] [Google Scholar]
- 21.Ngo-Giang-Huong N, Deveau C, Da Silva I, et al. Proviral HIV-1 DNA in subjects followed since primary HIV-1 infection who suppress plasma viral load after one year of highly active antiretroviral therapy. AIDS. 2001;15:665–73. doi: 10.1097/00002030-200104130-00001. [DOI] [PubMed] [Google Scholar]
- 22.Yerly S, Rutschmann OT, Opravil M, Marchal F, Hirschel B, Perrin L. Cell-associated HIV-1 RNA in blood as indicator of virus load in lymph nodes: the Swiss HIV Cohort Study. J Infect Dis. 1999;180:850–3. doi: 10.1086/314932. [DOI] [PubMed] [Google Scholar]
- 23.Yerly S, Perneger TV, Vora S, et al. Decay of cell-associated HIV-1 DNA correlates with residual replication in patients treated during acute HIV-1 infection. AIDS. 2000;14:2805–12. doi: 10.1097/00002030-200012220-00001. [DOI] [PubMed] [Google Scholar]
- 24.Natarajan V, Bosche M, Metcalf JA, Ward DJ, Lane HC, Kovacs JA. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Highly active antiretroviral therapy. Lancet. 1999;353:119–20. doi: 10.1016/s0140-6736(05)76156-0. [DOI] [PubMed] [Google Scholar]
- 25.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 26.Cavert W, Notermans DW, Staskus K, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–4. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 27.Tenner-Racz K, Stellbrink HJ, van Lunzen J, et al. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4T cell proliferation:the impact of highly active antiretroviral therapy. J Exp Med. 1998;187:949–59. doi: 10.1084/jem.187.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 29.Embretson J, Zupancic M, Ribas JL, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–62. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 31.Lafeuillade A, Chollet L, Hittinger G, Profizi N, Costes O, Poggi C. Residual human immunodeficiency virus type 1 RNA in lymphoid tissue of patients with sustained plasma RNA of <200 copies/mL. J Infect Dis. 1998;177:235–8. doi: 10.1086/517362. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz L, van Lunzen J, Arno A, et al. Protease inhibitor-containing regimens compared with nucleoside analogues alone in the suppression of persistent HIV-1 replication in lymphoid tissue. AIDS. 1999;13:F1–8. doi: 10.1097/00002030-199901140-00001. [DOI] [PubMed] [Google Scholar]
- 33.Lafeuillade A, Tamalet C, Poggi C, Pellegrino P, Tourres C, Izopet J. Antiretroviral effect of zidovudine-didanosine combination on blood and lymph nodes. AIDS. 1997;11:67–72. doi: 10.1097/00002030-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Gunthard HF, Wong JK, Ignacio CC, et al. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J Virol. 1998;72:2422–8. doi: 10.1128/jvi.72.3.2422-2428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong JK, Gunthard HF, Havlir DV, et al. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci USA. 1997;94:12574–9. doi: 10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–32. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith KY, Valdez H, Landay A, et al. Thymic size and lymphocyte restoration in patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine, and ritonavir therapy. J Infect Dis. 2000;181:141–7. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 39.Franco JM, Rubio A, Martinez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99:3702–6. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 40.Schacker TW, Nguyen PL, Martinez E, et al. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. J Infect Dis. 2002;186:1092–7. doi: 10.1086/343802. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Schacker T, Carlis J, et al. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis. 2004;189:572–82. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- 42.Kuritzkes DR. Less than the sum of its parts: failure of a tenofovirabacavir-Lamivudine triple-nucleoside regimen. J Infect Dis. 2005;192:1867–8. doi: 10.1086/498070. [DOI] [PubMed] [Google Scholar]
- 43.Khanlou H, Yeh V, Guyer B, et al. Early virologic failure in a pilot study evaluating the efficacy of therapy containing once-daily abacavir, lamivudine, and tenofovir DF in treatment-naive HIV-infected patients. AIDS Patient Care STDS. 2005;19:135–40. doi: 10.1089/apc.2005.19.135. [DOI] [PubMed] [Google Scholar]
- 44.Pantaleo G, Graziosi C, Demarest JF, et al. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev. 1994;140:105–30. doi: 10.1111/j.1600-065x.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 45.Cohen OJ, Pantaleo G, Lam GK, et al. Studies on lymphoid tissue from HIV-infected individuals: implications for the design of therapeutic strategies. Springer Semin Immunopathol. 1997;18:305–22. doi: 10.1007/BF00813500. [DOI] [PubMed] [Google Scholar]
- 46.Cohen OJ, Pantaleo G, Schwartzentruber DJ, et al. Pathogenic insights from studies of lymphoid tissue from HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl 1):S6–14. [PubMed] [Google Scholar]
- 47.Barrios A, Rendón A, Negredo E, et al. Paradoxical CD4+ T-cell decline in HIV-infected patients with complete virus suppression taking tenofovir and didanosine. AIDS. 2005;19:1722–351. doi: 10.1097/01.aids.0000163933.14649.93. [DOI] [PubMed] [Google Scholar]
- 48.Negredo E, Moltó J, Burger D, et al. Unexpected CD4 cell count decline in patients receiving didanosine and tenofovir-based regimens despite undetectable viral load. AIDS. 2004;18:459–63. doi: 10.1097/00002030-200402200-00012. [DOI] [PubMed] [Google Scholar]
- 49.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattapallil JJ, Douek DC, Hill B, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]