Abstract
The plasma coagulation system in mammalian blood consists of a cascade of enzyme activation events in which serine proteases activate the proteins (proenzymes and procofactors) in the next step of the cascade via limited proteolysis. The ultimate outcome is the polymerization of fibrin and the activation of platelets, leading to a blood clot. This process is protective, as it prevents excessive blood loss following injury (normal hemostasis). Unfortunately, the blood clotting system can also lead to unwanted blood clots inside blood vessels (pathologic thrombosis), which is a leading cause of disability and death in the developed world. There are two main mechanisms for triggering the blood clotting, termed the tissue factor pathway and the contact pathway. Only one of these pathways (the tissue factor pathway) functions in normal hemostasis. Both pathways, however, are thought to contribute to thrombosis. An emerging concept is that the contact pathway functions in host pathogen-defenses. This review focuses on how the initiation phase of the blood clotting cascade is regulated in both pathways, with a discussion of the contributions of these pathways to hemostasis versus thrombosis.
Keywords: Blood coagulation, tissue factor, factor VII, contact pathway, factor XII, polyphosphate
Introduction
Blood is a liquid that circulates under pressure through the vasculature. Following vascular injury, any escaping blood must rapidly be converted into a gel (“clot”) to plug the hole and minimize further blood loss. The plasma portion of blood contains a collection of soluble proteins that act together in a cascade of enzyme activation events, culminating in the formation of a fibrin clot. This review addresses the mechanisms by which the blood clotting cascade is initiated in both hemostasis and pathologic thrombosis. Hemostasis is the normal process by which the clotting cascade seals up vascular damage to limit blood loss following injury. Thrombosis is a group of pathologic conditions in which the clotting cascade is triggered inside the lumen of a blood vessel, leading to the formation of a blood clot (known, in this case, as a “thrombus”) that can impede the flow of blood within a vessel. Severe thrombosis can block the flow of blood to a tissue, leading to ischemia and tissue death.
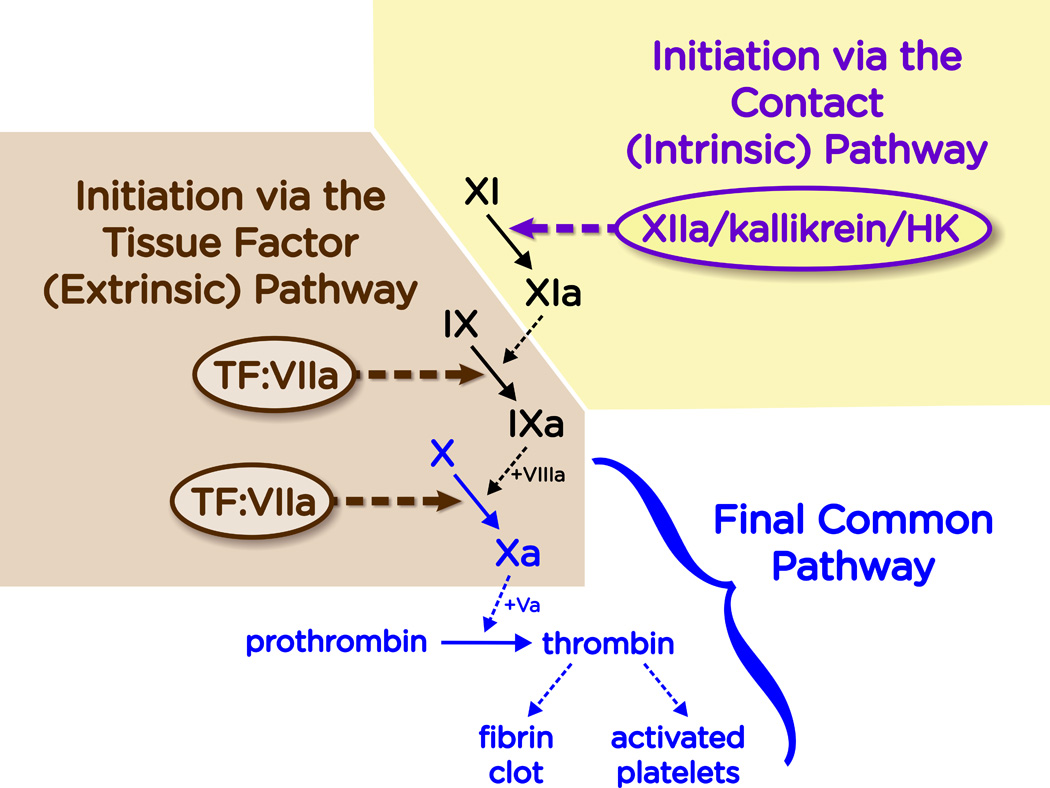
Two major pathways exist for triggering the blood clotting cascade, known as the tissue factor pathway and the contact pathway. Figure 1 shows a somewhat simplified version of the clotting cascade, emphasizing these two mechanisms for initiating blood clotting.
Figure 1.
Overview of the blood clotting cascade. The plasma clotting system is initiated in two distinct mechanisms: the Tissue Factor (TF) Pathway and the Contact Pathway. The TF pathway is triggered when the cell-surface complex of TF and fVIIa (TF:VIIa) activates fIX and/or fX by limited proteolysis. The contact pathway is triggered when fXII, PK and HK assemble on a suitable surface or polymer. This results in the reciprocal activation of fXII to fXIIa by kallikrein, and PK to kallikrein by fXIIa. The resulting generation of fXIIa activates fXI to fXIa, which then converts fIX to fIXa. Both pathways converge at the production of fXa. This final common pathway results in the generation of a burst of thrombin, which converts fibrinogen to fibrin and activates platelets (among many other actions of thrombin that, for simplicity, are not shown here).
The tissue factor pathway is named for the protein that triggers it—a cell-surface, integral-membrane protein known as tissue factor (TF)(Morrissey & Broze, 2013). This way of triggering blood clotting is also sometimes called the Extrinsic Pathway, because it requires that plasma come into contact with something “extrinsic”—i.e.,TF—to trigger it. The TF pathway is the mechanism of triggering blood clotting that functions in normal hemostasis, and probably also in many types of thrombosis. Thus, when cells expressing TF are exposed to blood, this event immediately triggers the clotting cascade as indicated in Figure 1. This pathway is discussed in much greater detail below.
The contact pathway of triggering blood clotting has also been termed the “intrinsic” pathway, since it can be triggered without adding a source of TF to the blood or plasma. This pathway is actually triggered when plasma comes into contact with certain types of artificial surfaces. Glass test tubes, diatomaceous earth (celite) and finely ground clay are especially good activators of the contact pathway (Nossel, 1967). This mechanism of initiating the clotting cascade is indicated in Figure 1, and is discussed in greater detail below. While this pathway does not contribute to normal hemostasis, it is thought to participate in thrombotic diseases (Renné, 2013).
The extrinsic, or TF pathway
The plasma clotting cascade consists of a series of reactions involving the activation of zymogens (inert precursors of enzymes) via limited proteolysis. The resulting enzymes are catalytically active serine proteases, yet they have low inherent enzymatic activity as isolated proteins. Binding of a typical clotting protease to a specific protein cofactor on a suitable membrane surface markedly potentiates the protease’s activity, often by as much as five orders of magnitude or more. The protein cofactors of the blood clotting cascade also generally circulate in the plasma as inert procofactors that must be converted into active cofactors via limited proteolysis. Most blood clotting proteins (both zymogens and procofactors) are represented by Roman numerals, with a lower case “a” appended to the numeral once the protein has been proteolytically converted to the active form. For example, the first serine protease in the extrinsic or TF pathway of blood clotting is coagulation factor VIIa (fVIIa), which circulates in plasma largely in the inactive, zymogen form (fVII).
The enzyme that actually triggers the TF pathway of blood clotting thus consists of two subunits: the catalytic subunit is the trypsin-like serine protease, fVIIa, and the positively-acting regulatory subunit (“protein cofactor”) is the cell-surface protein, TF. The complex between TF and fVIIa (TF:VIIa) is anchored to the cell surface, because TF is an integral membrane protein (Morrissey & Broze, 2013). Free fVIIa is a very weak enzyme, but the TF:VIIa complex is an extremely potent activator of coagulation. Once formed, the TF:VIIa complex activates two downstream substrates in the coagulation cascade via limited proteolysis: factor IX (fIX) is converted to fIXa, and fX is converted to fXa (Figure 1). Both of these active enzymes must assemble on suitable membrane surfaces together with their own protein cofactors (fVIIIa in the case of fIXa; or fVa in the case of fXa) in order to propagate the clotting cascade. This ultimately leads to a large burst of thrombin, the last serine protease in the clotting cascade. Thrombin efficiently processes fibrinogen into fibrin via limited proteolysis, which in turn spontaneously assembles into a fibrin clot. Thrombin is also a potent activator of platelets, further contributing to the formation of a protective hemostatic plug (in normal hemostasis) or a thrombus (in pathologic activation of clotting).
TF
TF, also sometimes known as thromboplastin (perhaps more correctly, tissue thromboplastin), coagulation factor III, or CD142, is a glycosylated, integral-membrane protein of about 46 kDa, consisting of a single polypeptide chain of 261 or 263 amino acids (the two forms are nearly equal in expression) (Morrissey et al., 1987; Spicer et al., 1987; Scarpati et al., 1987). Membrane anchoring of TF via its single membrane-spanning domain is essential for full procoagulant activity (Paborsky et al., 1991). TF is unusual among the protein cofactors of the plasma clotting cascade in that it is an integral membrane protein, and also that it is does not require proteolysis for activity.
TF is abundant in adventitial cells surrounding all blood vessels larger than capillaries, in keratinocytes in the skin, and in a variety of epithelial layers such as organ capsules (Drake et al., 1989a; Wilcox et al., 1989; Fleck et al., 1990). This pattern of expression is consistent with the role of TF as a protective “hemostatic envelope” surrounding the vasculature, organ structures, and the organism in its entirety (Drake et al., 1989a). Further, there is especially abundant TF at anatomic sites where hemorrhage is likely to result in disastrous consequences, such as kidney and brain (Drake et al., 1989a; Fleck et al., 1990). TF expression is quite low in skeletal muscle and synovial tissues (Drake et al., 1989a; Fleck et al., 1990). Interestingly, these are two anatomic sites of spontaneous bleeding in hemophilic patients (who lack either fVIII or fIX). A plausible explanation for bleeding at these sites is that activation of fIX by the TF:VIIa complex provides an additional amplification step, compared with direct activation of fX (see Figure 1). This may explain why hemophilic patients do not tend to bleed excessively from superficial cuts in the skin (which has high levels of TF, allowing for direct, abundant activation of fX by TF:VIIa). On the other hand, sites such as skeletal muscle and joints, where TF levels are low, may require the additional amplification of the clot-initiating signal gained from activating fIX by TF:VIIa. The newly-generated fIXa then assembles with fVIIIa to generate larger quantities of fXa than could be generated directly by low levels of TF:VIIa alone.
In cross-sections of normal blood vessels, TF is readily detectable only in the adventitial cells that make up the outermost layers of the vessel wall (Drake et al., 1989a; Wilcox et al., 1989; Fleck et al., 1990). Circulating blood cells, as well as the endothelial cells that line the blood vessels, do not usually express TF (as detected by antibody staining). However, certain inflammatory mediators (Geczy, 1994; Camerer et al., 1996) or hypoxia (Yan et al., 1999) can stimulate cultured peripheral blood monocytes and endothelial cells to express significant amounts of TF. Other blood cell types such as neutrophils, eosinophils and platelets have been reported to express TF under some circumstances (Camera et al., 2012; Moosbauer et al., 2007; Todoroki et al., 1998; Giesen et al., 1999), although this is somewhat controversial (Østerud, 2012). Induced expression of TF in the vasculature by inflammatory mediators may play important roles in thrombotic diseases.
Urine and plasma may contain low levels of TF antigen, although the source of this “blood-borne” TF is a matter of some controversy (Giesen et al., 1999). An alternatively-spliced, soluble form of TF has been described (Bogdanov et al., 2003), and microparticles shed from activated leucocytes likely contribute to blood-borne TF (Sabatier et al., 2002).
FVII/VIIa
Zymogen fVII is a glycosylated protein of approximately 50 kDa, consisting of a single polypeptide chain of 406 amino acids (Radcliffe & Nemerson, 1975; Kisiel & Davie, 1975; Broze & Majerus, 1980). FVII is synthesized in the liver and circulates in plasma at a concentration of about 10 nM (Fair, 1983). When initially synthesized inside the endoplasmic reticulum of hepatocytes, fVII contains a signal peptide and a propeptide (removed intracellularly) that mediate, respectively, secretion and a specific type of post-translational modification (γ-carboxylation) of all the glutamate residues within about 45 amino acids of the N-terminus of the mature protein. Like other related vitamin-K dependent coagulation proteins, fVII contains an N-terminal γ-carboxyglutamate–rich domain (GLA domain). The fVII GLA domain contains ten γ-carboxyglutamate (Gla) residues that are essential for the clotting activity of this protein. The GLA domain confers reversible, Ca2+-dependent binding of fVII to membranes containing negatively charged phospholipids such as phosphatidylserine or phosphatidic acid (Neuenschwander & Morrissey, 1994; Tavoosi et al., 2013).
FVII, like all the coagulation serine proteases, circulates in the plasma chiefly as an inert zymogen. Unlike most other plasma serine proteases, however, fVII also circulates in its active enzymatic form (fVIIa). Zymogen fVII is converted to its enzymatic form, fVIIa, by proteolysis of a single peptide bond, resulting in two disulfide-linked polypeptide chains. The light chain, approximately 20 kDa, has 152 amino acids and contains the GLA domain and two epidermal growth factor (EGF)-like domains. The heavy chain, approximately 30 kDa, has 254 amino acids and contains the trypsin-like serine protease domain.
The active forms of most coagulation serine proteases have extremely short plasma half-lives (measured in seconds to minutes) because plasma contains high concentrations of protease inhibitors. However, free fVIIa is not susceptible to most plasma protease inhibitors (Kondo & Kisiel, 1987). It consequently circulates with a half-life of approximately 2 hours, similar to the approximately 5-hour half-life of zymogen fVII (Seligsohn et al., 1979a). Approximately 1% of the fVII in plasma circulates in the activated form in normal humans (Morrissey et al., 1993).
The precise source of circulating fVIIa in vivo is not clear. Proteases that are able to activate fVII in vitro include fIXa, fXa, fXIIa, thrombin, plasmin, fVII–activating protease, and the TF:VIIa complex (Nemerson & Repke, 1985; Römisch, 2002; Rao & Rapaport, 1988; Radcliffe & Nemerson, 1975; Kisiel et al., 1977; Seligsohn et al., 1979b; Masys et al., 1982; Tsujioka et al., 1999; Yamamoto et al., 1992; Neuenschwander et al., 1993). Interestingly, patients deficient in fIX (hemophilia B) have approximately a tenfold reduction in plasma fVIIa levels (Wildgoose et al., 1992). This suggests that fIX (presumably, as fIXa) contributes substantially to activation of fVII in vivo. FVIIa concentrations increase during the post-prandial period, in a fIX-dependent manner (Miller et al., 1996), especially after fatty meals (Lefevre et al., 2004; Miller, 1998). This suggests that generation of circulating fVIIa may involve both fIXa and lipoproteins.
The TF:VIIa complex in hemostasis
TF binds either fVII or fVIIa with high affinity, resulting in a 1:1 complex on the cell surface. Once fVII binds to TF, it is rapidly converted to fVIIa by limited proteolysis (Nemerson & Repke, 1985). There are consequently two ways to form the TF:VIIa complex: through direct capture of fVIIa by TF, or by capture of fVII and subsequent conversion to fVIIa.
Free fVIIa activates its substrates (fVII, fIX, or fX) extremely slowly, but assembling the TF:VIIa complex on a suitable phospholipid membrane enhances the activity of fVIIa by at least five orders of magnitude (Nemerson & Gentry, 1986; Bom & Bertina, 1990; Komiyama et al., 1990). Negatively charged phospholipids, most particularly phosphatidylserine, are required for binding of the substrates, fIX or fX, to the phospholipid surface. Quiescent, intact cells expressing TF on their surfaces have much lower procoagulant activity than do damaged or activated cells (Maynard et al., 1977). TF on a quiescent cell is not fully active until the membrane properties of the cell are altered (Drake et al., 1989f; Bach & Rifkin, 1990). This process, sometimes called decryption of “encrypted” cell-surface TF, is incompletely understood. “Decryption” of TF is, at least in part, due to exposure of negatively charged phospholipids on the outer leaflet of the plasma membrane, resulting in expression of efficient binding sites for the substrates of the TF:VIIa complex. Additional proposed mechanisms for encryption/decryption of cell-surface TF include: association with caveolae where lipid composition is altered (Sevinsky et al., 1996; Mulder et al., 1996); dimerization or oligomerization of TF with reduced enzymatic activity (Bach & Moldow, 1997); and reduction or oxidation of a specific disulfide bond in TF that is required for cofactor function (Ahamed et al., 2006) (Versteeg & Ruf, 2011).
Regulation of the TF:VIIa complex
The TF:VIIa complex is primarily inhibited by the plasma serine protease inhibitor, tissue factor pathway inhibitor (TFPI), which has two isoforms in humans: TFPIα (32 kDa) and TFPIβ (22 kDa) (Piro & Broze, 2005). TFPI is a Kunitz-type inhibitor, with the Kunitz-2 domain mediating binding and inhibition of fXa, and the Kunitz-1 domain required for inhibition of fVIIa in the TF:VIIa complex (Girard et al., 1989). The majority of TFPI in vivo is associated with the microvascular endothelium (Bajaj et al., 1999), but a small amount of TFPI circulates in the plasma at a concentration of around ~1.6 nM. Most (~80%) circulating TFPI is lipoprotein-bound (Novotny et al., 1989; Sandset et al., 1991; Hansen et al., 1994). TFPI is also expressed by megakaryocytes, stored in platelets, and secreted upon platelet activation (Novotny et al., 1988; Maroney et al., 2007). A substantial fraction of the TFPI produced by endothelial cells remains at the cell surface, associates with caveolae, and is released by phosphatidylinositol-specific phospholipase C. Thrombin and shear increase the expression and release of TFPI in vitro (Lupu et al., 1995; Lupu et al., 1999; Hansen et al., 2000; Grabowski et al., 1993; Westmuckett et al., 2000; Zhang et al., 2003; Piro & Broze, 2004; Chouhan et al., 1999), and the administration of heparin causes a rapid increase in the circulating levels of total TFPI in plasma in vivo (Sandset et al., 1988; Novotny et al., 1991; Walenga et al., 2002; Naumnik et al., 2011).
TFPI regulates coagulation via direct inhibition of fXa, and via fXa-dependent feedback-inhibition of TF:VIIa. The TFPIβ isoform is a weaker inhibitor of fXa than is TFPIα (Chang et al., 1999). Protein S substantially enhances the inhibition of fXa by TFPIα (Hackeng et al., 2006). Heparin and other polyanions accelerate fXa inhibition by TFPIα in a template-dependent manner (Huang et al., 1993; Wesselschmidt et al., 1993). FXa-dependent inhibition of TF:VIIa by TFPI involves the formation of a quaternary complex consisting of TFPI, fVIIa, TF, and fXa. TFPI-mediated regulation of coagulation is critically important, as evidenced by the effects of disruption of this protein in mouse models, where TFPI-deficient mice die in utero from a consumptive coagulopathy (Huang et al., 1997), but can be rescued by concomitant fVII or TF deficiency (Chan et al., 1997; Pedersen et al., 2005). Antithrombin, in the presence of heparin, is also able to inhibit the TF:VIIa complex (Rao et al., 1993; Lawson et al., 1993).
TF:VIIa in disease
While the TF:VIIa complex is the crucial trigger for hemostatic responses in vivo, excessive initiation of coagulation via the extrinsic pathway can lead to thrombosis, consumptive coagulopathy, or inflammation. Increased complex formation can be the result of loss of vascular wall integrity, increased TF expression, or increased levels (or activity) of fVII/fVIIa.
Atherosclerotic plaques contain significant levels of TF, generally associated with monocytes/foam cells and smooth muscle cells (Wilcox et al., 1989; Tipping et al., 1989; Ichikawa et al., 1996; Marmur et al., 1996; Thiruvikraman et al., 1996). TF antigen may also be found in the acellular core of atheromas, most likely from necrotic cells. Plaque TF is functional and can bind fVIIa (Marmur et al., 1996; Thiruvikraman et al., 1996). In atherosclerosis, the blood is separated from TF by only a thin monolayer of endothelial cells. Myocardial infarction is thought to be triggered by rupture of an atherosclerotic plaque in a coronary artery (Forrester et al., 1987), with the consequent exposure of TF to fVII/fVIIa within the blood. If this coagulation activation is extensive enough to form an occlusive thrombosis within the coronary vessel, myocardial infarction ensues.
TF expression can also be increased with malignancy, potentially leading to cancer-associated thrombosis (also known as Trousseau syndrome)(Thaler et al., 2012). The neoplastic cell itself can express TF, or tumor TF can be associated with infiltrating activated monocytes or stromal cells.
During sepsis, TF is expressed on monocytes, but is also expressed by endothelial cells in some areas, such as the splenic microvasculature (Drake et al., 1993). In primate models, coagulopathies associated with sepsis and septic shock are mediated by TF, and TF:VIIa contributes directly to mortality in sepsis (Taylor et al., 1991; Taylor, 1996).
Epidemiologic studies have indicated that elevated plasma fVII may be a risk factor for thrombotic disease (Meade et al., 1986; Balleisen et al., 1987; Ruddock & Meade, 1994). Elevated plasma fVII coagulant activity (fVII:C) or elevated levels of circulating fVIIa have also been described with angina pectoris, transient ischemic attacks, diabetes, uremia, and peripheral vascular disease (Broadhurst et al., 1990; Carvalho de Sousa et al., 1988; Cortellaro et al., 1992; Hoffman et al., 1988; Hoffman et al., 1989; Kario et al., 1993; Kario et al., 1994; Kario et al., 1995; Orlando et al., 1987; Suzuki et al., 1991). In contrast, some studies have failed to find a relationship between fVII levels and thrombotic disease (Hultin, 1991; Grant, 2003). Population studies have reported that fVII levels are unrelated to the degree of carotid artery thickness or other manifestations of vascular disease (Folsom et al., 1993; Koster et al., 1994; Moor et al., 1995; Sosef et al., 1994; Vaziri et al., 1992). Results have been mixed with regard to a potential correlation between fVIIa levels and the risk of thrombotic disease (Kalaria et al., 2000) (Danielsen et al., 1998; Cooper et al., 2000).
The contact pathway
The contact pathway of coagulation is initiated by activation of factor XII (fXII) in a process that also involves high-molecular-weight kininogen (HK) and plasma prekallikrein (PK). Contact of blood with an artificial surface leads to a change in the conformation of fXII, resulting in the generation of small amounts of active factor XII (fXIIa) (Silverberg et al., 1980; Tankersley & Finlayson, 1984). This enzyme then activates PK to kallikrein. Further reciprocal activation of fXII by kallikrein, and PK by fXIIa, results in a positive feedback loop (Müller et al., 2011). The fXIIa that is generated then activates its downstream substrate, fXI, to fXIa (Figure 1). Limited proteolysis of fIX to fIXa by fXIa then allows for formation of the “intrinsic tenase” complex (i.e., the cell-surface complex of fIXa and fVIIIa), which in turn activates fX to fXa. The final common pathway of blood clotting then leads to thrombin generation and a blood clot.
Despite its important role in clot formation in vitro, contact activation appears to have no contribution to hemostasis in vivo. This conclusions comes from the fact that mice and humans lacking fXII have no bleeding tendencies (Renné et al., 2012). Rather, one of the functions of the contact pathway in vivo appears to be the generation of bradykinin, a vital inflammatory mediator that is produced when kallikrein cleaves HK. This small peptide is the ligand for the kinin B2 receptor on endothelial cells. Binding of bradykinin to its receptor results in vasodilation, increased vascular permeability, pain, and neutrophil chemotaxis. Components of the contact system also contribute to fibrinolysis, and inhibit thrombin-induced platelet activation, angiogenesis, and adhesive interactions (Renné, 2013).
FXII/XIIa
FXII is an approximately 80 kDa protein consisting of a single polypeptide chain of 596 amino acids (Renné, 2013). It is synthesized in the liver and circulates in plasma at a concentration of around 375 nM. FXII is activated via limited proteolysis by kallikrein, plasmin, and fXIIa (autoactivation), resulting in a two-chain molecule (αfXIIa) consisting of a 353 amino acid heavy chain and a 243 amino acid light chain, which contains the serine protease domain.
PK/kallikrein
PK is also made in the liver. Prekallikrein contains 609 amino acids, but due to variable glycosylation may have a molecular weight of either 85kDa and/or 88 kDa (Mandle & Kaplan, 1977). It circulates in plasma at a concentration of around 490 nM, with 75% bound to HK (Mandle & Kaplan, 1977). Prekallikrein is activated via limited proteolysis by fXIIa, resulting in a two-chain enzyme (kallikrein) consisting of a 371 amino acid heavy chain and a 248 amino acid light chain, which contains the serine protease domain.
HK
HK is a 120 kDa protein with a plasma concentration of about 670 nM. Granulocytes, platelets and endothelial cells contain HK, but plasma HK is most likely synthesized in the liver. HK binds to cell surfaces in a zinc-dependent manner. The major contribution of HK to the contact pathway is facilitation of substrate presentation to fXIIa (Renné, 2013). HK is required for efficient formation of kallikrein in surface-activated plasma (Griffin & Cochrane, 1976)
Activators of the contact pathway in vitro and ex vivo
Exposure of blood to an artificial surface invariably results in some activation of fXII to fXIIa. In fact, fXII activation is the mechanism by which clotting is initiated when blood is collected into glass tubes. Because activation of fXII is not calcium dependent, collection of blood into common anticoagulants that are metal-ion chelators (e.g., EDTA or citrate) does not block the formation of fXIIa. For typical clotting tests, however, this is not a problem since only low levels of fXIIa are generated in blood collection tubes in the absence of an added contact activator, and these low levels of fXIIa are continuously inhibited by the protease inhibitors in plasma.
Activation of fXII initiates clotting in the commonly used diagnostic plasma clotting test known as the activated partial thromboplastin time (aPTT). In this test, plasma fXII, PK, and HK assemble onto artificial surfaces such as finely dispersed kaolin, diatomaceous earth (celite), or ellagic acid. The fXIIa is generated via fXII autoactivation and via kallikrein-mediated reciprocal activation of fXII. The generated fXIIa initiates the coagulation cascade via activation of its downstream substrate fXI. Note that, despite having a markedly prolonged aPTT, individuals with fXII deficiency have no tendency for either spontaneous or trauma-induced bleeding (Renné et al., 2012).
Ex vivo activation of the contact pathway also occurs during hemodialysis, cardiopulmonary bypass, and extracorporeal membrane oxygenation (ECMO), where blood comes into contact with artificial surfaces. Anticoagulant therapy (e.g., with citrate or heparin) is required to maintain blood flow through the extracorporeal circuit, because fXIIa generation results in cleavage of downstream enzymes. Note that neither of these anticoagulants prevents contact activation, but rather inhibits the activity of downstream coagulation enzymes. Recently, a blocking antibody to fXIIa has shown utility in stopping unwanted blood clotting during extracorporeal membrane oxygenation without the usual bleeding risk associated with conventional anticoagulants (Larsson et al., 2014).
Activators of the contact pathway in vivo
Several candidate activators of the contact pathway have been proposed, but the precise (patho)physiologic activators in vivo have not been definitively identified. Suggested naturally occurring activators include specific proteins on mammalian cell surfaces (Schmaier, 2008), extracellular nucleic acids (Kannemeier et al., 2007), inorganic polyphosphate (polyP) (Müller et al., 2009) misfolded proteins (Maas et al., 2008), glycosaminoglycans (Brunnée et al., 1997; Hojima et al., 1984), and bacterial surface proteins (Herwald et al., 1998; Nickel & Renné, 2012).
Contact activation occurs on the surface of endothelial cells in vitro in a zinc-dependent manner (Joseph et al., 2001). Endothelial cell binding sites for HK and fXII that have been identified include the C1q receptor, cytokeratin 1, and the urokinase plasminogen activator receptor (Kaplan & Ghebrehiwet, 2010).
Nucleic acids are released from cells due to apoptosis, necrosis, or extrusion of nuclear material by activated neutrophils—a process termed neutrophil extracellular traps, or NETs (Martinod & Wagner, 2014). Extracellular nucleic acids can bind to either fXII or fXI, and in vitro studies indicate that they are capable of enhancing fXII activation (Geddings & Mackman, 2014; Kannemeier et al., 2007). The potency of nucleic acids as contact activators in vitro is somewhat weak, being some two orders of magnitude lower than that of kaolin on a weight basis (Kannemeier et al., 2007). Nevertheless, this mechanism for triggering blood clotting may be quite significant, as animal models employing administration of either exogenous RNA or RNase support a possible role for RNA as a contact activator in vivo (Kannemeier et al., 2007).
PolyP
Inorganic polyP is an intensely anionic, linear polymer of orthophosphate units linked by high-energy phosphoanhydride bonds. PolyP is widespread in biology, with polymer sizes ranging from a few phosphates up to hundreds or even thousands of phosphates in length, depending on the organism and type of cell (Ault-Riché et al., 1998; Brown & Kornberg, 2004). PolyP has mostly been studied in prokaryotes and unicellular eukaryotes, but roles for polyP in mammalian systems are rapidly emerging. Microorganisms store polyP in granules (Docampo & Moreno, 2011), which typically contain very long-chain polyP, ranging in length from hundreds to thousands of phosphate units (Kornberg et al., 1999). Mammalian cellular compartments that contain polyP include platelet dense granules (Ruiz et al., 2004), a subset of mast cell granules (Moreno-Sanchez et al., 2012), lysosomes (Pisoni & Lindley, 1992), mitochondria, and nuclei (Kumble & Kornberg, 1995). Upon activation, platelets and mast cells release polyP of about 60–100 units in length (Ruiz et al., 2004; Moreno-Sanchez et al., 2012). Tissue extracts from mammalian heart, liver, lung and kidneys contain heterogeneous polyP of 50 to 800 phosphate units long, while brain polyP is longer, at about 800 phosphates long (Kumble & Kornberg, 1995).
PolyP binds with high affinity to certain proteins of the contact pathway of blood clotting (Smith et al., 2006; Choi et al., 2010; Smith et al., 2010), and is a very strong activator of the contact pathway in vitro in both plasma and purified protein systems (Smith et al., 2006; Müller et al., 2009). Contact activation by polyP is profoundly dependent on polymer length, with optimal activity requiring very long polyP polymers (Smith et al., 2010) which, on a weight basis, have potencies greater than that of the artificial activator, kaolin. Platelet-derived polyP, which is much shorter in length, is able to weakly activate contact factors, but is markedly less potent than long-chain polyP (Smith et al., 2010). PolyP activates the contact pathway in vivo in mouse models as evidenced by development of cutaneous vascular leakage that is bradykinin- and fXII-dependent (Müller et al., 2009; Smith et al., 2012).
Misfolded protein
Aggregated amyloid β peptide (Aβ) is known to activate fXII in vitro (Shibayama et al., 1999), and patients with Alzheimer’s disease have evidence indicating increased in vivo generation of fXIIa, particularly in the central nervous system (Bergamaschini et al., 1998; Bergamaschini et al., 2001). Amorphous aggregates of Aβ and large amyloid fibrils are also both present in patients with systemic amyloidosis, who also experience increased in vivo activation of both fXII and PK (Maas et al., 2008). The generation of kallikrein by misfolded protein aggregates is dependent on fXII, but does not result in increased activation of fXI. Interestingly, the activation of the contact pathway by misfolded proteins does not appear to be procoagulant, suggesting that kallikrein-kinin pathway is regulated differently than the intrinsic pathway of coagulation in vivo (Maas et al., 2008).
Glycosaminoglycans
Heparin has been long known to be capable of supporting autoactivation of fXII in vitro (Silverberg & Diehl, 1987; Noga et al., 1999). Heparin released from allergen-activated mast cells initiates fXIIa-mediated activation of plasma PK to kallikrein, but without activating fXI (Brunnée et al., 1997). More recent evidence suggests that glycosaminoglycans can contribute to pathologic activation of the contact system in vivo. In particular, contamination of pharmaceutical heparin with an over-sulfated chondroitin sulfate led to serious adverse effects in patients receiving heparin therapy, from excessive contact activation (Kishimoto et al., 2008). Activation of fXII and kallikrein, and cleavage of HK, all occur in patients with anaphylaxis, and are accompanied by increased levels of heparin (Sala-Cunill et al., 2014).
Regulation of the contact pathway
The plasma protease inhibitor, C1-inhibitor, is a crucial regulator of the contact pathway, inhibiting fXIIa, kallikrein, and fXIa, as well as several members of the complement cascade. Inhibitory activity is potently enhanced by the binding of glycosaminoglycans. C1-inhibitor is a member of the serpin superfamily (Zeerleder, 2011). It is a heavily glycosylated protein of 478 amino acids, with an apparent molecular weight of about 104 kDa and a normal circulating plasma concentration of approximately 1.8 µM. Since C1-inhibitor is an acute phase protein, the plasma concentration can be markedly higher with inflammatory conditions (Zeerleder, 2011).
The contact pathway in disease
The contact pathway (perhaps better termed the plasma kallikrein-kinin system), although dispensable for normal hemostasis, is thought to play important roles in host-responses to pathogens and regulation of inflammatory pathways, topics outside the scope of this article but reviewed in detail recently by others (Renné et al., 2012; Schmaier, 2008; Schmaier & McCrae, 2007). Activation of the contact pathway in vivo leads to release of the vasoactive peptide bradykinin. The importance of this pathway is clearly indicated by the clinical manifestations in patients with hereditary angioedema. These individuals experience intermittent episodes of edema and pain due to dysregulation of the contact pathway, usually caused by deficiency of C1 inhibitor (Walford & Zuraw, 2014). Contact activation also occurs in sepsis and other infectious causes of systemic inflammatory response syndrome (Karlsrud et al., 1996b; Karlsrud et al., 1996a), in which continued generation of fXIIa and kallikrein can deplete zymogen levels (Kaufman et al., 1991).
Although the contact pathway is not required for normal hemostasis, recent evidence indicates that it contributes to thrombotic disorders. Deficiency of fXII is protective against thrombus formation in both arteries and veins in animal models (Gailani & Renné, 2007; Müller & Renné, 2008), and increased plasma fXII, fXI, or kallikrein activity is associated with atherosclerosis (Colhoun et al., 2002) or myocardial infarction (Grundt et al., 2004; Doggen et al., 2006; Merlo et al., 2002). Individuals with severe fXI deficiency have reduced risk of stroke (Salomon et al., 2008). In animal models of thrombosis, fXII deficiency decreases formation of arterial thrombi (Renné et al., 2005) and protects the animals from ischemic brain injury (Kleinschnitz et al., 2006). Activation of the contact pathway in vivo via intravenous administration of RNA (Kannemeier et al., 2007), or polyP (Müller et al., 2009) triggers pulmonary embolism in animal models. And finally, inhibitors of polyP are antithrombotic in arterial and venous thrombosis models in mice, with reduced bleeding side-effects compared to heparin (Smith et al., 2012; Jain et al., 2012; Travers et al., 2014).
Concluding remarks
As our understanding of the myriad processes involved in the initiation of coagulation in mammalian blood continues to grow, so does our understanding of the complex relationship between hemostasis and pathological thrombosis. Under the original assumption that these processes were inseparable, it made sense to target the most important enzymes in the final common pathway of blood coagulation. This includes fXa and thrombin (targeted with heparin and the new direct oral anticoagulants), and GLA-domain containing proteins in general (targeted by warfarin). Classical anticoagulant drugs are some of the most widely prescribed medications today, even with the knowledge that they necessitate straddling a sharp line between too much anticoagulation (risk of bleeding) and to little anticoagulation (risk of thrombosis). Recent advances in our understanding of the role of the contact pathway in thrombosis has led to the intriguing possibility that drugs that inhibit initiation of the contact pathway may be effective antithrombotics with little or no bleeding side effects.
For example, a novel human monoclonal antibody targeting the active site of fXIIa developed via phage display has recently been shown to inhibit venous and arterial thrombosis during both experimental injury and extracorporeal circulation (ECMO) in animal models (Larsson et al., 2014). This antibody was just as effective as heparin without the concurrent risk of bleeding, and the fact that it specifically targets fXIIa rather than both the activated and zymogen forms of this enzyme means that it can be effective at much lower doses than inhibitors that bind to the zymogen and inhibit activation. Another exciting anticoagulant therapy based on inhibiting the contact (and thrombin-feedback) pathway relies on using antisense oligonucleotides to inhibit the biosynthesis of fXI. This method has shown to be safe and effective in rabbits (Yau et al., 2014), primates (Crosby et al., 2013), and even humans (Büller et al., 2015). These oligonucleotides specifically target fXI mRNA and cause its degradation, leading to a dose-dependent decrease in fXI levels and resulting in decreased risk of thrombosis with less risk of bleeding compared to conventional therapeutics.
Inhibition of the contact pathway as a method of anticoagulation not only carries less risk of bleeding than current therapeutics, it also has the potential to reduce the often damaging connections (mediated by the fXIIa/kallikrein/bradykinin pathway) between coagulation and inflammation in human disease. Such novel anticoagulation approaches therefore have the potential to expand the health benefits of antithrombotic therapy to a much wider set of patients (who would otherwise be at severe risk of bleeding from conventional therapeutics) in a safer and more effective manner than is currently possible. Though research in human blood coagulation has a long and successful history, novel research in the mechanisms of thrombosis and hemostasis continues to reveal surprising and exciting insights into human biology that have the potential to save lives.
Acknowledgments
Declaration of interest
Studies in the authors’ laboratory were supported by grants R01 HL047014 and R01 HL103999 from the National Heart Lung and Blood Institute of NIH, and by Predoctoral Fellowship 13PRE14550007 from the American Heart Association. All three authors are co-inventors on pending patent applications on medical uses of polyphosphate and polyphosphate inhibitors. JHM receives patent royalties from sales of kits for measuring plasma fVIIa levels.
References
- Ahamed J, Versteeg HH, Kerver M, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault-Riché D, Fraley CD, Tzeng CM, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: Regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–3276. [PubMed] [Google Scholar]
- Bajaj MS, Kuppuswamy MN, Manepalli AN, Bajaj SP. Transcriptional expression of tissue factor pathway inhibitor, thrombomodulin and von Willebrand factor in normal human tissues. Thromb Haemost. 1999;82:1047–1052. [PubMed] [Google Scholar]
- Balleisen L, Schulte H, Assmann G, et al. Coagulation factors and the progress of coronary heart disease. Lancet. 1987;2:461. doi: 10.1016/s0140-6736(87)91004-x. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Donarini C, Foddi C, et al. The region 1–11 of Alzheimer amyloid-beta is critical for activation of contact-kinin system. Neurobiol Aging. 2001;22:63–69. doi: 10.1016/s0197-4580(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Parnetti L, Pareyson D, et al. Activation of the contact system in cerebrospinal fluid of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:102–108. doi: 10.1097/00002093-199806000-00008. [DOI] [PubMed] [Google Scholar]
- Bogdanov VY, Balasubramanian V, Hathcock J, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- Bom VJ, Bertina RM. The contributions of Ca2+, phospholipids and tissue-factor apoprotein to the activation of human blood-coagulation factor X by activated factor VII. Biochem J. 1990;265:327–336. doi: 10.1042/bj2650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst P, Kelleher C, Hughes L, et al. Fibrinogen, factor VII clotting activity and coronary artery disease severity. Atherosclerosis. 1990;85:169–173. doi: 10.1016/0021-9150(90)90108-u. [DOI] [PubMed] [Google Scholar]
- Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci U S A. 2004;101:16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broze GJ, Jr, Majerus PW. Purification and properties of human coagulation factor VII. J Biol Chem. 1980;255:1242–1247. [PubMed] [Google Scholar]
- Brunnée T, Reddigari SR, Shibayama Y, et al. Mast cell derived heparin activates the contact system: a link to kinin generation in allergic reactions. Clin Exp Allergy. 1997;27:653–663. [PubMed] [Google Scholar]
- Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camera M, Brambilla M, Facchinetti L, et al. Tissue factor and atherosclerosis: not only vessel wall-derived TF, but also platelet-associated TF. Thromb Res. 2012;129:279–284. doi: 10.1016/j.thromres.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Camerer E, Kolsto AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Carvalho De Sousa J, Azevedo J, Soria C, et al. Factor VII hyperactivity in acute myocardial thrombosis: A relation to the coagulation activation. Thromb Res. 1988;51:165–173. doi: 10.1016/0049-3848(88)90060-6. [DOI] [PubMed] [Google Scholar]
- Chan JCY, Rosen ED, Carmeliet P, et al. Factor VII deficiency rescues the intrauterine lethality of tissue factor pathway inhibitor deficient mice. Blood. 1997;90:1775. [Google Scholar]
- Chang JY, Monroe DM, Oliver JA, Roberts HR. TFPIbeta, a second product from the mouse tissue factor pathway inhibitor (TFPI) gene. Thromb Haemost. 1999;81:45–49. [PubMed] [Google Scholar]
- Choi SH, Collins JN, Smith SA, et al. Phosphoramidate end labeling of inorganic polyphosphates: facile manipulation of polyphosphate for investigating and modulating its biological activities. Biochemistry. 2010;49:9935–9941. doi: 10.1021/bi1014437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan VD, Comerota AJ, Sun L, et al. Inhibition of tissue factor pathway during intermittent pneumatic compression: A possible mechanism for antithrombotic effect. Arterioscler Thromb Vasc Biol. 1999;19:2812–2817. doi: 10.1161/01.atv.19.11.2812. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Zito F, Norman Chan N, et al. Activated factor XII levels and factor XII 46C>T genotype in relation to coronary artery calcification in patients with type 1 diabetes and healthy subjects. Atherosclerosis. 2002;163:363–369. doi: 10.1016/s0021-9150(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Miller GJ, Bauer KA, et al. Comparison of novel hemostatic factors and conventional risk factors for prediction of coronary heart disease. Circulation. 2000;102:2816–2822. doi: 10.1161/01.cir.102.23.2816. [DOI] [PubMed] [Google Scholar]
- Cortellaro M, Boschetti C, Cofrancesco E, et al. The PLAT Study: Hemostatic function in relation to atherothrombotic ischemic events in vascular disease patients. Principal results. Arterioscler Thromb. 1992;12:1063–1070. doi: 10.1161/01.atv.12.9.1063. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Marzec U, Revenko AS, et al. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.301282. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen R, Onundarson PT, Thors H, et al. Activated and total coagulation factor VII, and fibrinogen in coronary artery disease. Scand Cardiovasc J. 1998;32:87–95. doi: 10.1080/14017439850140238. [DOI] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989a;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Drake TA, Ruf W, Morrissey JH, Edgington TS. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide-stimulated human blood monocytes and a constitutively tissue factor-producing neoplastic cell line. J Cell Biol. 1989f;109:389–395. doi: 10.1083/jcb.109.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DS. Quantitation of factor VII in the plasma of normal and warfarin-treated individuals by radioimmunoassay. Blood. 1983;62:784–791. [PubMed] [Google Scholar]
- Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Wu KK, Shahar E, Davis CE. Association of hemostatic variables with prevalent cardiovascular disease and asymptomatic carotid artery atherosclerosis. Arterioscler Thromb. 1993;13:1829–1836. doi: 10.1161/01.atv.13.12.1829. [DOI] [PubMed] [Google Scholar]
- Forrester JS, Litvack F, Grundfest W, Hickey A. A perspective of coronary disease seen through the arteries of living man. Circulation. 1987;75:505–513. doi: 10.1161/01.cir.75.3.505. [DOI] [PubMed] [Google Scholar]
- Gailani D, Renné T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- Geczy CL. Cellular mechanisms for the activation of blood coagulation. Int Rev Cytol. 1994;152:49–108. doi: 10.1016/s0074-7696(08)62554-1. [DOI] [PubMed] [Google Scholar]
- Geddings JE, Mackman N. New players in haemostasis and thrombosis. Thromb Haemost. 2014;111:570–574. doi: 10.1160/TH13-10-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen PLA, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: Another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TJ, Warren LA, Novotny WF, et al. Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- Grabowski EF, Zuckerman DB, Nemerson Y. The functional expression of tissue factor by fibroblasts and endothelial cells under flow conditions. Blood. 1993;81:3265–3270. [PubMed] [Google Scholar]
- Grant PJ. The genetics of atherothrombotic disorders: A clinician’s view. J Thromb Haemost. 2003;1:1381–1390. doi: 10.1046/j.1538-7836.2003.00276.x. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Cochrane CG. Mechanisms for the involvement of high molecular weight kininogen in surface-dependent reactions of Hageman factor. Proc Natl Acad Sci U S A. 1976;73:2554–2558. doi: 10.1073/pnas.73.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt H, Nilsen DW, Hetland O, et al. Activated factor 12 (FXIIa) predicts recurrent coronary events after an acute myocardial infarction. Am Heart J. 2004;147:260–266. doi: 10.1016/j.ahj.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci U S A. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JB, Huseby NE, Sandset PM, et al. Tissue-factor pathway inhibitor and lipoproteins. Evidence for association with and regulation by LDL in human plasma. Arterioscler Thromb. 1994;14:223–229. doi: 10.1161/01.atv.14.2.223. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Svensson B, Olsen R, et al. Heparin induces synthesis and secretion of tissue factor pathway inhibitor from endothelial cells in vitro. Thromb Haemost. 2000;83:937–943. [PubMed] [Google Scholar]
- Herwald H, Mörgelin M, Olsén A, et al. Activation of the contact-phase system on bacterial surfaces—a clue to serious complications in infectious diseases. Nat Med. 1998;4:298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
- Hoffman C, Shah A, Sodums M, Hultin MB. Factor VII activity state in coronary artery disease. J Lab Clin Med. 1988;111:475–481. [PubMed] [Google Scholar]
- Hoffman CJ, Miller RH, Lawson WE, Hultin MB. Elevation of factor VII activity and mass in young adults at risk of ischemic heart disease. J Am Coll Cardiol. 1989;14:941–946. doi: 10.1016/0735-1097(89)90470-1. [DOI] [PubMed] [Google Scholar]
- Hojima Y, Cochrane CG, Wiggins RC, et al. In vitro activation of the contact (Hageman factor) system of plasma by heparin and chondroitin sulfate E. Blood. 1984;63:1453–1459. [PubMed] [Google Scholar]
- Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- Huang ZF, Wun TC, Broze GJ., Jr Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem. 1993;268:26950–26955. [PubMed] [Google Scholar]
- Hultin MB. Fibrinogen and factor VII as risk factors in vascular disease. Prog Hemost Thromb. 1991;10:215–241. [PubMed] [Google Scholar]
- Ichikawa K, Nakagawa K, Hirano K, Sueishi K. The localization of tissue factor and apolipoprotein(a) in atherosclerotic lesions of the human aorta and their relation to fibrinogen-fibrin transition. Pathol Res Pract. 1996;192:224–232. doi: 10.1016/S0344-0338(96)80225-1. [DOI] [PubMed] [Google Scholar]
- Jain S, Pitoc GA, Holl EK, et al. Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proc Natl Acad Sci U S A. 2012;109:12938–12943. doi: 10.1073/pnas.1204928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph K, Ghebrehiwet B, Kaplan AP. Activation of the kinin-forming cascade on the surface of endothelial cells. Biol Chem. 2001;382:71–75. doi: 10.1515/BC.2001.012. [DOI] [PubMed] [Google Scholar]
- Kalaria VG, Zareba W, Moss AJ, et al. Gender-related differences in thrombogenic factors predicting recurrent cardiac events in patients after acute myocardial infarction. The THROMBO Investigators. Am J Cardiol. 2000;85:1401–1408. doi: 10.1016/s0002-9149(00)00785-2. [DOI] [PubMed] [Google Scholar]
- Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AP, Ghebrehiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47:2161–2169. doi: 10.1016/j.molimm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Kario K, Matsuo T, Matsuo M, et al. Marked increase of activated factor VII in uremic patients. Thromb Haemost. 1995;73:763–767. [PubMed] [Google Scholar]
- Kario K, Matsuo T, Sakata T, Miyata T. Factor VII hyperactivity and ischaemic heart disease. Lancet. 1994;343:233. doi: 10.1016/s0140-6736(94)91017-0. [DOI] [PubMed] [Google Scholar]
- Kario K, Sakata T, Matsuo T, Miyata T. Factor VII in non-insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:1552. doi: 10.1016/s0140-6736(05)80119-9. [DOI] [PubMed] [Google Scholar]
- Karlsrud TS, Buo L, Aasen AO, Johansen HT. Bradykinin release from high molecular weight kininogen in surgical ICU patients. Immunopharmacology. 1996a;33:365–368. doi: 10.1016/0162-3109(96)00087-2. [DOI] [PubMed] [Google Scholar]
- Karlsrud TS, Buo L, Aasen AO, Johansen HT. Cleavage of plasma high molecular weight kininogen in surgical ICU patients. Intensive Care Med. 1996b;22:760–765. doi: 10.1007/BF01709518. [DOI] [PubMed] [Google Scholar]
- Kaufman N, Page JD, Pixley RA, et al. Alpha 2-macroglobulin-kallikrein complexes detect contact system activation in hereditary angioedema and human sepsis. Blood. 1991;77:2660–2667. [PubMed] [Google Scholar]
- Kishimoto TK, Viswanathan K, Ganguly T, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel W, Davie EW. Isolation and characterization of bovine factor VII. Biochemistry. 1975;14:4928–4934. doi: 10.1021/bi00693a023. [DOI] [PubMed] [Google Scholar]
- Kisiel W, Fujikawa K, Davie EW. Activation of bovine factor VII (proconvertin) by factor XIIa (activated Hageman factor) Biochemistry. 1977;16:4189–4194. doi: 10.1021/bi00638a009. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Stoll G, Bendszus M, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Pedersen AH, Kisiel W. Proteolytic activation of human factors IX and X by recombinant human factor VIIa: Effects of calcium, phospholipids, and tissue factor. Biochemistry. 1990;29:9418–9425. doi: 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kisiel W. Regulation of factor VIIa activity in plasma: Evidence that antithrombin III is the sole plasma protease inhibitor of human factor VIIa. Thromb Res. 1987;46:325–335. doi: 10.1016/0049-3848(87)90294-5. [DOI] [PubMed] [Google Scholar]
- Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- Koster T, Rosendaal FR, Reitsma PH, et al. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA polymorphisms--the Leiden Thrombophilia Study (LETS) Thromb Haemost. 1994;71:719–722. [PubMed] [Google Scholar]
- Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- Lawson JH, Butenas S, Ribarik N, Mann KG. Complex-dependent inhibition of factor VIIa by antithrombin III and heparin. J Biol Chem. 1993;268:767–770. [PubMed] [Google Scholar]
- Lefevre M, Kris-Etherton PM, Zhao G, Tracy RP. Dietary fatty acids, hemostasis, and cardiovascular disease risk. J Am Diet Assoc. 2004;104:410–419. doi: 10.1016/j.jada.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Lupu C, Lupu F, Dennehy U, et al. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- Lupu C, Poulsen E, Roquefeuil S, et al. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1999;19:2251–2262. doi: 10.1161/01.atv.19.9.2251. [DOI] [PubMed] [Google Scholar]
- Maas C, Govers-Riemslag JW, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandle R, Jr, Kaplan AP. Hageman factor substrates. Human plasma prekallikrein: mechanism of activation by Hageman factor and participation in Hageman factor-dependent fibrinolysis. J Biol Chem. 1977;252:6097–6104. [PubMed] [Google Scholar]
- Marmur JD, Thiruvikraman SV, Fyfe BS, et al. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- Maroney SA, Haberichter SL, Friese P, et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masys DR, Bajaj SP, Rapaport SI. Activation of human factor VII by activated factors IX and X. Blood. 1982;60:1143–1150. [PubMed] [Google Scholar]
- Maynard JR, Dreyer BE, Stemerman MB, Pitlick FA. Tissue-factor coagulant activity of cultured human endothelial and smooth muscle cells and fibroblasts. Blood. 1977;50:387–396. [PubMed] [Google Scholar]
- Meade TW, Mellows S, Brozovic M, et al. Haemostatic function and ischaemic heart disease: Principal results of the Northwick Park Heart Study. Lancet. 1986;2:533–537. doi: 10.1016/s0140-6736(86)90111-x. [DOI] [PubMed] [Google Scholar]
- Merlo C, Wuillemin WA, Redondo M, et al. Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease. Atherosclerosis. 2002;161:261–267. doi: 10.1016/s0021-9150(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Miller GJ. Postprandial lipaemia and haemostatic factors. Atherosclerosis. 1998;141:S47–S51. doi: 10.1016/s0021-9150(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Miller GJ, Martin JC, Mitropoulos KA, et al. Activation of factor VII during alimentary lipemia occurs in healthy adults and patients with congenital factor XII or factor XI deficiency, but not in patients with factor IX deficiency. Blood. 1996;87:4187–4196. [PubMed] [Google Scholar]
- Moor E, Silveira A, Van’t Hooft F, et al. Coagulation factor VII mass and activity in young men with myocardial infarction at a young age. Role of plasma lipoproteins and factor VII genotype. Arterioscler Thromb Vasc Biol. 1995;15:655–664. doi: 10.1161/01.atv.15.5.655. [DOI] [PubMed] [Google Scholar]
- Moosbauer C, Morgenstern E, Cuvelier SL, et al. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey JH, Broze GJ., Jr . Tissue factor and the initiation and regulation (TFPI) of coagulation. In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC 2nd, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 6th. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- Morrissey JH, Fakhrai H, Edgington TS. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987;50:129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–744. [PubMed] [Google Scholar]
- Mulder AB, Smit JW, Bom VJ, et al. Association of smooth muscle cell tissue factor with caveolae. Blood. 1996;88:1306–1313. [PubMed] [Google Scholar]
- Müller F, Gailani D, Renné T. Factor XI and XII as antithrombotic targets. Curr Opin Hematol. 2011;18:349–355. doi: 10.1097/MOH.0b013e3283497e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Renné T. Novel roles for factor XII-driven plasma contact activation system. Curr Opin Hematol. 2008;15:516–521. doi: 10.1097/MOH.0b013e328309ec85. [DOI] [PubMed] [Google Scholar]
- Naumnik B, Rydzewska-Rosolowska A, Mysliwiec M. Different effects of enoxaparin, nadroparin, and dalteparin on plasma TFPI during hemodialysis: a prospective crossover randomized study. Clin Appl Thromb Hemost. 2011;17:480–486. doi: 10.1177/1076029610376936. [DOI] [PubMed] [Google Scholar]
- Nemerson Y, Gentry R. An ordered addition, essential activation model of the tissue factor pathway of coagulation: Evidence for a conformational cage. Biochemistry. 1986;25:4020–4033. doi: 10.1021/bi00362a006. [DOI] [PubMed] [Google Scholar]
- Nemerson Y, Repke D. Tissue factor accelerates the activation of coagulation factor VII: The role of a bifunctional coagulation cofactor. Thromb Res. 1985;40:351–358. doi: 10.1016/0049-3848(85)90270-1. [DOI] [PubMed] [Google Scholar]
- Neuenschwander PF, Fiore MM, Morrissey JH. Factor VII autoactivation proceeds via interaction of distinct protease-cofactor and zymogen-cofactor complexes. Implications of a two-dimensional enzyme kinetic mechanism. J Biol Chem. 1993;268:21489–214892. [PubMed] [Google Scholar]
- Neuenschwander PF, Morrissey JH. Roles of the membrane-interactive regions of factor VIIa and tissue factor. The factor VIIa Gla domain is dispensable for binding to tissue factor but important for activation of factor X. J Biol Chem. 1994;269:8007–8013. [PubMed] [Google Scholar]
- Nickel KF, Renné T. Crosstalk of the plasma contact system with bacteria. Thromb Res. 2012;130(Suppl 1):S78–S83. doi: 10.1016/j.thromres.2012.08.284. [DOI] [PubMed] [Google Scholar]
- Noga O, Brunnée T, Schäper C, Kunkel G. Heparin, derived from the mast cells of human lungs is responsible for the generation of kinins in allergic reactions due to the activation of the contact system. Int Arch Allergy Immunol. 1999;120:310–316. doi: 10.1159/000024284. [DOI] [PubMed] [Google Scholar]
- Nossel HL. Differential consumption of coagulation factors resulting from activation of the extrinsic (tissue thromboplastin) or the intrinsic (foreign surface contact) pathways. Blood. 1967;29:331–340. [PubMed] [Google Scholar]
- Novotny WF, Brown SG, Miletich JP, et al. Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Purification and characterization of the lipoprotein-associated coagulation inhibitor from human plasma. J Biol Chem. 1989;264:18832–18837. [PubMed] [Google Scholar]
- Orlando M, Leri O, Macioce G, et al. Factor VII in subjects at risk for thromboembolism: Activation or increased synthesis? Haemostasis. 1987;17:340–343. doi: 10.1159/000215767. [DOI] [PubMed] [Google Scholar]
- Østerud B. Tissue factor/TFPI and blood cells. Thromb Res. 2012;129:274–278. doi: 10.1016/j.thromres.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Paborsky LR, Caras IW, Fisher KL, Gorman CM. Lipid association, but not the transmembrane domain, is required for tissue factor activity. Substitution of the transmembrane domain with a phosphatidylinositol anchor. J Biol Chem. 1991;266:21911–21916. [PubMed] [Google Scholar]
- Pedersen B, Holscher T, Sato Y, et al. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105:2777–2782. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- Piro O, Broze GJ., Jr Role for the Kunitz-3 domain of tissue factor pathway inhibitor-alpha in cell surface binding. Circulation. 2004;110:3567–3572. doi: 10.1161/01.CIR.0000148778.76917.89. [DOI] [PubMed] [Google Scholar]
- Piro O, Broze GJ., Jr Comparison of cell-surface TFPIalpha and beta. J Thromb Haemost. 2005;3:2677–2683. doi: 10.1111/j.1538-7836.2005.01636.x. [DOI] [PubMed] [Google Scholar]
- Pisoni RL, Lindley ER. Incorporation of [32P]orthophosphate into long chains of inorganic polyphosphate within lysosomes of human fibroblasts. J Biol Chem. 1992;267:3626–3631. [PubMed] [Google Scholar]
- Radcliffe R, Nemerson Y. Activation and control of factor VII by activated factor X and thrombin: Isolation and characterization of a single chain form of factor VII. J Biol Chem. 1975;250:388–395. [PubMed] [Google Scholar]
- Rao LV, Rapaport SI. Activation of factor VII bound to tissue factor: A key early step in the tissue factor pathway of blood coagulation. Proc Natl Acad Sci U S A. 1988;85:6687–6691. doi: 10.1073/pnas.85.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao LV, Rapaport SI, Hoang AD. Binding of factor VIIa to tissue factor permits rapid antithrombin III/heparin inhibition of factor VIIa. Blood. 1993;81:2600–2607. [PubMed] [Google Scholar]
- Renné T. The factor XII-driven plasma contact system. In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC 2nd, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 6th. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renné T, Schmaier AH, Nickel KF, et al. In vivo roles of factor XII. Blood. 2012;120:4296–4303. doi: 10.1182/blood-2012-07-292094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römisch J. Factor VII activating protease (FSAP): A novel protease in hemostasis. Biol Chem. 2002;383:1119–1124. doi: 10.1515/BC.2002.121. [DOI] [PubMed] [Google Scholar]
- Ruddock V, Meade TW. Factor-VII activity and ischaemic heart disease: Fatal and non-fatal events. Q J Med. 1994;87:403–406. [PubMed] [Google Scholar]
- Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- Sabatier F, Roux V, Anfosso F, et al. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–3970. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- Sala-Cunill A, Bjorkqvist J, Senter R, et al. Plasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactions. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.07.057. [DOI] [PubMed] [Google Scholar]
- Salomon O, Steinberg DM, Koren-Morag N, et al. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI) Thromb Res. 1988;50:803–813. doi: 10.1016/0049-3848(88)90340-4. [DOI] [PubMed] [Google Scholar]
- Sandset PM, Lund H, Norseth J, et al. Treatment with hydroxymethylglutaryl-coenzyme A reductase inhibitors in hypercholesterolemia induces changes in the components of the extrinsic coagulation system. Arterioscler Thromb. 1991;11:138–145. doi: 10.1161/01.atv.11.1.138. [DOI] [PubMed] [Google Scholar]
- Scarpati EM, Wen D, Broze GJ, Jr, et al. Human tissue factor: cDNA sequence and chromosome localization of the gene. Biochemistry. 1987;26:5234–5238. doi: 10.1021/bi00391a004. [DOI] [PubMed] [Google Scholar]
- Schmaier AH. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int Immunopharmacol. 2008;8:161–165. doi: 10.1016/j.intimp.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaier AH, Mccrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5:2323–2329. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- Seligsohn U, Kasper CK, Østerud B, Rapaport SI. Activated factor VII: Presence in factor IX concentrates and persistence in the circulation after infusion. Blood. 1979a;53:828–837. [PubMed] [Google Scholar]
- Seligsohn U, Østerud B, Brown SF, et al. Activation of human factor VII in plasma and in purified systems: Roles of activated factor IX, kallikrein, and activated factor XII. J Clin Invest. 1979b;64:1056–1065. doi: 10.1172/JCI109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama Y, Joseph K, Nakazawa Y, et al. Zinc-dependent activation of the plasma kinin-forming cascade by aggregated beta amyloid protein. Clin Immunol. 1999;90:89–99. doi: 10.1006/clim.1998.4621. [DOI] [PubMed] [Google Scholar]
- Silverberg M, Diehl SV. The autoactivation of factor XII (Hageman factor) induced by low-Mr heparin and dextran sulphate. The effect of the Mr of the activating polyanion. Biochem J. 1987;248:715–720. doi: 10.1042/bj2480715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg M, Dunn JT, Garen L, Kaplan AP. Autoactivation of human Hageman factor. Demonstration utilizing a synthetic substrate. J Biol Chem. 1980;255:7281–7286. [PubMed] [Google Scholar]
- Smith SA, Choi SH, Collins JN, et al. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood. 2012;120:5103–5110. doi: 10.1182/blood-2012-07-444935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Choi SH, Davis-Harrison R, et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mutch NJ, Baskar D, et al. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosef MN, Bosch JG, Van Oostayen J, et al. Relation of plasma coagulation factor VII and fibrinogen to carotid artery intima-media thickness. Thromb Haemost. 1994;72:250–254. [PubMed] [Google Scholar]
- Spicer EK, Horton R, Bloem L, et al. Isolation of cDNA clones coding for human tissue factor: Primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987;84:5148–4152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yamauchi K, Matsushita T, et al. Elevation of factor VII activity and mass in coronary artery disease of varying severity. Clin Cardiol. 1991;14:731–736. doi: 10.1002/clc.4960140907. [DOI] [PubMed] [Google Scholar]
- Tankersley DL, Finlayson JS. Kinetics of activation and autoactivation of human factor XII. Biochemistry. 1984;23:273–279. doi: 10.1021/bi00297a016. [DOI] [PubMed] [Google Scholar]
- Tavoosi N, Smith SA, Davis-Harrison RL, Morrissey JH. Factor VII and protein C are phosphatidic acid-binding proteins. Biochemistry. 2013;52:5545–5552. doi: 10.1021/bi4006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB., Jr Role of tissue factor and factor VIIa in the coagulant and inflammatory response to LD100 Escherichia coli in the baboon. Haemostasis. 1996;26:83–91. doi: 10.1159/000217246. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Jr, Chang A, Ruf W, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- Thaler J, Ay C, Mackman N, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- Thiruvikraman SV, Guha A, Roboz J, et al. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab Invest. 1996;75:451–461. [PubMed] [Google Scholar]
- Tipping PG, Malliaros J, Holdsworth SR. Procoagulant activity expression by macrophages from atheromatous vascular plaques. Atherosclerosis. 1989;79:237–243. doi: 10.1016/0021-9150(89)90129-9. [DOI] [PubMed] [Google Scholar]
- Todoroki H, Higure A, Okamoto K, et al. Possible role of platelet-activating factor in the in vivo expression of tissue factor in neutrophils. J Surg Res. 1998;80:149–155. doi: 10.1006/jsre.1998.5348. [DOI] [PubMed] [Google Scholar]
- Travers RJ, Shenoi RA, Kalathottukaren MT, et al. Nontoxic polyphosphate inhibitors reduce thrombosis while sparing hemostasis. Blood. 2014;124:3183–3190. doi: 10.1182/blood-2014-05-577932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujioka H, Suehiro A, Kakishita E. Activation of coagulation factor VII by tissue-type plasminogen activator. Am J Hematol. 1999;61:34–39. doi: 10.1002/(sici)1096-8652(199905)61:1<34::aid-ajh7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Kennedy SC, Kennedy D, Gonzales E. Coagulation, fibrinolytic, and inhibitory proteins in acute myocardial infarction and angina pectoris. Am J Med. 1992;93:651–657. doi: 10.1016/0002-9343(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Ruf W. Thiol pathways in the regulation of tissue factor prothrombotic activity. Curr Opin Hematol. 2011;18:343–348. doi: 10.1097/MOH.0b013e32834981de. [DOI] [PubMed] [Google Scholar]
- Walenga JM, Jeske WP, Samama MM, et al. Fondaparinux: a synthetic heparin pentasaccharide as a new antithrombotic agent. Expert Opin Investig Drugs. 2002;11:397–407. doi: 10.1517/13543784.11.3.397. [DOI] [PubMed] [Google Scholar]
- Walford HH, Zuraw BL. Current update on cellular and molecular mechanisms of hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112:413–418. doi: 10.1016/j.anai.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Wesselschmidt R, Likert K, Huang Z, et al. Structural requirements for tissue factor pathway inhibitor interactions with factor Xa and heparin. Blood Coagul Fibrinolysis. 1993;4:661–669. [PubMed] [Google Scholar]
- Westmuckett AD, Lupu C, Roquefeuil S, et al. Fluid flow induces upregulation of synthesis and release of tissue factor pathway inhibitor in vitro. Arterioscler Thromb Vasc Biol. 2000;20:2474–2482. doi: 10.1161/01.atv.20.11.2474. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildgoose P, Nemerson Y, Hansen LL, et al. Measurement of basal levels of factor VIIa in hemophilia A and B patients. Blood. 1992;80:25–28. [PubMed] [Google Scholar]
- Yamamoto M, Nakagaki T, Kisiel W. Tissue factor-dependent autoactivation of human blood coagulation factor VII. J Biol Chem. 1992;267:19089–19094. [PubMed] [Google Scholar]
- Yan SF, Mackman N, Kisiel W, et al. Hypoxia/hypoxemia-induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arterioscler Thromb Vasc Biol. 1999;19:2029–2035. doi: 10.1161/01.atv.19.9.2029. [DOI] [PubMed] [Google Scholar]
- Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123:2102–2107. doi: 10.1182/blood-2013-12-540872. [DOI] [PubMed] [Google Scholar]
- Zeerleder S. C1-inhibitor: more than a serine protease inhibitor. Semin Thromb Hemost. 2011;37:362–374. doi: 10.1055/s-0031-1276585. [DOI] [PubMed] [Google Scholar]
- Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108:623–627. doi: 10.1161/01.CIR.0000078642.45127.7B. [DOI] [PubMed] [Google Scholar]