Abstract
Objective
Changes in smooth muscle cell (SMC) membrane potential (Em) are critical to vasomotor responses. As a fluorescent indicator approach would lessen limitations of glass electrodes in contracting preparations, we aimed to develop a Forster (or fluorescence) resonance energy transfer (FRET)-based measurement for Em.
Methods
The FRET pair used in this study (donor CC2-DMPE [excitation 405 nm] and acceptor DisBAC4(3)) provide rapid measurements at a sensitivity not achievable with many ratiometric indicators. The method also combined measurement changes in Ca2+i using fluo-4 and excitation at 490 nm.
Results
After establishing loading conditions, a linear relationship was demonstrated between Em and fluorescence signal in FRET dye-loaded HEK cells held under voltage clamp. Over the voltage range from −70 to +30 mV, slope (of FRET signal vs. voltage, m) = 0.49 ± 0.07, r2 = 0.96 ± 0.025. Similar data were obtained in cerebral artery SMCs, slope (m) = 0.30 ± 0.02, r2 = 0.98 ± 0.02. Change in FRET emission ratio over the holding potential of −70 to +30 mV was 41.7 ± 4.9% for HEK cells and 30.0 ± 2.3% for arterial SMCs. The FRET signal was also shown to be modulated by KCl-induced depolarization in a concentration-dependent manner. Further, in isolated arterial SMCs, KCl-induced depolarization (60 mM) measurements occurred with increased fluo-4 fluorescence emission (62 ± 9%) and contraction (−27 ± 4.2%).
Conclusions
The data support the FRET-based approach for measuring changes in Em in arterial SMCs. Further, image-based measurements of Em can be combined with analysis of temporal changes in Ca2+i and contraction.
Keywords: forster resonance energy transfer, vascular smooth muscle, membrane potential, intracellular Ca2+, imaging
INTRODUCTION
Changes in arteriolar SMC membrane potential (Em) and intracellular Ca2+ (Ca2+i) are key events in vasoconstrictor and vasodilator responses to a wide variety of vasoactive stimuli. For example, membrane depolarization and increases in Ca2+i have been shown to underlie vasoconstrictor responses to increased intraluminal pressure, adrenergic agonists, and KCl [1–5]. Similarly, SMC hyper-polarization and decreased Ca2+i underly dilation to agents including small increases in extracellular K+, acetylcholine, levcromakalim, and decreased intraluminal pressure [5–11]. Despite the importance of these signaling events, the availability of temporal information on changes in Em has been limited by technical difficulties related to the use of conventional sharp glass microelectrodes. In particular, movement during contraction or dilation tends to cause loss of cellular impalement necessary for Em recording. For this reason, a number of attempts have been made to use voltage-sensitive fluorescent indicators.
Voltage-sensitive dyes have largely been categorized based on their broad kinetic properties as slow or fast dyes. The slow dyes, such as the oxonol DiBAC4, show high sensitivity, relying on Em-dependent partitioning from the extracellular to the intracellular environment. In contrast, fast dyes tend to show only relatively small changes in fluorescence for a large change in Em. For general reviews on the use of early voltage-sensitive dyes see references [12] and [13].
Single wavelength dyes such as oxonol derivatives have been used to study Em changes in smooth muscle in response to a variety of depolarizing stimuli [14,15]. Typically such measurements reached a steady-state after a number of minutes (10–30 minutes for bis-oxonol in the study of Neylon et al. [14]) consistent with their description as slow dyes and casting doubt on their use for following dynamic temporal signal events in response to physiological stimuli. A further difficulty in using single wavelength fluorescent dyes is the added complication provided by contraction or dilation. In such situations a change in fluorescence may be recorded, but this can only be interpreted as a relative change due to the change in volume of the dye compartment.
To overcome some of the limitations of single wavelength fluorescent dyes, microvascular studies have more recently been performed using a ratiometric approach (see Montana et al. [16] for background methodology in HeLa cells and lipid vesicles). Such an approach is advantageous in situations where variability in dye loading may occur and, as outlined above, where cell movement is evident [17]. Typically these studies have utilized the styryl-based fast response dyes such as di-8-ANEPPS. Beach et al. [18] initially described this approach for measurement of changes in membrane potential hamster in cheek pouch arterioles and later in capillaries [19]. More recently, Chen and Rivers [20] developed a dual intensified CCD approach (focusing specifically on the vessel wall) to measure arteriolar smooth muscle Em in an in vivo preparation loaded with di-8-ANEPPS. Fluorescence emission ratio (620:560 nm) was observed to decrease by approximately 6% in response to 100 mM KCl corresponding to a 6 mV/% change in ratio or 0.17% change in ratio per mV change in Em. Methacholine (10−4 M), a muscarinic receptor agonist, resulted in smooth muscle hyperpolarization with an increase in fluorescence ratio of 2.3 ± 0.3%. This level of sensitivity would thus likely be limiting when examining Em in situations where a stimulus results in a change in the order of a few millivolts.
More recently, FRET-based systems have been used to measure changes in Em [21–25]. In particular, the FRET pair CC2-DMPE (coumarin-labeled phospholipid donor molecule) and DisBAC4(3) (oxonol acceptor molecule) have been used in high-throughput screening applications in CHO-K1 and RBL-2H3 cells [24,26] and studies of agonist-induced changes in Em in keratinocytes [23] and pancreatic β cells [25]. In this approach, the donor molecule is anchored to the outer leaflet of the plasma membrane due to its phospholipid tail while the mobile DisBAC4(3) acceptor molecule moves toward the inner leaflet of the plasma membrane on depolarization. As FRET is critically dependent on the distance between donor and acceptor molecules, depolarization reduces the FRET signal with an increase in donor emission intensity while decreasing emission intensity from the acceptor molecule. Importantly, these studies [23,25] demonstrated the FRET approach to overcome some of the limitations of slow or fast fluorescent indicators.
The principal aim of the present study was to design and implement an optical system that would allow FRET-based measurements of Em to be performed on arteriolar SMC. Experiments were conducted on freshly isolated SMC from both cremaster muscle arterioles and cerebral arteries to demonstrate the general applicability of the approach and HEK cells (under whole cell voltage clamp) to enable the relationship between Em and fluorescence to be studied. A secondary aim was to extend the utility of the imaging system to allow recording of changes in Ca2+i and dimensions in addition to Em. The rationale for this approach is that measurements of these variables in single microvascular cells would enhance future temporal studies of agonist- and mechanically induced vasomotor mechanisms.
MATERIALS AND METHODS
Animal Handling
Vascular tissues used in the studies were obtained from male Sprague Dawley rats (weight 150–250 g). On the day of experiment, rats were anaesthetized with sodium pento-barbital (60 mg/kg, intraperitoneal). Upon reaching a surgical plane of anesthesia, the left and right cremaster muscles were removed and placed in a cooled (<4°C) dissection chamber. Rats were then killed by an overdose of anesthesia and the brain removed for dissection of cerebral arteries.
Prior to use, rats were maintained in a controlled environment animal facility and given free access to standard chow and water. All procedures were approved by the Animal Care and Use Committee of the University of Missouri, USA.
Isolated Cell Preparations
Small arteries from cremaster muscle and the cerebral circulation were micro-dissected as previously described [17]. Arteriolar myocytes from first- and second-order arterioles (1A/2A) were isolated according to the published methods [27–29]. In brief, cremaster muscles were excised and pinned flat for vessel dissection at 4°C in Ca2+-free physiological saline solution (PSS) containing (mM): 140 NaCl, 5.6 KCl, 1.0 MgCl2, 1.2 NaH2PO4, 5.0 d-glucose, 2.0 Na-pyruvate, 0.02 EDTA, 3 mM MOPS, plus 0.1 mg/mL bovine serum albumin (BSA; Amersham Life Science, Arlington Heights, IL, USA). Dissected segments of arterioles were transferred to a 1 mL tube of low-Ca2+ (0.1 mM) PSS containing (mM): 144 NaCl, 5.6 KCl, 0.1 CaCl2, 1.0 MgCl2, 0.42 Na2HPO4, 10 HEPES, 2 Na-pyruvate, and 1 mg/mL BSA at room temperature for 10 minutes. The solution was decanted and replaced with a similar solution containing 26 U/mL papain (Sigma, St Louis, MO, USA) and 1 mg/mL dithioerythritol. The vessels were incubated for 30 minutes at 37°C with occasional agitation, then transferred to a new tube containing low-Ca2+ PSS containing 1.95 U/mL collagenase (FALGPA; Sigma), 1 mg/mL soybean trypsin inhibitor (Sigma) and 75 U/mL elastase (Calbiochem, La Jolla, CA, USA), and incubated for 7–10 minutes at 37°C. After further digestion, the remaining fragments were gently rinsed 2–3 times with low-Ca2+ PSS without BSA and gently triturated using a fire-polished Pasteur pipette to release single cells. Spindle-shaped arteriolar myocytes were used within approximately 4 hours of isolation.
Smooth muscle cells from rat cerebral arteries were enzymatically isolated as previously described [28,30]. Briefly, arterial segments were placed in Ca2+-free PSS as above (37°C, 10 minutes). Vessels were then exposed to a two-step digestion process that involved [1] a 15-minute incubation in isolation media (37°C) containing 0.6 mg/mL papain and 1.8 mg/mL dithioerythritol, and [2] a 5–6 minutes incubation in low-Ca2+ PSS with 0.7 mg/mL type F collagenase, and 0.4 mg/mL type H collagenase. Following enzyme treatment, tissues were washed repeatedly with ice-cold low Ca2+ PSS and triturated with a fire-polished pipette. Isolated smooth muscle cells were stored in ice-cold isolation medium for use within 4 hours. Prior to imaging experiments smooth muscle cells were loaded with CC2-DMPE and DisBAC4(3) for 1 hour at room temperature as described under Results.
In preliminary studies, cultured cremaster muscle arteriolar SMCs, as previously described, were also used for optimizing indicator loading, demonstrating FRET and assessing toxicity of the dyes.
HEK Cells
HEK 293 cells (derived from tsA201 line) in DMEM (supplemented with 10% FBS, 1% penicillin–streptomycin and 1% l-glutamine) were maintained in an incubator at 37°C with 5% CO2. Cells between passages 8 and 24 were plated on the glass coverslip base of experimental chambers 12–24 hours before performing patch clamp and imaging. During this period the experimental chambers plated with cells were kept in a 28°C incubator (with 5% CO2) to control the cell density. Immediately prior to imaging experiments HEK cells were loaded with CC2-DMPE (5 μM) and DisBAC4(3) (3 μM) for 1 hour at room temperature.
Description of Microscope and Optical Path
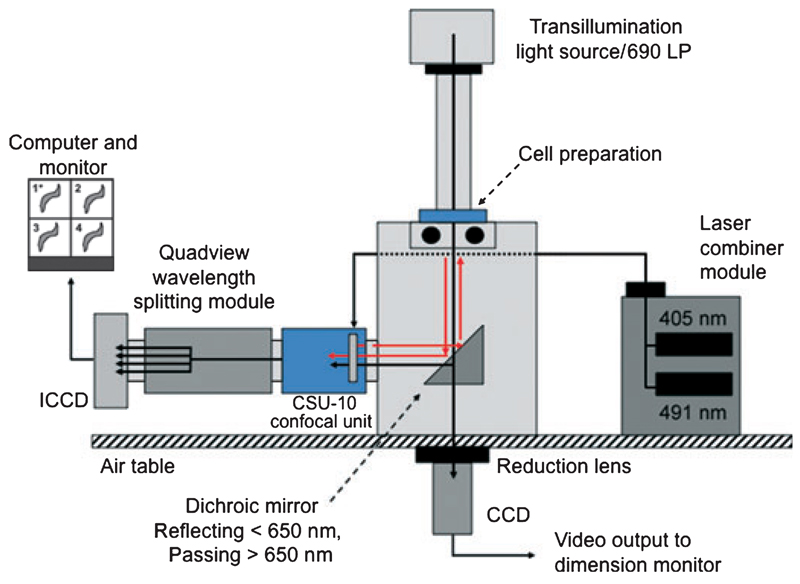
The optical system was based on an Olympus IX71 microscope, Hitschfel Instruments, St Louis, MO, USA. The left-hand side port was coupled to a Yokogawa CSU-10 Nipkow-confocal spinning disk unit (Solamere Technologies, Salt Lake City, Utah, USA), a Quadview image splitting device (Optical Insights, Tucson, AZ, USA) and an intensified CCD camera (Stanford XR-Mega 10-S30 Stanford Photonics, Palo Alto, CA, USA) (Figure 1 and Supplementary material). Illumination was provided by 405 and 491 nm solid-state lasers (50 and 100 mW, respectively) controlled by an acoustooptical tunable filter (AOTF; Solamere Technologies).
Figure 1.
Schematic diagram illustrating the optical set-up used for measurement of membrane potential (Em), Ca2+i and image dimensions. ICCD, intensified charged couple device; CSU-10, confocal scanning unit.
*(1) CC2-DMPE emission image; (2) wide field image; (3) fluo-4 emission image; (4) DisBAC4(3) emission image (FRET signal). See Supplementary material for additional detail of optical paths including dichroic mirrors and filters.
The microscope was fitted with 40× (Olympus UApo/340, N.A. 1.15, working distance 0.25 mm) and 60× (Olympus UPlan S Apo, N.A.1.2, working distance 0.21 mm) objective lenses and a long distance condenser. Details of dichroic mirrors and filters necessary for directing the light path and providing the necessary excitation and emission wavelengths for separating fluorescent signals are given in the Supplementary material.
To obtain an on-line image of cells for continual monitoring of dimensions, a transmitted light image was directed through the bottom port of the microscope to a CCD camera (Sony Exwave HAD) (Figure 1). The use of 40× and 60× objective lenses for the fluorescence imaging studies required the positioning of a reduction lens (0.38× Optem, Qioptiq Linos, Inc., Fairport, NY, USA; Figure 1 and Figure S1) in front of the bottom port CCD camera to obtain a workable image on a video monitor. The CCD was then connected to a time base generator, video caliper for measurement of dimensions, video monitor and recordable digital video disk recorder (DVD). Trans-illumination was provided by a 100 W halogen light source passed through a 690 nm long pass filter to prevent spectral interference with the laser illumination and/or fluorescence emission signals. Approximately 90% of the transmitted light image was passed to the bottom port with the remaining light passed through the left-side port to one quadrant of the intensified CCD chip. This was accomplished by using a dichroic mirror without antireflective (AR) coating (Reflect 585/40 to 400 nm, Trans 690/40 to 720 No AR; Chroma Technology Corp, Bellows Falls, VT, USA). The latter image was collected for situations where it was required to overlay the fluorescence emission signals on the image of a cell preparation.
Image Analysis and Processing
In experiments where only changes in Em were assessed, images were collected at 10 Hz and 1024 × 1024 pixels (binning 1 × 1). For quantification of the FRET emission signals, a region of interest (ROI) was drawn around a given cell and fluorescence intensities within the ROI recorded. All signals were corrected for background fluorescence by subtraction of identical ROIs drawn across the respective quadrants. A pictorial representation of the procedure for processing the signals is shown in Figure S2. Changes in Em were measured as the change in FRET signal which was quantified as the ratio of fluorescence emission signals (F = emission460/emission560). Thus, the calculated FRET emission ratio = (donor molecule emission fluorescence−background)/(acceptor molecule emission fluorescence−background).
For combined measurements of changes in Em and Ca2+i, cells were alternatively exposed to 405 and 491 nm excitation using the AOTF. Images were collected at 10 Hz and 512 × 512 pixels (binning 2 × 2) with switching of excitation sources occurring at 1 second intervals. The acquired sequential images represent those obtained via excitation of the 405 and 491 nm lasers, respectively. These images were then partitioned into FRET and fluo-4 emission stacks and analyzed as described earlier. For measurement of changes in Ca2+i, cellular fluorescence was expressed as F/F0 where F = fluorescence level in response to a given stimulus and F0 = fluorescence level prior to the application of the stimulus. All images were corrected for background fluorescence. As an index of cellular contraction, cross-sectional area of the cell image plane was quantified. This was performed by a software routine where a threshold was applied to distinguish background from the cell image and a ROI was automatically drawn at the boundary of these regions (i.e., the perimeter of the cell). A pixel count was then performed on the baseline images to give an initial cell cross-sectional area of 100%. Subsequent images were then reported relative to this value.
Images were acquired using Quad In Vivo (Media Cybernetics, MD, USA) and Piper (Stanford Photonics, Palo Alto, CA, USA) software processed off-line using Image J (NIH, Bethesda, MD, USA).
Experimental Protocols
All experiments were performed in either multi-well cell chambers with glass bottoms (Lab Tek II, Nalge Nunc International Corp., IL, USA), or single well perfusion chambers designed for patch clamp studies. Experiments were performed at room temperature (22°C). KCl (10–60 mM), valinomycin (10 μM) and the myosin light chain kinase inhibitor, ML-7 (10 μM), were added directly to the chambers to achieve appropriate concentrations.
In experiments where set levels of membrane potential were applied to either HEK or freshly isolated SMC, cells were voltage clamped in the whole cell mode using a patch clamp amplifier (HEKA EPC-7, Germany). The bath solution contained (in mM): 140 NaCl, 5.4 KCl, 1.0 CaCl2, 1 MgCl2, 10 Glucose, 10 Hepes, 2 Na-pyruvate (pH 7.4). The pipette solution contained (in mM): 140 KCl, 8 NaCl, 2 EGTA, 3 Mg-ATP, 10 HEPES (pH 7.2); CaCl2 was added to bring free [Ca2+] to 100 nM. The cells were held at −70 mV and depolarization steps were applied from −50 to +30 mV in 20 mV increments, with duration of 5 seconds for each step. Signals were filtered at 3 kHz. Pipette tip resistances ranged from 4.0 to 5.0 MΩ. For cerebral artery SMC, those cells undergoing a marked change in morphology on initial patching (i.e., rounding up as opposed to maintaining an elongated shape) were removed from subsequent data analysis.
Chemicals
Unless stated otherwise, general chemicals and reagents were purchased from Sigma–Aldrich (St Louis, MO, USA). The FRET indicators were generously supplied by Dr Stephen Hess, Invitrogen (Madison, WI, USA) while fluo-4 was purchased from Invitrogen. ML-7 was purchased from Biomol (Woburn, MA, USA) and prepared as a stock solution in EtOH. Subsequent dilutions were made in physiological salt solution.
Statistical methods
Results are shown as mean ± SEM. Simple comparisons of two means were performed using the Student’s t-test while comparisons between more than two groups were performed using anova. Statistical significance was assumed at p < 0.05.
RESULTS
Loading of Donor and Acceptor Molecules and Demonstration of a Lack of Cellular Toxicity
Initial studies examined loading of various concentrations of the CC2-DMPE (1–30 μM) donor and DisBAC4(3) (1–10 μM) acceptor molecules to establish optimal loading conditions while avoiding toxicity. Nuclear propidium iodide staining, used as a marker of cellular damage, was not apparent in smooth muscle cells loaded with CC2-DMPE and DisBAC4(3) up to concentrations of 30 and 10 μM, respectively (see Figure S3). As a positive control, propidium iodide staining was performed on ethanol-treated SMC (Figure S3). On the basis of an apparent lack of cellular toxicity, a measurable fluorescence signal, and the available literature [23,25] optimal loading was judged to occur at CC2-DMPE (5 μM) and DisBAC4(3) (3 μM) for 1 hour at room temperature with CC2-DMPE and DisBAC4(3) being loaded simultaneously.
Demonstration of FRET Emission Signal
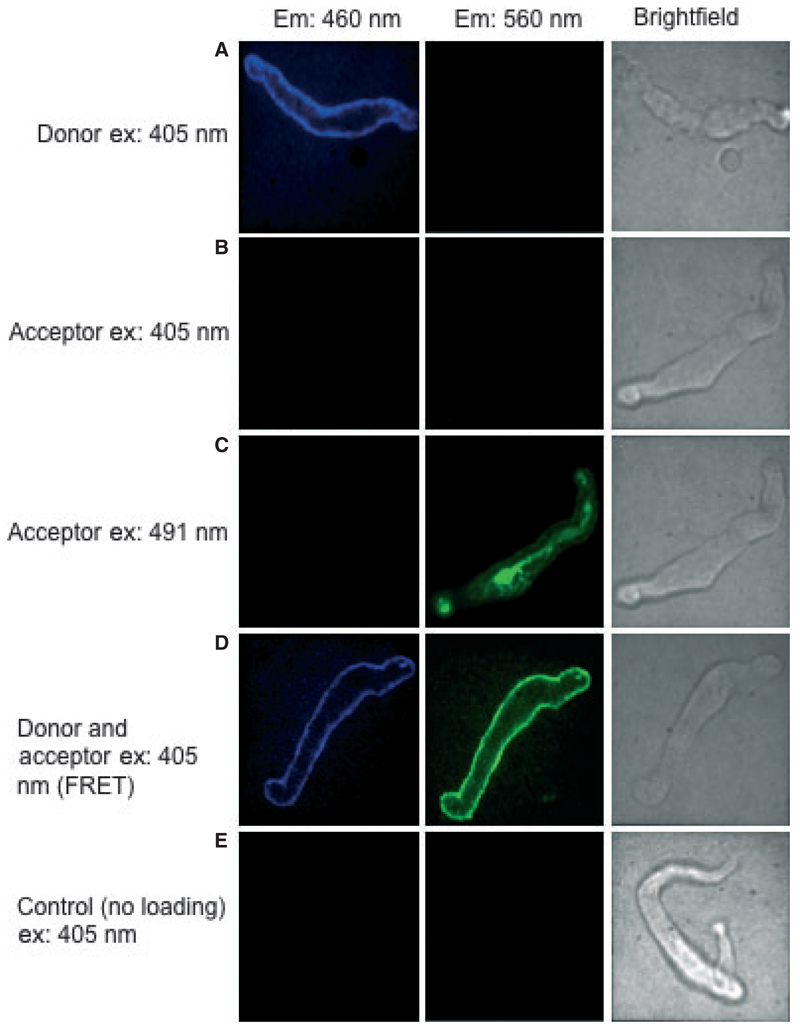
To establish the presence of a FRET signal and to examine potential bleed-through between detector windows, freshly isolated cremaster arteriolar SMC were loaded with CC2-DMPE (donor) alone; DisBAC4(3) (acceptor) alone; or the combination of the FRET pair. As shown in Figure 2, illumination of cells loaded with CC2-DMPE alone and excited by the 405 nm laser showed an emission image in the donor channel (460 nm; Figure 2, Row A) but not in the acceptor channel (580 nm; Figure 2, Row A). DisBAC4(3)-loaded cells illuminated with the 405 nm laser showed no apparent emission image (Figure 2, Row B) while producing an image in response to the 491 nm laser (Figure 2, Row C). In contrast, when cells loaded with both donor and acceptor were excited at 405 nm, an image was observed in the DisBAC4(3) emission channel indicative of FRET (Figure 2; Row D). Control cells not loaded with either indicator did not fluoresce in response to excitation at 405 nm and hence no images were detected at either emission wavelength (Row E). These studies were also performed in cultured cremaster arteriolar SMC to demonstrate the utility of the loading procedure in both freshly prepared and cultured cells (Figure S4).
Figure 2.
Demonstration of a specific FRET signal in freshly isolated cremaster smooth muscle cell (SMC). Cells were loaded with CC2-DMPE (donor; 5 µM) alone, DisBAC4(3) (acceptor; 3 μM) alone or a combination of the two molecules (for details see text). The figure shows pseudo-colored images (quadrants) taken from the original Quadview images. Excitation at 405 nm was shown to directly excite only the donor molecule (compare images in Rows A and B). In contrast, direct excitation of the acceptor was demonstrated with the 491 nm laser (Row C). In cells loaded with both fluorophores, FRET could be demonstrated following excitation at 405 nm (Row D). Control cells not loaded with either indicator showed no detectable fluorescence in response to excitation at 405 nm (Row E).
Measurement of FRET Emission Ratio in Voltage-Clamped HEK and VSM Cells
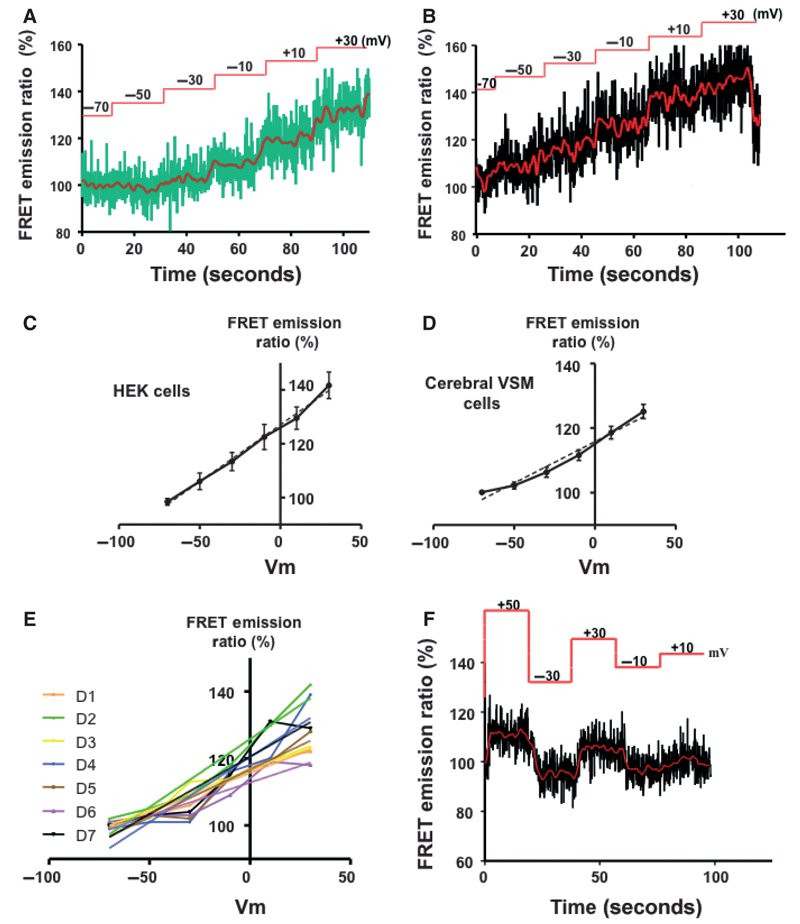
To quantify the relationship between the FRET signal and Em, FRET images were acquired from CC2-DMPE (5 μM) and DisBAC4(3) (3 μM) loaded cells held at different Em using whole cell voltage clamp. A linear relationship in the FRET emission ratio was observed, over an Em range from −70 to + 30 mV, both in cultured HEK (Figure 3A) and freshly isolated cerebral artery SMCs (Figure 3B) cells. Linear relationships (X = Em and Y = FRET ratio) were calculated as Y = 0.42X + 127.1 (r2 = 0.99) and Y = 0.30X + 115.8 (r2 = 0.98) for HEK and SMC, respectively. Change in FRET emission ratio over a holding potential of −70 to +30 mV was 41.7 ± 4.9 for HEK cells and 30.0 ± 2.3 for the SMC. Figures 3C and 3D, show the individual normalized regression relationships for two HEK (n = 16 cells) and seven cerebral SMC (n = 23 cells) preparations, respectively, studied on separate days. In HEK cells slope values varied from 0.18 to 0.78 (mean 0.49 ± 0.07, (n = 16)) and r2 values from 0.80 to 0.99 (mean 0.96 ± 0.025) suggesting a high degree of reproducibility. Similarly, in freshly prepared cerebral vascular SMCs, slope values varied from 0.15 to 0.46 (mean 0.30 ± 0.02 (n = 23)) and r2 values from 0.77 to 0.99 (mean 0.98 ± 0.02) (Figure 3E).
Figure 3.
Demonstration of a linear relationship between membrane potential (from −70 to +30 mV) and fluorescent signal. Studies were performed in both HEK cells (A, C) and freshly isolated cerebral VSM cells (B, D). Membrane potential was clamped at given levels using whole cell patch clamp techniques. Example tracings are shown in A and B with the redline showing a smoothed/averaged representation of the raw signal. Smoothing was accomplished by averaging the four measurements either side of a given data point. Results in C and D provide the group data and are shown as mean ± SEM. The solid lines show simple interpolation between data points while the dotted lines represent a linear fit to the data sets (see text for further details). Panel E illustrates day-to-day consistency of measurements. Two lines are given for each data set; showing raw data and a linear fit. Panel F illustrates temporal relationships between step changes in membrane holding potential and the measured FRET signals and further shows that the signal was reversible and not dependent on sequential application of the voltage steps.
To further examine the dynamic responsiveness of the FRET-based Em measurements, membrane potential was altered, via the patch pipette, in acute steps both in the negative and positive directions. Example responses are shown in Figure 3F where data from individual steps in holding potential are plotted against a fluorescence ratio–membrane voltage regression relationship constructed from sequential changes in potential (−70 to +50 mV). These data also showed that the measured changes in Em were reversible on return to the holding potential.
Measurement of Changes in FRET Emission Ratio Following KCl-Induced Depolarization; Effect of Cellular Contraction
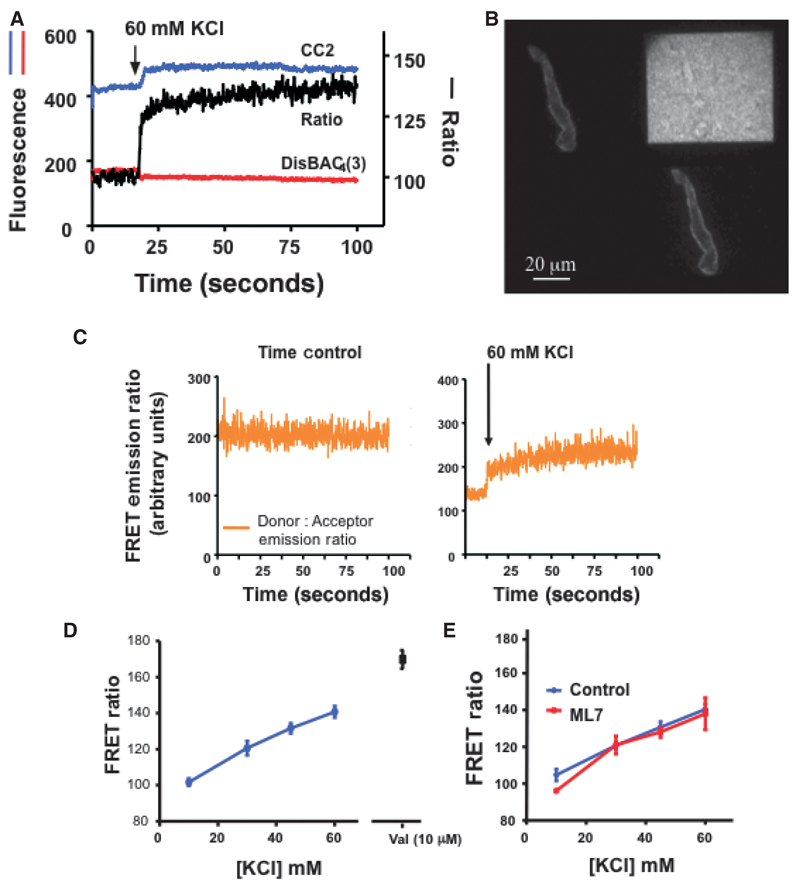
To demonstrate changes in FRET signals with a stimulus known to affect changes in Em, SMC loaded with CC2-DMPE (5 μM) and DisBAC4(3) (3 μM) were exposed to increasing concentrations of KCl (10, 30, 45 and 60 mM; Figure 4A–D). At the end of selected concentration– response protocol the K+ ionophore, valinomycin (10 μM) was added to demonstrate a maximal response on disruption of the K+ gradient. Time controls demonstrated stability of the emission and FRET ratio signals in the absence of stimulation (Figure 4C).
Figure 4.
FRET-based Em measurements in cremaster VSM cells following depolarization with KCl. Panel A shows example signals obtained following stimulation of a VSM cell with 60 mM KCl. Signals of the two fluorochromes are given on the left hand axis and the calculated FRET emission ratio on the right. Panel B shows a representative image as viewed on the computer monitor. Panel C shows a time control demonstrating the stability of the emission and FRET ratio signals in the absence of stimulation; a KCl-stimulated cell from the same cell isolation is shown for comparison. Figure D shows group FRET ratio data (expressed as a percentage of baseline fluorescence levels) to increasing concentrations of KCl (n = 63 cells from nine preparations) and the ionophore valimomycin (10 μM; n = 7 cells from three preparations). Panel E illustrates that preventing contraction with ML-7 (10 µM) does not substantially alter the FRET ratio (% baseline) signaling compared to that in the absence of the inhibitor (n = 4 cells in each group). Group data are shown as mean ± SEM.
To determine whether movement associated with KCl-induced contraction altered the fluorescence signal, control experiments were performed in the presence of the myosin light chain kinase inhibitor, ML-7 (10 μM). Preventing contraction did not markedly affect the FRET emission signal consistent with the ratio calculation being independent of movement artifact (Figure 4E).
Measurement of FRET Emission Ratio and Changes in Ca2+i in VSM Cells Following KCl-Induced Depolarization
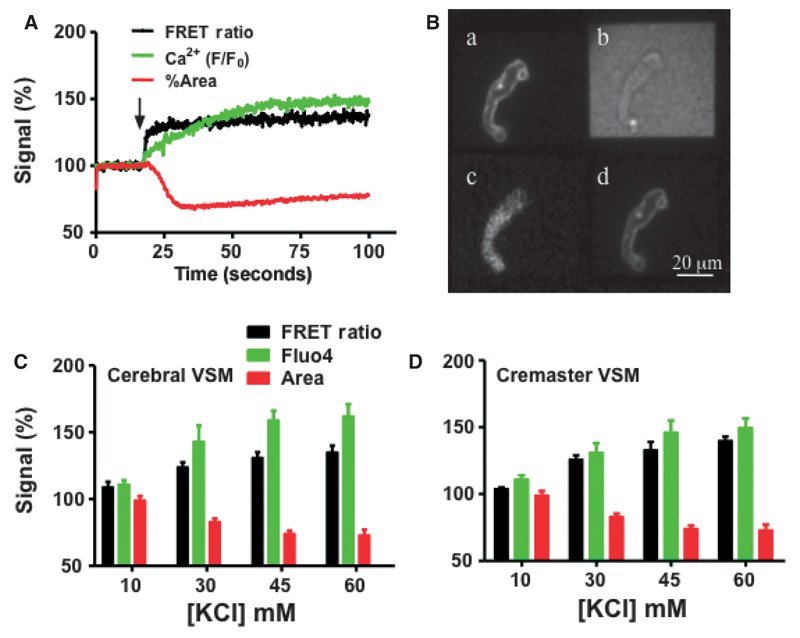
Preliminary studies demonstrated that fluo 4 loading did not alter the ability of the FRET indicators to detect KCl-induced changes in Em (Figure S5). Further, these experiments confirmed that the simultaneous loading of the three indicators did not impact cell viability within the context of our experimental protocols. Example tracings and group data for concurrent measurement of changes in Em, Ca2+i and contraction are shown in Figure 5. Contractile activity was assessed by changes in cell area calculated from the wide field images. Consistent with our understanding of the temporal nature of KCl-induced contraction in smooth muscle, acute exposure to KCl (60 mM) caused depolarization followed by a rise in Ca2+i and a decrease in cell area (Figure 5A). An example merged screen image representing excitation at 405 nm followed by excitation at 491 nm is also shown in Figure 5B. Responses were further shown to be KCl concentration-dependent and similar in cerebral and cremaster arteriolar myocytes (see Figure 5C,D for group data).
Figure 5.
Membrane potential (Em), Ca2+ and cell image cross-sectional area (measure of contraction) responses to increasing concentrations of KCl.
(A) representative trace of a cremaster SMC showing responses to 60 mM KCl applied at the arrow. For each signal data have been normalized such that baseline values equal 100%. (B) shows a merged picture of images collected following sequential 405 and 491 nm excitation. Panels A and D show the donor and acceptor fluorescence emission, respectively, in response to excitation at 405 nm. Panel C shows fluo-4 emission following excitation at 491 nm while Panel B shows the transmitted light image. (C and D) Group data showing responses of cremaster cerebral SMC, respectively. Steady-state data were calculated for each signal and normalized such that baseline values equaled 100%. Individual data points for each KCl concentration were obtained by averaging the final 5 seconds of the recording period as shown in A. For these grouped data results are shown as mean ± SEM.
DISCUSSION
A change in membrane potential plays a vital role in many vasomotor responses, being an early signaling event in pathways leading to vasoconstriction and vasodilation following either agonist or mechanical stimulation. Such changes are particularly relevant to resistance arteries and arterioles, where resting Em of vascular smooth muscle (at physiological levels of intraluminal pressure) is typically within the range from −30 to −40 mV [1,4,5], allowing both hyperpolarization and additional depolarization to affect changes in ion channel activity and consequent levels of vascular tone. Further, changes in membrane potential are important to endothelial cell function, contributing to phenomena such as endothelial-dependent dilation and propagated vasomotor responses [18,31–33]. The present study, therefore, examined the application of a FRET-based imaging approach for measurement of changes in Em in freshly isolated vascular SMC, with emphasis on ease of use and ability to follow changes during active contractile responses. A further objective was to combine different imaging techniques so that Em measurements could be performed in conjunction with measurements of changes in global Ca2+i and cell dimensions.
The FRET-based approach for assessing changes in Em has been previously employed in high throughput screening and drug discovery applications using cell lines (for example HEK cells) [24,34] as well as in cultured keratinocytes [23] and freshly isolated pancreatic islets [25]. To our knowledge, the current study is the first utilizing contractile cells and specifically those of the arteriolar wall. Importantly, the present studies showed that the fluorescence emission ratio approach for analyzing the FRET signals was not affected by contraction and is therefore suitable for use in mechanically active preparations.
The most common approach for direct measurement of Em in functional SMC preparations such as cannulated arterioles, including studies of our own, has involved the use of sharp glass intracellular electrodes [1,3–5]. However, difficulties arise when contraction occurs as the glass electrodes can easily be dislodged thus making temporal responses difficult to investigate. Further, microelectrodes are seldom used in studies of isolated SMC with most studies preferring to use patch pipettes in the current clamp mode [35,36]. As an alternative, a number of investigators have used fluorescent indicator-based approaches. These have typically involved single wavelength dyes including bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)) and di-8-ANEPPS [37]. The latter indicator has been adapted for use as a ratiometric dye to negate image-based inaccuracies associated with indicator distribution during cell/tissue contraction. Overall, these approaches have tended to be limited in applicability due to either the indicators being inherently slow to reach a steady-state or requiring a large change in Em to give a measurable change in fluorescence.
More recently several different approaches have been developed for an image-based method for measurement of Em. For example, studies in neurons have examined the use of anellated hemicyanine dyes (ANNINE-6 and ANNINE-6plus) as voltage-sensitive fluorescence probes [38–40]. These single wavelength indicators demonstrate both rapid (millisecond time frame) responses to changes in Em and high-voltage sensitivity. In regard to studies of SMC, the use of ANNINE-6 has been restricted to the measurement of electrotonic signal spread in afferent arterioles [41]. Despite the attractive properties of this indicator the single wavelength approach presents difficulties in contracting tissue as compared to a ratiometric method. An additional approach being actively pursued involves FRET between genetically expressed voltage-sensitive protein molecules [42,43]. While these studies have also been directed toward neurophysiology, they emphasize the interest in the development of optical methods for measurement of Em.
The FRET-based approach used in the current studies involved the relatively simple loading of a commercially available donor/acceptor pair, which appeared to not adversely affect cell viability and was not limited by dye loss or photobleaching during the course of our experiments. Further, the FRET-based Em signals were found to be reproducible and reversible. In particular, the slope of the relationship between fluorescence signal and membrane-holding potentials was consistent between cellular preparations and between days on which experiments were conducted. To provide a quantitative approach to assess variability we calculated slope and r2 values for data sets subjected to linear regression. A linear relationship in fluorescence signal was also observed in previous studies of mouse pancreatic β cells [25] although the apparent magnitude of change in fluorescence signal (relative to holding potential) appeared greater than in the present study. Such differences in absolute levels of fluorescence might be expected due to differences in the extent of dye loading between preparations and variation in instrumentation. It is also possible that differences in membrane properties between cell types may result in variation of the exact linear fluorescence relationship that we have described (compare Figure 3C,D for comparison of HEK and cerebral vascular smooth muscle cells).
In regard to sensitivity of the fluorescence-based measurements of Em, the change in FRET signal for a given change in Em obtained in this study appears to exceed that of other published studies using fast dyes in arteriolar SMC. Based on the data from patch clamped cerebral vascular SMC we observed a 0.29% change in FRET signal/mV in applied Em. Chen and Rivers reported an estimated 0.15% change in fluorescence/mV using exogenous KCl and applying the Nernst equation. Similarly, using di 8 ANEPPS in endothelial cells Beach et al. [18] reported a 0.1% change in fluorescence/mV change in Em. The use of KCl in this context, however, may suffer from a number of conceivable methodological difficulties. Indeed, considering our own data we appear to obtain relatively greater fluorescent signals from KCl as might be predicted from the voltage clamp approach. Thus, KCl may exert effects beyond simply changing Em including depolarization of other tissues (particularly in more complex preparations), such as nerves, which release vasoactive agents and by having affects on transporters such as the Na+K+ ATPase. Further, a calibration based on application of exogenous KCl can be limited by not knowing exact intra-cellular [K+].
An additional consideration for the usefulness of the FRET approach for measuring changes in Em in vascular smooth muscle is the time taken for the signal to reach a steady-state. Published data have shown that the indicator pair used in the present studies provides temporal responsiveness of approximately 4 measurements per second. This can be increased by using an oxonol with a longer alkyl carbon chain length [24], however, the increase in speed of the dye is offset by a decrease in sensitivity [24]. As shown in Figure 3E, the FRET signals, in both HEK and SMCs, rapidly changed in response to applied steps in membrane holding potential. Steady-state measurements were typically achieved within 5 seconds of changing Em consistent with previously published studies using the CC2-DMPE - Dis-BAC4(3) FRET indicators [23,25,26]. This time course is in a range that would be useful for physiological measurements of Ca2+and Em in vascular cells.
An important consideration is whether or not the FRET-based signals can, or should be, calibrated in an attempt to provide Em measurements in absolute terms of mV. Approximations could conceivably be made based on the relationship between holding potential, under patch clamp, and the FRET emission ratio. However, direct comparisons between these data and other approaches that evoke changes in Em (for example, depolarization by exogenous K+) assume that all factors impacting the behavior of the indicators are constant. An alternate approach is to apply a two point calibration curve as used by Cohen and Jackson [44] to calibrate Em signals using the fluorescent indicator di-8-ANEPPS. In this approach fluorescence values were collected in the presence of the K+ ionophore valinomycin (to negate the K+ gradient) and both low (5 mM) and high (145 mM) K+. Using this method, Cohen and Jackson [44] reported that a 1% change in di-8-ANEPPS fluorescence signal (calculated as 450/510 nm ratio) corresponded to a 8.8 mV change in Em. Alternatively the FRET data, as obtained in the present study, can simply be considered in relative terms to follow the temporal nature of changes in Em.
An advantage of our approach for studying vascular cells, particularly SMC, is the ability to measure changes in Em, intracellular Ca2+, and cellular dimensions in the same preparation. Importantly, this enables the measurement of several variables related to the contractile process with high temporal resolution. Similarly, the concurrent measurement of changes in Em and Ca2+i in pancreatic β cells was studied in relation to islet cell function and the mechanisms underlying insulin production [25]. A further advantage is that measurements can be performed on multiple cells within a field of view without the difficulty of having to use multiple glass recording electrodes [45].
The use of fluorescent vital dyes in contracting cells often presents problems for analysis. For example, contraction results in a change in the volume within which a cytosol-loaded indicator is distributed and similarly while membrane folding may conceivably impact on membrane-loaded molecules. While the use of ratiometric approaches is often used to circumvent such problems (and is used in the current FRET-based approach: donor emission/acceptor emission), we also compared Em changes in the absence and presence of an inhibitor of contraction. To accomplish this we used the myosin light chain kinase inhibitor, ML-7, as this agent inhibits contraction distally to changes in Em and Ca2+i [46]. As the KCl-induced changes in the FRET ratio signals were not significantly different we conclude that an artifact related to contraction, per se, did not affect the measurement of Em.
Forster (or fluorescence) resonance energy transfer (FRET)-based measurements can be complicated by overlap in the spectral characteristics of the donor and acceptor molecules; particularly when there is direct excitation of the acceptor molecule at donor excitation wavelengths. Under the conditions of our experiments, and with the imaging instrumentation used, we did not observe a significant level of direct excitation of the DisBAC4(3). In contrast, the 491 nm laser source used to excitate fluo-4 for the Ca2+ measurements caused direct excitation of the Dis-BAC4(3) molecule. Therefore, to circumvent this problem the computer controlled AOTF was used to switch between lasers (405 and 491 nm) such that Em and Ca2+ measurements were collected in an alternating pattern.
While the aim of this study was to develop a method for measuring changes in Em in studies of acutely dispersed vascular smooth muscle cells, during agonist and mechanical stimulation, its general applicability may be increased by extending its use to more intact vascular preparations. In this regard, we have observed adequate indicator loading and resultant FRET signals in partially digested sheets of cells (data not shown). Use in the intact arteriole preparation will require consideration of barriers to indicator loading such as those that result from the physical and charge properties of the adventitia and matrix elements.
In summary a FRET-based approach for measuring changes in Em in isolated SMC cells has been described. This method provides an alternative to glass electrode measurements which are often limited in mechanically active cells and tissues because movement interferes with impalement. Further, this method appears to be superior to previous image-based approaches in that it provides both reasonable speed and sensitivity (as evidenced by a change in fluorescence for a given change in Em). Importantly, the ratiometric approach does not appear to be markedly affected by cell contraction. The described method also enables image-based measurement of changes in intracellular Ca2+ and cellular dimensions allowing multiple variables to be examined in the one preparation and thus extending its utility in studies of SMC contractile signaling.
Supplementary Material
ACKNOWLEDGEMENTS
Design and implementation of the imaging system required the input of a number of people. We would like to thank Dr Andrea Trache (Texas A&M University) for contributing to the design of filters/dichroic mirrors, Dr Richard Heil-Chapdelaine (Hitschfel Instruments, MO) for assembly of the microscope and design of the light paths, Dr George Peeters (Solamere Technologies, UT) for designing the AOTF and configuring the laser control system and Dr Michael Buchon (Stanford Photonics, CA) for assistance with issues relating to software. Sincere thanks are also given to Dr Stephen Hess (Invitrogen, WI) for supplying the FRET indicators and to Dr Zhe Sun, University of Missouri who provided the cultured cremaster SMC. MAH (HL-92241), GAM (P01 HL095486)_and MJD (P01 HL095486) are supported by grants from NIH (NHLBI).
Abbreviations used
- CCD
Charge couple device
- FRET
Forster (Fluorescence) energy transfer
- Ca2+i
Intracellular Ca2+
- LP
Long pass filter
- Em
Membrane potential
- SMC
Smooth muscle cell
- VSM
Vascular smooth muscle
- HEK
Human embryonic kidney (cells)
References
- 1.Beach JM, McGahren ED, Xia J, Duling BR. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am J Physiol. 1996;270:H2216–2227. doi: 10.1152/ajpheart.1996.270.6.H2216. [DOI] [PubMed] [Google Scholar]
- 2.Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca(2+) entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- 3.Burgstahler R, Koegel H, Rucker F, Tracey D, Grafe P, Alzheimer C. Confocal ratiometric voltage imaging of cultured human keratinocytes reveals layer-specific responses to ATP. Am J Physiol Cell Physiol. 2003;284:C944–952. doi: 10.1152/ajpcell.00053.2002. [DOI] [PubMed] [Google Scholar]
- 4.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Rivers RJ. Measurement of membrane potential and intracellular Ca(2+) of arteriolar endothelium and smooth muscle in vivo. Microvasc Res. 2001;62:55–62. doi: 10.1006/mvre.2001.2315. [DOI] [PubMed] [Google Scholar]
- 6.Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells. Microcirculation. 2005;12:169–182. doi: 10.1080/10739680590904973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criddle DN, Greenwood IA, Weston AH. Levcromakalim-induced modulation of membrane potassium currents, intracellular calcium and mechanical activity in rat mesenteric artery. Naunyn Schmiede-bergs Arch Pharmacol. 1994;349:422–430. doi: 10.1007/BF00170890. [DOI] [PubMed] [Google Scholar]
- 8.Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285:H119–126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 9.Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol. 1995;46:463–506. doi: 10.1016/0301-0082(95)00010-s. [DOI] [PubMed] [Google Scholar]
- 10.Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol. 2002;283:H102–109. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- 11.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 12.Fromherz P, Hubener G, Kuhn B, Hinner MJ. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur Biophys J. 2008;37:509–514. doi: 10.1007/s00249-007-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez JE, Maher MP. Cellular fluorescent indicators and voltage/ion probe reader (VIPR) tools for ion channel and receptor drug discovery. Receptors Channels. 2002;8:283–295. [PubMed] [Google Scholar]
- 14.Gonzalez JE, Tsien RY. Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys J. 1995;69:1272–1280. doi: 10.1016/S0006-3495(95)80029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez JE, Tsien RY. Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem Biol. 1997;4:269–277. doi: 10.1016/s1074-5521(97)90070-3. [DOI] [PubMed] [Google Scholar]
- 16.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- 17.Harder DR. Pressure-induced myogenic activation of cat cerebral arteries is dependent on intact endothelium. Circ Res. 1987;60:102–107. doi: 10.1161/01.res.60.1.102. [DOI] [PubMed] [Google Scholar]
- 18.Harder DR. Increased sensitivity of cat cerebral arteries to serotonin upon elevation of transmural pressure. Pflugers Arch. 1988;411:698–700. doi: 10.1007/BF00580870. [DOI] [PubMed] [Google Scholar]
- 19.van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548:271–296. doi: 10.1113/jphysiol.2002.033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CJ, Harootunian A, Maher MP, Quan C, Raj CD, McCormack K, Numann R, Negulescu PA, Gonzalez JE. Characterization of voltage-gated sodium-channel blockers by electrical stimulation and fluorescence detection of membrane potential. Nat Biotechnol. 2006;24:439–446. doi: 10.1038/nbt1194. [DOI] [PubMed] [Google Scholar]
- 21.Jackson WF. Hypoxia does not activate ATP-sensitive K+ channels in arteriolar muscle cells. Microcirculation. 2000;7:137–145. [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 23.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1326–1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn B, Denk W, Bruno RM. In vivo two-photon voltage-sensitive dye imaging reveals top-down control of cortical layers 1 and 2 during wakefulness. Proc Natl Acad Sci U S A. 2008;105:7588–7593. doi: 10.1073/pnas.0802462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn B, Fromherz P, Denk W. High sensitivity of Stark-shift voltage-sensing dyes by one- or two-photon excitation near the red spectral edge. Biophys J. 2004;87:631–639. doi: 10.1529/biophysj.104.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznetsov A, Bindokas VP, Marks JD, Philipson LH. FRET-based voltage probes for confocal imaging: membrane potential oscillations throughout pancreatic islets. Am J Physiol Cell Physiol. 2005;289:C224–229. doi: 10.1152/ajpcell.00004.2005. [DOI] [PubMed] [Google Scholar]
- 28.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 29.Marsh DJ, Toma I, Sosnovtseva OV, Peti-Peterdi J, Holstein-Rathlou NH. Electrotonic vascular signal conduction and nephron synchronization. Am J Physiol Renal Physiol. 2009;296:F751–761. doi: 10.1152/ajprenal.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGahren ED, Beach JM, Duling BR. Capillaries demonstrate changes in membrane potential in response to pharmacological stimuli. Am J Physiol. 1998;274:H60–65. doi: 10.1152/ajpheart.1998.274.1.H60. [DOI] [PubMed] [Google Scholar]
- 31.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol. 1991;261:H950–959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- 32.Montana V, Farkas DL, Loew LM. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry. 1989;28:4536–4539. doi: 10.1021/bi00437a003. [DOI] [PubMed] [Google Scholar]
- 33.Mutoh H, Perron A, Dimitrov D, Iwamoto Y, Akemann W, Chudakov DM, Knopfel T. Spectrally-resolved response properties of the three most advanced FRET based fluorescent protein voltage probes. PLoS One. 2009;4:e4555. doi: 10.1371/journal.pone.0004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neylon CB, Avdonin PV, Dilley RJ, Larsen MA, Tkachuk VA, Bobik A. Different electrical responses to vasoactive agonists in morphologically distinct smooth muscle cell types. Circ Res. 1994;75:733–741. doi: 10.1161/01.res.75.4.733. [DOI] [PubMed] [Google Scholar]
- 35.Perron A, Mutoh H, Akemann W, Gautam SG, Dimitrov D, Iwamoto Y, Knopfel T. Second and third generation voltage-sensitive fluorescent proteins for monitoring membrane potential. Front Mol Neurosci. 2009;2:5. doi: 10.3389/neuro.02.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potocnik SJ, McSherry I, Ding H, Murphy TV, Kotecha N, Dora KA, Yuill KH, Triggle CR, Hill MA. Endothelium-dependent vasodilation in myogenically active mouse skeletal muscle arterioles: role of EDH and K(+) channels. Microcirculation. 2009;16:377–390. doi: 10.1080/10739680902804042. 1 p following 390. [DOI] [PubMed] [Google Scholar]
- 37.Sguilla FS, Tedesco AC, Bendhack LM. A membrane potentialsensitive dye for vascular smooth muscle cells assays. Biochem Biophys Res Commun. 2003;301:113–118. doi: 10.1016/s0006-291x(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 38.Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1634–1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 39.Ungvari Z, Koller A. Mediation of EDHF-induced reduction of smooth muscle [Ca(2+)](i) and arteriolar dilation by K(+) channels, 5,6-EET, and gap junctions. Microcirculation. 2001;8:265–274. doi: 10.1038/sj/mn/7800080. [DOI] [PubMed] [Google Scholar]
- 40.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178–186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 41.Wolff C, Fuks B, Chatelain P. Comparative study of membrane potential-sensitive fluorescent probes and their use in ion channel screening assays. J Biomol Screen. 2003;8:533–543. doi: 10.1177/1087057103257806. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276:30285–30292. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- 43.Wu BN, Luykenaar KD, Brayden JE, Giles WR, Corteling RL, Wiehler WB, Welsh DG. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H1085–1094. doi: 10.1152/ajpheart.00926.2006. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Ella SR, Murphy TV, Grayson TH, Hwang Y, Peichun G, Braun AP, Korhuis RJ, Davis MJ, Hill MA. Relative lack of β1-subunit-mediated regulation of BKCa in cremaster arteriolar smooth muscle. J Physiol. 2009;587:3025–3044. doi: 10.1113/jphysiol.2009.169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic S, Zecevic D. Imaging membrane potential with voltage-sensitive dyes. Biol Bull. 2000;198:1–21. doi: 10.2307/1542798. [DOI] [PubMed] [Google Scholar]
- 46.Zou H, Ratz PH, Hill MA. Role of myosin phosphorylation and [Ca2+]i in myogenic reactivity and arteriolar tone. Am J Physiol. 1995;269:H1590–1596. doi: 10.1152/ajpheart.1995.269.5.H1590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.