Abstract
Scope
The potential benefit of vitamin K as a therapeutic in osteoporosis is controversial and the vitamin K regimen being used clinically (45 mg/day) employs doses that are many times higher than required to ensure maximal gamma-carboxylation of the vitamin K-dependent bone proteins. We therefore tested the hypothesis that vitamin K catabolites, 5-carbon (CAN5C) and 7-carbon carboxylic acid (CAN7C) aliphatic side-chain derivatives of the naphthoquinone moiety exert an osteotrophic role consistent with the treatment of osteoporosis.
Methods and results
Osteoblast-like MG63 cell cultures were challenged with lipopolysaccharide and the levels of interleukin-6, an osteoclastogenic cytokine, measured with and without catabolites; low concentrations of CAN7C significantly inhibited interleukin-6 release, but CAN5C did not. In models of bone loss induced by ovariectomy or sciatic neurectomy in C57BL/6 mice, we found that the rarer CAN7C catabolite markedly restricted ovariectomy-induced bone loss and possibly limited sciatic neurectomy-induced bone loss. CAN7C activity depends on a free carboxylic acid and its particular side-chain structure.
Conclusion
These in vivo data indicate for the first time that the clinical utility of vitamin K for osteoporosis may reside in an unusual catabolite.
Keywords: Bone loss, Catabolite, Naphthoquinone, Ovariectomy, Vitamin K
1 Introduction
Vitamin K is required for the post-translational gamma-carboxylation of specific glutamic acid residues in several bone proteins. These observations initiated investigations into the potential role of vitamin K in skeletal tissue development and homeostasis and its use in the treatment of osteoporosis [1, 2]. Clinical therapeutic use of vitamin K in osteoporosis has predominately been used in the Far East, and in particular Japan, as a high-dose regimen consisting of 45 mg/day menaquinone-4; a vitamin K2 homologue. This dose was found clinically to be the lowest effective therapeutic dose [3]. Although the significance of this therapeutic modality for bone mineral density benefit remains controversial, the reduction of fracture incidence supports its value as a clinical tool in the management of osteoporosis [4–6]. In contrast, other studies have shown no beneficial impact of vitamin K therapy on skeletal health [7–9] and its therapeutic value has been called into question until definitive investigations have been completed [10].
An intriguing paradox emerges, however, when the potential therapeutic mechanisms of high-dose vitamin K are considered. It is clear, for instance, that the dose used to treat osteoporosis in Japan is significantly greater than is required to achieve maximal post-translational gamma-carboxylation of the transformable glutamyl residues in the bone protein osteocalcin [11]. Similarly, we have found in a cohort of mature healthy women that administration of 300 µg vitamin K1 can reduce the levels of undercarboxylated osteocalcin down to those commonly found in younger adult women (Hodges and Soper, unpublished data). This indicates that high-dose vitamin K may have an alternative mechanism of action in skeletal biology that is unrelated to the classical biochemical function of vitamin K and gamma-carboxyglutamic acid formation, and suggests a novel mechanism is potentially responsible for the beneficial effects of high-dose vitamin K in Japanese osteoporotic patients.
Vitamin K is subject to metabolic processing involving an initial ω-oxidation of its aliphatic side chain, followed by successive β-oxidation steps to generate short branch-chained aliphatic carboxylic acid side chain at position 3 of the naphthoquinone [12–15]. This area has been recently been the subject of a thorough review by Harrington et al. [16].
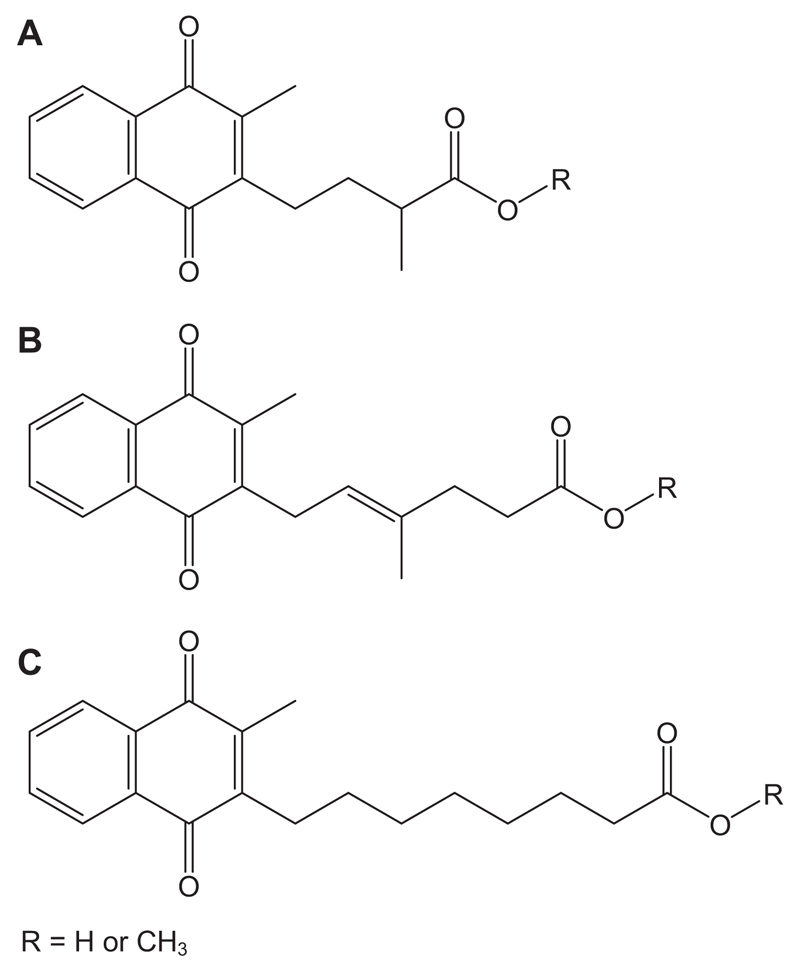
Nutritional vitamin K intake metabolism produces mostly a 5-carbon, branched side chain, aliphatic carboxylic acid at position 3 on the 2-methyl-1,4-naphthoquinone moiety (CAN5C) and notably lesser amounts of a 7-carbon aliphatic carboxylic acid homologue (CAN7C) (Fig. 1) [12–14]. Levels of CAN7C are substantially increased, however, following pharmacological doses of vitamin K1 or K2 [14]. These data have led us to hypothesize that vitamin K catabolites, namely CAN5C and CAN7C, exert actions in bone and provide an alternative mechanism of action for high-dose vitamin K therapy. We explored this hypothesis by examining whether these vitamin K catabolites can modify agonist-induced production of interleukin-6 (IL-6), a known osteoclastogenic cytokine implicated in the pathophysiology of osteoporosis [17–20], in osteoblast-like cells (MG63) in vitro and by determining their in vivo effects in two recognised murine models of bone loss induced by either ovariectomy (OVX) or neurectomy. Our studies indicate that the CAN7C catabolite of vitamin K suppresses IL-6 production by osteoblast-like cells in vitro and exerts a protective role against OVX-induced bone loss.
Figure 1.
Naphthoquinone compounds used to investigated the potential role of this class of molecules in bone biology. (A) The principal 5-carbon carboxylic acid natural metabolite of vitamin K (R = H). (B) The less-abundant 7-carbon carboxylic acid natural metabolite of vitamin K (R = H). (C) A synthetic octyl aliphatic 2-methyl-1,4-naphthoquinone carboxylic acid not found in nature. The methyl esters of these compounds are not found naturally.
2 Materials and methods
2.1 Naphthoquinone compound syntheses
The natural CAN5C, CAN7C catabolites of vitamin K and a novel straight chain 8-carbon aliphatic carboxylic acid 2-methyl-1,4-naphthoquinone compound (CAN8C; a more hydrophobic homologue not found in nature) as well as the methyl ester form of each (CAN5C-Me, CAN7C-Me and CAN8C-Me; Fig. 1) were synthesised using standard chemical procedures. All chemicals and materials were purchased from Sigma-Aldrich, Dorset, UK unless otherwise stated.
Synthesis involved joining respective side chains to a cyclopentadiene-naphthoquinone adduct, formed by a Diels–Alder reaction. A retro Diels–Alder reaction was used to produce the methyl esters, which were subsequently each enzymatically hydrolysed to yield the corresponding carboxylic acids.
The side chain for CAN7C was produced through a multistep synthesis. Briefly, the alcohol group of 3-methylbut-2-ene-1-ol was protected with tert-butyldimethylsilylchloride, then meta-chloroperoxybenzoic acid was used to epoxidate this protected alcohol, the resultant tert-butyl-(3,3-dimethyloxiranylmethoxy)dimethylsilane was subsequently converted to tert-butyldimethyl-(2-hydroxy-3-methylbut-3-enyloxy)silane by regioselective thermal rearrangement. Production of (E)-methyl 6-(tert-butyldimethylsilanyloxy)-4-methylhex-4-enoate was achieved by a Claisen rearrangement using trimethylorthoacetate and proprionic acid. The tert-butyldimethylsilane group was then cleaved from the ester using tetrabutylammonium fluoride to yield the alcohol. The final bromination step in the side-chain synthesis was performed using carbon tetrabromide and triphenylphosphine to yield (E)-methyl 6-bromo-4-methylhex-4-enoate.
The synthesis of the 5-carbon carboxylic acid side chain was initiated by the production of methyl-4-bromo-2(R,S)-methylbutanoate from 2(R,S)-methyl-4-butyrolactone using phosphorus tribromide and methanol. The resultant bromide was converted into methyl-4-iodo-2(R,S)-methylbutanoate by a Finkelstein reaction using sodium iodide in dry acetone. The 8-carbon carboxylic acid side chain was synthesised using essentially the same method except that 8-bromooctanoic acid was used as the starting material.
Each of these compounds was purified by sequential column chromatography and their authenticities and purities verified by NMR spectroscopy on a Jeol EX 270 MHz spectrometer and mass spectra obtained using VG Biotech Quattrol 2 instrument and confirmed by HPLC with electrochemical detection. The compounds used in all experiments were 98% pure.
2.2 Osteoblast cell culture
Human osteosarcoma-derived osteoblast-like MG63 cells (American Type Culture Collection) were cultured at a seeding density of 200 000 in 24-well plates (Nunc) in Dulbecco’s modified essential medium (DMEM; Gibco) supplemented with 10% fetal calf serum (FCS; Hyclone), L-glutamine (4 mM; Gibco), penicillin (200 units/mL) and streptomycin (200 µg/mL; Gibco) at 37°C in a humidified atmosphere of 5% CO2, until they achieved 80–85% confluence. At this point, the cell growth cycles were synchronised in DMEM lacking FCS for 24 h and then cultured in DMEM containing 2% FCS for a further 24 h. Medium was then replaced with fresh DMEM supplemented with 2% FCS as a negative control or the cultures were challenged with bacterial lipopolysaccharide (LPS). LPS or surface-associated material from several bacterial species was examined for the ability to release IL-6 from cultured MG63 cells before selection of Escherichia coli serotypes 0111:B4 (25 ng/mL: 50% maximal IL-6 stimulation concentration) for the positive control. Media were further supplemented with each of the vitamin K catabolites (CAN5C, CAN7C or CAN8C or their respective methyl esters) at between 10−8 to 10−5 M in the presence of LPS (25 ng/mL). The vitamin K catabolites were soluble in ethanol and final ethanol concentration was 2%; the negative and positive controls also contained 2% ethanol. The cells were then incubated for a further 24 h. After this time, the media was aspirated and stored at –80°C until analysed by ELISA for the osteoclastogenic cytokine IL-6.
2.3 ELISA assay
An in-house ELISA assay was developed [21], briefly, Nunc Maxisorp 96-well plates were coated with 100 µL/well of anti-IL-6 coating antibody at (1 µg/mL) in standard assay diluent (PBS) and stored overnight at 4°C. All antibodies and conjugated streptavidin-horseradish peroxidase were purchased from Biosource, SARL, Belgium. The plates were blocked with 300 µL/well of standard assay diluent containing 5 g/L BSA (Fraction V; Sigma-Aldrich, Dorset, UK) and left for 2 h at room temperature. The plates were then washed with PBS containing 0.1% Tween 20 v/v, followed by the addition of diluted standards or samples. Immediately following this biotinylated IL-6 detection antibody (0.4 µg/mL) in standard assay diluent containing 5 g/L BSA was added and the plates left to stand for 2 h at room temperature. The plates were then washed before the addition of streptavidin-horseradish peroxidase in standard assay diluent containing 5 g/L BSA and the plate left to incubate for 20 min at room temperature. After washing the plates, o-phenylenediamine in substrate buffer (0.05 M phosphate-citrate buffer (pH 5.0) containing 0.03% sodium perborate) was added. The reaction was stopped by the addition of 1 M sulphuric acid and the plates were read at 450 nm, referenced at 630 nm.
2.4 Proliferation assay
The effects of the vitamin K catabolites on osteoblast proliferation were examined using the cell culture procedure described above. After synchronisation of cell growth cycles, MG63 cells were cultured in DMEM containing 2% FCS for 18 h with and without LPS (E. coli serotype 0111:B4), and with or without supplementation with each of the vitamin K catabolites, before addition of 3H-thymidine in DMEM (0.37 MBq/mL) to all the wells for a further 6 h. Media were removed, cells washed with PBS and the plates freeze-thawed in PBS containing 1% Tween 20. The cell lysate was aspirated through a printed filter-mat using a cell harvester and radioactivity (cpm) measured on a scintillation counter.
2.5 Animal studies
2.5.1 OVX model
Female C57BL/6 mice were obtained from Charles Rivers at 8 weeks age and housed under standard conditions according to local and UK Home Office regulations. All experiments were done under Home Office licence and local, Royal Veterinary College, ethical regulations governing animal experimentation.
After acclimatisation in 12-h dark/light conditions in groups of four for 2 weeks the animals were randomly assigned to, sham-operated, ovariectomised and ovariectomised-treated [22]. The day after surgery, animals received 15 µg of freshly prepared naphthoquinone compounds (CAN7C and CAN8C or their respective methyl esters) per day (ethanol/saline 2% v/v solution) by intraperitoneal injection. A higher dose of 30 µg CAN7C was also investigated in a group of ovariectomised mice. Controls received ethanol/saline (2% v/v) solution daily. The animals were presented ad libitum water and standard chow and weighed daily. After 5 weeks, the mice were sacrificed and the right tibiae removed, cleaned of soft tissues and prepared for micro-computerised tomography analyses.
2.5.2 Neurectomy model
Following evaluation of the results from the OVX studies, only CAN7C was used to evaluate effects on neurectomy-induced bone loss. Female C57BL/6 mice were housed as described above. Using methods previously described [23], all mice had the right sciatic nerve severed and a 2 mm section removed, thereafter mice were randomly assigned to one of four groups; control untreated and two treatment groups. The day after surgery, the treated animals received either 15 or 30 µg of freshly made CAN7C per day by intraperitoneally in an ethanol/saline (2% v/v) solution. The untreated animals received an ethanol/saline (2% v/v) vehicle solution daily. Two weeks after surgery, the animals were sacrificed and both tibiae removed cleaned of soft tissues and prepared for micro-computed tomography (mCT) analyses.
2.6 mCT analysis
Right tibiae from ovariectomised mice and both tibiae from neurectomised mice were dissected, fixed in 10% neutral buffered formalin for 48 h, rinsed in tap water for 3 h and stored in 70% ethanol. The tibiae were briefly rehydrated in physiological saline (0.9%) prior to being scanned using a mCT system (Skyscan 1172 X-Ray Microtomograph, Aartse-laar, Belgium) to evaluate trabecular architecture and geometry using methods described by us elsewhere [23, 24]. Briefly, high-resolution scans with an isotropic voxel size of 5 µm were acquired (scan time approximately 1 h 30 min for each bone), then reconstructed using NRecon (Skyscan) and analysed using CTAn (Skyscan). In the trabecular bone compartment, bone volume to total volume ratio (BV/TV), trabecular number (Tb.No.; /mm), trabecular thickness (Tb.Th; mm), trabecular separation (Tb.Sp) were evaluated. Coefficients of variation for these parameters were within empirical tolerance for this technique: BV/TV = 1.65%; Tb.No. = 1.72%; Tb.Tk = 0.85%; Tb.Sp = 0.85%.
2.7 Statistical analyses
The data were initially examined for normality of distribution and equal variance. The in vitro cell culture data were found to be normally distributed and had equal variances within each experiment. These results were analysed by one-way ANOVA followed by a Bonferroni multiple comparison tests. The in vivo data required non-parametric analyses due to failing to meet normality tests that could not be normalised by log transformation. Therefore Kruskal–Wallis one-way ANOVA test were done followed by Dunn’s multiple comparison test. All analyses were done using GraphPad Prism 6.0 statistical package and the results are shown in Table 1 (GraphPad Software, Inc., La Jolla, CA).
Table 1.
The calculated probabilities for differences between the control and treated groups of four tibial architectural trabecular parameters measured by micro-computed tomography. The upper section shows results for comparisons between sham-operated ovariectomised mice and the treated ovariectomised mice and the lower section shows results for the experiments with sciaticectomised mice
| Calculated probabilities |
||||
|---|---|---|---|---|
| BV/TV | Tb.Sp | Tb.No. | Tb.Th | |
| Ovariectomy | ||||
| Sham versus OVX | 0.04 | 0.32 | 0.26 | 0.99 |
| Sham versus OVX + 15 µg CAN7C | 0.99 | 0.99 | 0.99 | 0.48 |
| Sham versus OVX + 30 µg CAN7C | 0.99 | 0.83 | 0.99 | 0.06 |
| Sham versus OVX + 15 µg CAN7CMe | 0.01 | 0.08 | 0.11 | 0.23 |
| Sham versus OVX + 15 µg CAN8C | 0.02 | 0.24 | 0.02 | 0.23 |
| Sham versus OVX + 15 µg CAN8C Me | 0.41 | 0.55 | 0.54 | 0.99 |
| Neurectomy | ||||
| Untreated operated versus unoperated tibiae | <0.001 | 0.23 | 0.001 | <0.001 |
| Ratio operated to unoperated limbs | ||||
| Untreated versus 15 µg CAN7C | 0.14 | 0.99 | 0.21 | 0.99 |
| Untreated versus 30 µg CAN7C | 0.99 | 0.99 | 0.99 | 0.99 |
3 Results
3.1 The vitamin K catabolite CAN7C inhibits in vitro IL-6 release from osteoblasts
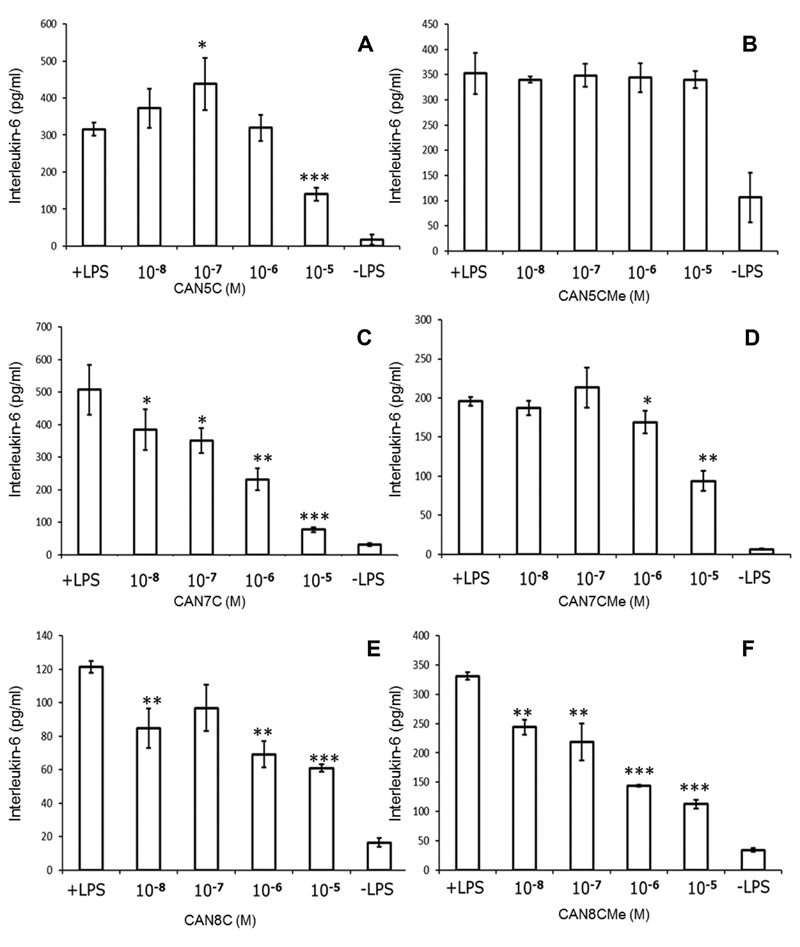
The osteoblast-like cell line MG63 produces IL-6 when challenged with LPS, the responses were found to be variable between batches of LPS and the most reproducible results were obtained with E. coli serotype 0111:B4. Six naphthoquinone compounds (CAN5/7/8C and their methyl esters) were tested for their capacity to inhibit IL-6 release from LPS-challenged MG63 cells, several experiments were done for each compound and representative results are shown in Fig. 2. It is interesting to observe that the two most closely related compounds CAN5C and CAN7C consistently had substantially different activities with respect to their inhibition of IL-6 release from LPS-challenged MG63 cells; the former being less active. We also found that these inhibitory activities were considerably blunted in experiments using the corresponding methyl ester naphthoquinone compounds. There was an inhibitory activity of the octyl carboxylic acid naphthoquinone, which is less pronounced than that seen with CAN7C and appears to be enhanced in this assay by the methyl ester form of this compound. Clearly reduced production of IL-6 could result from a general cytotoxic reaction to the naphthoquinone compounds, however, the proliferation assays demonstrated that the compounds had no detectable cytotoxic activity up to 10−5 M (data not shown). Incubation of MG63 cells with the individual naphthoquinone compounds over the described concentration range, in the absence of LPS, did not cause the release of IL-6 (data not shown).
Figure 2.
Individual representative results from interleukin-6 release from cultured MG63 osteoblast-like cells (pg/ml) challenged with E. coli lipopolysaccharide (25 ng/mL: +LPS) and by LPS in the presence of the naphthoquinone compounds or without LPS or compound (-LPS) (mean ± SE for four replicates). (A) The 5-carbon carboxylic metabolite of vitamin K (CAN5C). (B) The methyl ester of the 5-carbon carboxylic metabolite (CAN5CMe). (C) The 7-carbon carboxylic metabolite of vitamin K (CAN7C). (D) The methyl ester of the 7-carbon carboxylic metabolite (CAN7CMe). (E) The aliphatic octyl carboxylic acid naphthoquinone (CAN8C). (F) The methyl ester of the octyl carboxylic acid naphthoquinone (CAN8CMe): *p < 0.05; **p < 0.005; ***p < 0.001, all compound data were compared against the positive (+LPS) control.
These in vitro results led to the selection of CAN7C, its methyl ester, and the equivalent pairing of the octyl carboxylic acid naphthoquinone compound (CAN8C) and its methyl ester to investigate their potential for protecting against the induced bone loss in the in vivo OVX model.
3.2 Vitamin K catabolite CAN7C inhibits murine bone loss induced by OVX
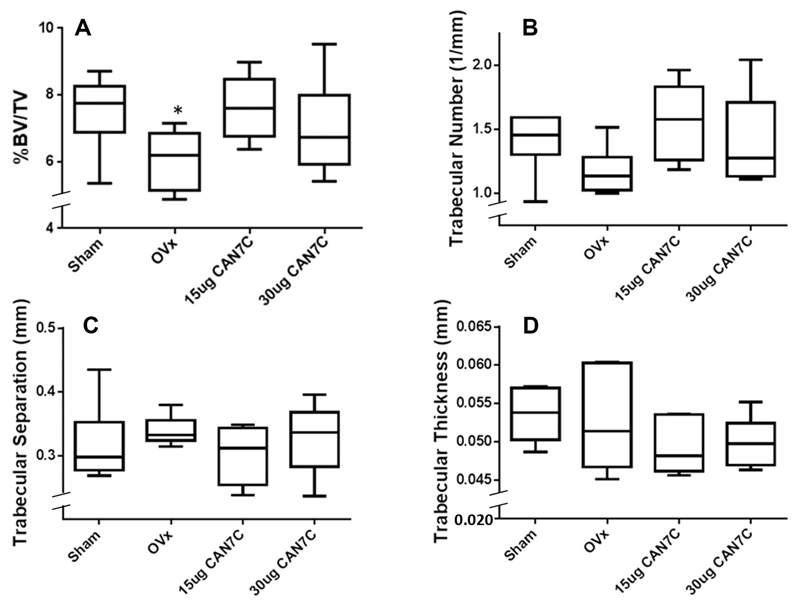
None of the animals showed overt signs of deleterious effects from the administration of the naphthoquinone compounds or the vehicle control solutions and all the mice went through to the completion of the experiments. Adopting the suggestion of Bouxsein et al. [25], the four prominent architectural trabecular parameters derived from the mCT analyses are presented in Fig. 3 from the OVX-induced bone loss experiments with CAN7C compared to controls. The calculated probabilities for the differences between sham-operated and ovariectomised mice (Table 1) reveal that untreated ovariectomised mice exhibited statistically significant cancellous bone loss, as witnessed by the reduction in percentage bone volume in the total trabecular compartment. Treatment with CAN7C causes the cancellous bone in the tibiae in the treated mice to be statistically indistinguishable from that in the sham-operated animals (Table 1). There was attenuation of the OVX-induced bone loss following treatment with CAN7C at both 15 and 30 µg/day (Table 1; Fig. 3). Consistent with our in vitro data, methylation of the carboxylic acid greatly limits the protection from OVX-induced cancellous bone loss observed by their free carboxylic acid homologues. Additionally, making the side chain more hydrophobic, as in the synthetic octyl-carboxylic acid naphthoquinone compound (CAN8C) and its methyl derivative did not produce any notable protective action (Table 1).
Figure 3.
The four principle architectural parameters from micro-computerised tomography measurements of mice tibae after sham operation or ovariectomy, with or without naphthoquinone administration. Two of the ovariectomy groups were administered by i.p. injection the 7-carbon carboxylic acid catabolite (CAN7C) of vitamin K at 15 or 30 µg/day. Data are plotted as box and whisker plots with maximum and minimum extremes on discontinuous y-axes; *p < 0.05 compared to sham-operated mice.
3.3 Vitamin K catabolite CAN7C restricts sciatic neurectomy-induced bone loss
Sciatic neurectomy produces a more intense bone loss than OVX [26]. This is evident in our experiments as the marked difference in BV/TV, Tb.No. and Tb.Th between operated and unoperated limbs (Table 1). Comparison of BV/TV in the unoperated limbs of untreated and treated groups indicates that these vitamin K derivatives failed to significantly modify basal bone architecture and mass, suggestive of limited change in bone turnover in these otherwise normal tibiae (data not shown). Comparisons between the operated, disused, limbs of the untreated and treated groups indicate potential amelioration of bone loss in the neurectomised limbs by 15 µg/day administration of CAN7C although this did not reach levels of statistical significance, while the higher dose of 30 µg/day showed no potential to limit bone loss.
4 Discussion
Our findings indicate that vitamin K catabolites are effective in restricting LPS-induced IL-6 production by osteoblast-like cells in vitro and in reducing OVX-induced bone loss in vivo. We find that these effects are relatively limited to the CAN7C catabolite and these data suggest an alternative mechanism by which vitamin K may exert osteotrophic effects. Use of high-dose vitamin K as a therapeutic for osteoporosis was found to be optimal at 45 mg/day [3] and it has also been suggested that this regimen may be more beneficial in patients who are actively experiencing increased bone turnover [27], which would more accurately parallel our OVX model. While 300 µg/day vitamin K, a supra-normal nutritional quantity, is sufficient to gamma-carboxylate the vitamin K-dependent bone protein osteocalcin, it does question if the remaining 44.7 mg (99.3%) of the therapeutic 45 mg/day human vitamin K dose may contribute to other biological functions. Our findings are consistent with the hypothesis that this may be due, at least in part, to the production of elevated levels of vitamin K catabolites in this high-dose regimen [14].
These studies demonstrate that a catabolite of vitamin K, CAN7C, which is not normally found in appreciable quantities, but which shows increased levels with pharmaceutical doses [14], has substantial osteotrophic activity. This compound inhibits IL-6 release from challenged MG63 osteoblast-like cells supporting our earlier finding of the inhibition of this inflammatory cytokine from challenged cultured primary human gingival fibroblasts [28]. While IL-6 is only one of many factors that can induce osteoclastogenesis, the observation in distinct cell populations may implicate a common, undisclosed, mechanism in activated cells.
With a normal diet, most of vitamin K is shuttled through to CAN5C [12–14]. However, except in high concentrations, we found that CAN5C has little inhibitory activity on IL-6 release in the challenged MG63 culture experiments. Thus, catabolism of native vitamin K predominantly into the relatively inert CAN5C catabolite may therefore act to conserve any non-pathological IL-6 cytokine production that is part of the control of normal bone turnover. The observation of substantial in vitro activity difference between CAN5C and CAN7C, which only differ by two additional carbon atoms and a double bond, remains enigmatic as the increased hydrophobicity of CAN8C does not confer greater activity over CAN7C, suggesting that this small structural feature is likely to have profound biologically relevance. Furthermore, the IL-6 inhibitory action of CAN7C appears to require a free carboxylic acid group for full function.
IL-6 has previously been suggested to have a role in the progression to presenting with osteoporosis [17–20]. It is possible that senescence-regulated DNA damage leads to a local inflammatory response operating through IL-6, TNF-α, RANKL, etc., which activates the NFκβ pathway [29]. It was therefore considered appropriate to undertake provisional investigations of CAN7C and CAN8C for their ability to inhibit OVX- and neurectomy-induced bone loss. Inhibition of this pathway has been noted for other 1,4-naphthoquinone natural products, notably plumbagin, where significant attenuation of osteoclastogenesis through IkappaB kinase (IKK) signalling has been demonstrated [30]. The role of IKK in OVX-induced bone loss is emerging [31], however, there is a paucity of published information concerning IKK in neurectomy-induced bone loss.
In addition to CAN7C showing an inhibition of the bone loss in the ovariectomised mouse (Fig. 3) we also noted that, as with the osteoblast in vitro observations, a free carboxylic acid moiety is required for the molecules to be fully functional and that a branched-chain substituent at position 3 on the naphthoquinone is more important than a hydrophobic straight chain aliphatic carbon structure (Table 1). It is possible that the residual actions of the methyl esters could be due to an esterase cleavage of the molecules to reveal the free acid compounds.
It is well established that neurectomy-induced disuse produces a dramatic loss of cancellous bone and this is consistent with our data comparing right tibiae to their respective contralateral control, non-operated limb (Table 1). Our data reveal that 15 µg/day CAN7C exerts some limited impact on the neurectomy-induced bone loss which, although not reaching levels of statistical significance for BV/TV (p = 0.14; Table 1) may suggest potential activity in this model where bone formation may be of greater relevance. Interestingly, the higher dose of 30 µg/day CAN7C appears to be completely ineffective in this model. Both of these models of induced bone loss are associated with increased bone turnover and diminished bone mass, with OVX-induced bone loss showing a higher propensity to bone resorption, relative to bone formation, [32–34], while neurectomy-induced bone loss shows a decreased bone formation rate with respect to bone resorption [26, 35].
Administration of high-dose menaquinone-4, a form of vitamin K, (50 mg/kg/day) has previously been shown to suppress elevated levels of osteoclast-mediated resorption without modifying increased osteoblast function in ovariectomised rodents [36]. In another study, again using menaquinone-4 (30 mg/kg/day; 3× week), in sciatic neurectomised rats, Iwamoto et al. found an attenuation of neurectomy-induced cancellous bone loss and increased bone formation rate [37]. As rats can break down vitamin K into carboxylic acid catabolites [38], it is, of course, possible that this attenuation of cancellous bone turnover is due to increased endogenous production of CAN7C.
The results presented here also need to be viewed in a broader context. The potential to deliver a consistent supply of the CAN7C from the administration of high doses of vitamin K will be subject to several variables; including taking vitamin K with food to aid gut absorption, compliance and other factors such as liver function. With specific reference to liver function, hepatectomy of the rat has shown that it is the dominant organ for vitamin K storage, catabolism and excretion [39–41]. Ageing is associated with decreasing general hepatic function [42] and a decreasing ability to metabolise xenobiotic molecules [43]. Although vitamin K metabolism has not been specifically addressed in an older human cohort, the newborn infant has been shown by Harrington et al. [44] to have some degree of impaired hepatic vitamin K catabolic activity, reflected as a greater CAN7C production following a pharmaceutical dose. Equally, if catabolism of vitamin K can occur in other tissues, the molecular architecture for this capability remains to be disclosed, which, should the enzymes be found in extra-hepatic tissues, bone is an interesting candidate organ as it is a rich depository for vitamin K [45]. Finally, as CAN7C is broken down into the relatively inert CAN5C or excreted directly [12–14, 39], it presents an interesting molecule to consider from a safety perspective for the therapeutic modulation of osteopenia and osteoporosis.
In conclusion, this pilot study highlights a possible role for a specific vitamin K catabolite in the regulation of bone metabolism. This catabolite is only appreciably encountered when high doses of vitamin K are administered. This remains to be vigorously investigated through the use of a broader range of CAN7C doses, different models, longer duration of the models, larger group sizes coupled to pharmacodynamics, pharmacokinetic and mechanistic investigations.
We gratefully acknowledge the partial support of the Heptagon Fund and we thank Drs. Chantal Chenu and Martin Shearer for their helpful suggestions with the manuscript.
Potential conflict of interest statement: The authors have minor equity holdings in Haoma Medica Ltd., which has the rights to the patents for carboxylic acid naphthoquinones in the treatment of osteoporosis.
Abbreviations
- CAN5C
5-carbon vitamin K catabolite with 3-methyl-butanoic acid at position 3 on the 2-methyl-1,4-naphthoquinone moiety
- CAN7C
7-carbon vitamin K catabolite with 4-methylhex-4-enoic acid at position 3 on the 2-methyl-1,4-naphthoquinone moiety
- CAN8C
8-carbon compound with octanoic acid at position 3 on 2-methyl-1,4-naphthoquinone moiety
- IL-6
interleukin-6
- IKK
IkappaB kinase
- LPS
lipopolysaccharide
- mCT
micro-computed tomography
- OVX
ovariectomy
References
- 1.Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care. 2000;3:433–438. doi: 10.1097/00075197-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ferland G. The discovery of vitamin K and its clinical applications. Ann Nutr Metab. 2012;61:213–218. doi: 10.1159/000343108. [DOI] [PubMed] [Google Scholar]
- 3.Orimo H, Fujita T, Onomura T, Inoue T, et al. Clinical effect of menatetrenone on osteoporosis [In Japanese] J New Remedies Clin. 1992;41:1249–1279. [Google Scholar]
- 4.Gajic-eljanoski O, Bayoumi AM, Tomlinson G, Khan K, et al. Vitamin K supplementation for the primary prevention of osteoporotic fractures: is it cost-effective and is future research warranted? Osteoporos Int. 2012;23:2681–2692. doi: 10.1007/s00198-012-1939-4. [DOI] [PubMed] [Google Scholar]
- 5.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (Menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515–521. doi: 10.1359/jbmr.2000.15.3.515. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto J, Sato Y. Menatetrenone for the treatment of osteoporosis. Expert Opin Pharmacother. 2013;14:449–458. doi: 10.1517/14656566.2013.763796. [DOI] [PubMed] [Google Scholar]
- 7.Booth SL, Dallal G, Shea MK, Gundberg C, et al. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–1223. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binkley N, Harke J, Krueger D, Engelke J, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res. 2009;24:983–991. doi: 10.1359/JBMR.081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emaus N, Gjesdal CG, Almås B, Christensen M, et al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised doubleblind placebo-controlled trial. Osteoporos Int. 2010;21:1731–1740. doi: 10.1007/s00198-009-1126-4. [DOI] [PubMed] [Google Scholar]
- 10.Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom. 2013;16:409–413. doi: 10.1016/j.jocd.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Binkley NC, Krueger DC, Kawahara TN, Engelke JA, et al. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76:1055–1060. doi: 10.1093/ajcn/76.5.1055. [DOI] [PubMed] [Google Scholar]
- 12.Shearer MJ, Barkhan P. Studies on the metabolites of phylloquinone (vitamin K 1) in the urine of man. Biochim Biophys Acta. 1973;297:300–312. doi: 10.1016/0304-4165(73)90077-9. [DOI] [PubMed] [Google Scholar]
- 13.Shearer MJ, McBurney A, Barkhan P. Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitam Horm. 1974;32:513–542. doi: 10.1016/s0083-6729(08)60025-4. [DOI] [PubMed] [Google Scholar]
- 14.Harrington DJ, Soper R, Edwards C, Savidge GF, et al. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J Lipid Res. 2005;46:1053–1060. doi: 10.1194/jlr.D400033-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Harrington DJ, Booth SL, Card DJ, Shearer MJ. Excretion of the urinary 5C- and 7C-aglycone metabolites of vitamin K by young adults responds to changes in dietary phylloquinone and dihydrophylloquinone intakes. J Nutr. 2007;137:1763–1768. doi: 10.1093/jn/137.7.1763. [DOI] [PubMed] [Google Scholar]
- 16.Card DJ, Gorska R, Cutler J, Harrington DJ. Vitamin K metabolism: current knowledge and future research. Mol Nutr Food Res. 2014;58:1590–1600. doi: 10.1002/mnfr.201300683. [DOI] [PubMed] [Google Scholar]
- 17.Jilka RL, Hangoc G, Girasole G, Passeri G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 18.Passeri G, Girasole G, Jilka RL, Manolagas SC. Increased interleukin-6 production by murine bone marrow and bone cells after estrogen withdrawal. Endocrinology. 1993;133:822–888. doi: 10.1210/endo.133.2.8393776. [DOI] [PubMed] [Google Scholar]
- 19.Poli V, Balena R, Fattori E, Markatos A, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 21.Soper RJ. The effects of natural quinones on cytokine release. PhD thesis; University of Essex UK: 2004. [Google Scholar]
- 22.de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, et al. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005;20:2159–2168. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- 23.Zaman G, Jessop HL, Muzylak M, De Souza RL, et al. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res. 2006;21:1297–1306. doi: 10.1359/jbmr.060504. [DOI] [PubMed] [Google Scholar]
- 24.Macrae VE, Horvat S, Pells SC, Dale H, et al. Increased bone mass, altered trabecular architecture and modified growth plate organization in the growing skeleton of SOCS2 deficient mice. J Cell Physiol. 2009;218:276–284. doi: 10.1002/jcp.21593. [DOI] [PubMed] [Google Scholar]
- 25.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 26.Brouwers JE, Lambers FM, van Rietbergen B, Ito K, et al. Comparison of bone loss induced by ovariectomy and neurectomy in rats analyzed by in vivo micro-CT. J Orthop Res. 2009;27:1521–1527. doi: 10.1002/jor.20913. [DOI] [PubMed] [Google Scholar]
- 27.Eisai Co. Ltd. [Accessed June 2014];announces the intermediate analysis of anti-osteoporosis treatment post-marketing research to investigate the benefits of menatetrenone as part of the Ministry of Health, Labour and Welfare’s pharmacoepidemiological drug preview program. Available online at www.eisai.co.jp/news/news200506.html.
- 28.Reddi K, Henderson B, Meghji S, Wilson M, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995;7:287–290. doi: 10.1006/cyto.1995.0034. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Liu K, Robinson AR, Clauson CL, et al. DNA damage drives accelerated bone aging via an NF-κB-dependent mechanism. J Bone Miner Res. 2013;28:1214–1228. doi: 10.1002/jbmr.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung B, Oyajobi B, Aggarwal BB. Plumbagin inhibits osteoclastogenesis and reduces human breast cancer-induced osteolytic bone metastasis in mice through suppression of RANKL signaling. Mol Cancer Ther. 2012;11:350–359. doi: 10.1158/1535-7163.MCT-11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idris AI, Krishnan M, Simic P, Landao-Bassonga E, et al. Small molecule inhibitors of IkappaB kinase signalling inhibit osteoclast formation in vitro and prevent ovariectomy-induced bone loss in vivo. FASEB J. 2010;24:4545–4555. doi: 10.1096/fj.10-164095. [DOI] [PubMed] [Google Scholar]
- 32.Gürkan L, Ekeland A, Gautvik KM, Langeland N, et al. Bone changes after castration in rats. A model for osteoporosis. Acta Orthop Scand. 1986;57:67–70. doi: 10.3109/17453678608993219. [DOI] [PubMed] [Google Scholar]
- 33.Wronski TJ, Cintrón M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988;123:681–686. doi: 10.1210/endo-123-2-681. [DOI] [PubMed] [Google Scholar]
- 34.Schot LP, Schuurs AH. Pathophysiology of bone loss in castrated animals. J Steroid Biochem Mol Biol. 1990;37:461–465. doi: 10.1016/0960-0760(90)90499-b. [DOI] [PubMed] [Google Scholar]
- 35.Weinreb M, Rodan GA, Thompson DD. Osteopenia in the immobilized rat hind limb is associated with increased bone resorption and decreased bone formation. Bone. 1989;10:187–194. doi: 10.1016/8756-3282(89)90052-5. [DOI] [PubMed] [Google Scholar]
- 36.Asawa Y, Amizuka N, Hara K, Kobayashi M, et al. Histochemical evaluation for the biological effect of menatetrenone on metaphyseal trabeculae of ovariectomized rats. Bone. 2004;35:870–880. doi: 10.1016/j.bone.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Iwamoto J, Matsumoto H, Takeda T, Sato Y, et al. Effects of vitamin K2 on cortical and cancellous bone mass, cortical osteocyte and lacunar system, and porosity in sciatic neurectomized rats. Calcif Tissue Int. 2010;87:254–262. doi: 10.1007/s00223-010-9387-7. [DOI] [PubMed] [Google Scholar]
- 38.Tadano K, Yuzuriha T, Sato T, Fujita T, et al. Identification of menaquinone-4 metabolites in the rat. J Pharmacobiodyn. 1989;12:640–645. doi: 10.1248/bpb1978.12.640. [DOI] [PubMed] [Google Scholar]
- 39.Losito R, Owen CA, Jr, Flock EV. Metabolic studies of vitamin K1–14C and menadione-14C in the normal and hepatectomized rats. Thromb Diath Haemorrh. 1968;19:383–388. [PubMed] [Google Scholar]
- 40.Shearer MJ, Mallinson CN, Webster GR, Barkhan P. Clearance from plasma and excretion in urine, faeces and bile of an intravenous dose of tritiated vitamin K1 in man. Br J Haematol. 1972;22:579–588. doi: 10.1111/j.1365-2141.1972.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 41.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3:182–195. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. 2010;5:e14254. doi: 10.1371/journal.pone.0014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polasek TM, Patel F, Jensen BP, Sorich MJ, et al. Predicted metabolic drug clearance with increasing adult age. Br J Clin Pharmacol. 2013;75:1019–1028. doi: 10.1111/j.1365-2125.2012.04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington DJ, Clarke P, Card DJ, Mitchell J, et al. Urinary excretion of vitamin K metabolites in term and preterm infants: relationship to vitamin K status and prophylaxis. Pediatr Res. 2010;68:508–512. doi: 10.1203/PDR.0b013e3181f981c7. [DOI] [PubMed] [Google Scholar]
- 45.Hodges SJ, Bejui J, Leclercq M, Delmas PD. Detection and measurement of vitamins K1 and K2 in human cortical and trabecular Bone. J Bone Miner Res. 1993;8:1005–1008. doi: 10.1002/jbmr.5650080814. [DOI] [PubMed] [Google Scholar]