Abstract
This paper presents a description of the hitherto unknown larvae of Drusus franzi Schmid 1956, and Drusus alpinus (Meyer-Dür 1875). Information on the morphological and genetic identification of both species is given, and the most important diagnostic features are illustrated. Their systematic position within the genus Drusus is affirmed and zoogeographical and ecological notes are added.
Keywords: identification, distribution, ecology, mitochondrial DNA
Introduction
The small caddisfly subfamily Drusinae is of European origin with some endemic species also occurring in Asia Minor and the Caucasus region. Their patchy distribution and high proportion of micro-endemic species makes the group ideal for studying processes of isolation, specialisation and speciation. Most Drusinae are restricted to crenal areas at altitudes above 1000 m.asl.
Larval Drusinae are commonly classified as algal scrapers as most of them have spoon-shaped mandibles (e.g., Décamps & Pujol 1975, Szczesny 1978, Waringer 1985, Graf 1993). Feeding studies and descriptions of larvae of D. discolor (Rambur 1842) (Bohle 1983) and D. romanicus Murgoci & Botosaneanu 1953 (Botosaneanu 1959), Crypthotrix nebulicola Mclachlan 1867 (Bohle 1987), D. chrysotus (Rambur 1842) (Waringer 1987) and D. muelleri McLachlan 1868 (Graf et al. 2005) revealed a second ecological line, which consists of predators that catch their prey with special adapted devices such as filtering bristles on legs and characteristic head and pronotal designs which are unique morphological features within the family Limnephilidae.
So far, 24 Drusinae species are reported from Austria, Germany and Switzerland (Lubini-Ferlin & Vicentini 2005; Malicky 1999, 2004; Robert 2001, 2004). Five of them (Drusus alpinus, D. chapmani McLachlan 1901, D. franzi and D. noricus Malicky 1981) are still unknown in the larval stage. Recently, we investigated larval specimens of D. franzi from Austria and Drusus alpinus from Switzerland whose identities were confirmed by genetic association with adults. With respect to mandible shape, both species can be assigned to an unexpected third group, the omnivorous shredders. The newly presented material enabled us to discover reliable diagnostic characters, permitting integration of both species into the larval key by Waringer and Graf (1997, 2004).
Material and methods
To support conspecific association between larval and adult specimens of D. alpinus and D. franzi we sequenced a 498bp long fragment of the mitochondrial cytochrome oxidase I gene (mtCOI) of larvae and adults of both species following the methods outlined by Pauls et al. (2006). We collected two males and one larva of D. alpinus from one locality and another male and larva from two further localities in the Swiss Alps (Table 1). For D. franzi we analyzed two males and one female from one locality, two males from a second locality and a larva and a male from a third locality. We analyzed these sequences in a pairwise genetic distance framework including further, previously published sequence data. As there are no very close relatives in the Drusinae to D. alpinus and D. franzi (Schmid 1956, Pauls et al. 2008) four other taxa from each of the other two main feeding ecology types in the Drusinae were selected for these analyses: carnivorous filterers (D. chrysotus, D. discolor, D. muelleri and D. romanicus) and grazers (D. annulatus (Stephens 1837), D. melanchaetes McLachlan 1876, D. nigrescens Meyer-Dür 1875 and D. trifidus McLachlan 1868) (Table 1). We generated uncorrected proportional pairwise distances among sequences (p) between individuals using the DNADist function as implemented in BioEdit 7.0.5.3 (Hall 1999). We used the resulting distance matrix to calculate a principal components analysis (PCA) without data standardization using the GenAlEx 6.2 software (Peakall & Smouse 2006).
Table 1.
Specimen information, locality data and GenBank Accession numbers.
| Drusus sp. | Stage** | Locality | GenBank Accession*** |
|---|---|---|---|
| Drusus alpinus | M | CH, Furkapass, Sidelen tributary, 17.07.2004 | EU637362A |
| D. alpinus | M | CH, Furkapass, Sidelen tributary, 17.07.2004 | EU215084D |
| D. alpinus | M | CH, St. Gotthardt Pass, 21.07.2006 | EU215085D |
| D. alpinus * | L | CH, Val Bedretto, Nufenen pass, 19.07.2007 | EU637363A |
| D. alpinus * | L | CH, Furkapass, Sidelen tributary, 2006 | EU637369A |
| D. annulatus | M | D, Black Forest, Brotenaubach, 11.05.2006 | EU215087D |
| D. chrysotus | M | AT, Saualpe, Quellbäche bei Ladinger Hütte, 30.06.2006 | EU143739C |
| D. discolor | M | ES, Cantabrian Mts., Cabanas Vellas, Rio Ancaras | AY954397B |
| D. franzi | F | AT, Klugveitl, 26.4.05 | EU637364A |
| D. franzi | M | AT, Klugveitl, 26.4.05 | EU637365A |
| D. franzi | M | AT, Klugveitl, 26.4.05 | EU637366A |
| D. franzi | M | AT, Koralpe, Weinebene, 27.5.2006 | EU637368A |
| D. franzi | M | AT, Koralpe, Weinebene, 27.5.2006, | EU215100D |
| D. franzi | M | AT, Saualpe, 29.5.2006, | EU215099D |
| D. franzi * | L | AT, Saualpe, 29.5.2006 | EU637367A |
| D. melanchaetes | M | CH, Meienreuss, East of Sustenpass, 16.07.2004 | EU143740C |
| D. muelleri | M | CH, Furkapass, Mutt tributary, 17.07.2004 | AY954398B |
| D. nigrescens | M | CH, Furkapass 21.7. 2006 | EF464565E |
| D. r. meridionalis | M | BG, Pirin Mts, Banderishka River, 18.08.2003 | AY954402B |
| D. r. romanicus | M | RO, Fagaras Mts, Valea Buda, 07.08.2003 | AY954403B |
| D. trifidus | M | AT, Ennstaler Alps, Gesäuse, 02.07.2006 | EU215109D |
Larva associated within this study
“M” = male, “F” = female, “L” = larva
A sequences generated in this study; B sequences from Graf et al. (2005); C sequences from Waringer et al. (2008); D sequences from Pauls et al. (2008); E sequences from Waringer et al. (2007).
To describe and characterize the larval morphology of D. alpinus and D. franzi and identify differentiating characters, we examined the following material: Drusus alpinus: 1 fifth instar larva together with numerous adults from Furka Pass (Switzerland, Uri, 46° 35.3′ N, 08° 25.8′ E, 2348 m asl, 21.7.2006), 2 fifth instar larvae, 1 male and 1 female from Nufenen Pass (Switzerland, Ticino, 46°28.4′ N, 08°26.2′ E, 20.7.2007) and 1 male, 2 females from Gotthard Pass (Switzerland, Ticino, 46°32.753′ N, 8°34.056 E, 21.7.2006); Drusus franzi: 10 fifth instar larvae from a nameless brook near Reinischkogel (Austria, Styria: 15°08′24″ E, 46°56′06′ N, 1112 m asl, 15.5.2006). Males and females were collected from the same place as well as from the Saualpe (Austria, Carinthia, 14°40′14″ E, 46°50′38″ N; 14°39′42″ E, 46°50′06″ N, 20.5.2006) and the Weinebene (Austria, Carinthia, 15° 00′ 20″ E, 46°50′31″N, 27.5.2006). Last and penultimate instar larvae of D. alpinus were collected on 21 July 2006 at the Furka-Paß, and on 20th July 2007 at the Nufenen-Paß, Switzerland. All material was collected by W.G. and Phillip Wenzl.
Results
Genetic association
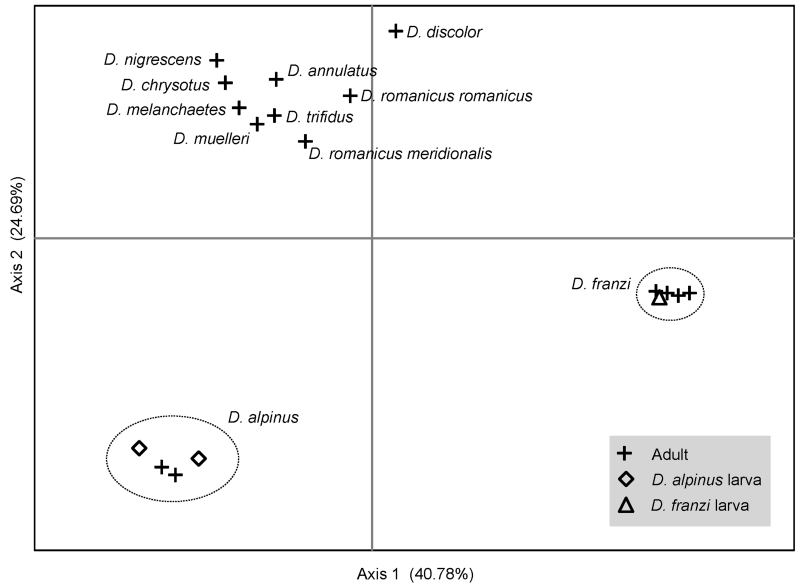
Together axis 1 and axis 2 of the PCA explain 65.47% of the variation; with axis 3, 81.81% of the variation is explained. The PCA shows two relatively tight and one more widespread cluster, when examining axis 1 vs axis 2 (Figure 1). The two smaller clusters group all adults of D. alpinus and D. franzi, respectively. The larvae examined in this study cluster closely together with the adults, confirming the associations to either D. alpinus or D. franzi. All adults from other species cluster in a loose cluster at the top of Figure 1. When examining axis 1 vs. axis 3, or axis 2 vs. 3 (data not shown), this larger cluster is divided into two smaller and tighter clusters, each of which groups the taxa belonging to one of the other feeding types.
Figure 1.
Principal Components Analysis (PCA) of 21 specimens of Drusinae, based on proportional pairwise genetic distances (p). Shown is the proximity of specimens relative to axis 1 vs. axis 2.
Uncorrected proportional pairwise distances (p) ranged from 0 to 0.0375 within D. alpinus and from 0 to 0.0101 within D. franzi (Table 2). The maximum distance between associated larvae with conspecific adults ranged from 0.002 to 0.0375 in D. alpinus and 0 to 0.081 in D. franzi. Between all examined species, minimal p was 0.0718 between D. discolor and D. romanicus meridionalis Kumanski. Minimal p between D. alpinus and the other species examined was 0.1104 (D. romanicus meridionalis). Minimal p between D. franzi and the other species examined in the study was 0.1103 (D. discolor). These values of inter- and intraspecific variation exceed those observed in D. monticola Meyer-Dür and D. nigrescens (Waringer et al. 2007), but are within the range of values observed in other studies of the group (Graf et al. 2005; Waringer et al. 2008).
Table 2.
Proportional pairwise genetic distances of 21 specimens of Drusinae based on 498 bp fragment of mtCOI.
| Drusus alpinus | EU637362 | 0 | ||||||||||||||||||||
| D. alpinus | EU215084 | 0 | 0 | |||||||||||||||||||
| D. alpinus | EU215085 | 0.002 | 0.002 | 0 | ||||||||||||||||||
| D. alpinus * | EU637363 | 0.0352 | 0.0352 | 0.0331 | 0 | |||||||||||||||||
| D. alpinus * | EU637369 | 0.002 | 0.002 | 0.0041 | 0.0375 | 0 | ||||||||||||||||
| D. annulatus | EU215087 | 0.1269 | 0.1269 | 0.1245 | 0.1317 | 0.1181 | 0 | |||||||||||||||
| D. chrysotus | EU143739 | 0.1181 | 0.1181 | 0.1205 | 0.1276 | 0.1188 | 0.1264 | 0 | ||||||||||||||
| D. discolor | AY954397 | 0.1398 | 0.1398 | 0.1374 | 0.1302 | 0.1358 | 0.1149 | 0.0983 | 0 | |||||||||||||
| D. franzi | EU637364 | 0.1233 | 0.1233 | 0.1257 | 0.1328 | 0.1168 | 0.1246 | 0.1336 | 0.1103 | 0 | ||||||||||||
| D. franzi | EU637365 | 0.131 | 0.131 | 0.1335 | 0.1407 | 0.1246 | 0.1325 | 0.1392 | 0.1178 | 0.0101 | 0 | |||||||||||
| D. franzi | EU637366 | 0.1256 | 0.1256 | 0.128 | 0.1351 | 0.1191 | 0.1269 | 0.1359 | 0.1125 | 0.002 | 0.0081 | 0 | ||||||||||
| D. franzi | EU637368 | 0.128 | 0.128 | 0.1305 | 0.1376 | 0.1216 | 0.1294 | 0.1384 | 0.1149 | 0.004 | 0.0101 | 0.002 | 0 | |||||||||
| D. franzi | EU215100 | 0.1256 | 0.1256 | 0.128 | 0.1351 | 0.1191 | 0.1269 | 0.1359 | 0.1125 | 0.002 | 0.0081 | 0 | 0.002 | 0 | ||||||||
| D. franzi | EU215099 | 0.1256 | 0.1256 | 0.128 | 0.1351 | 0.1191 | 0.1269 | 0.1359 | 0.1125 | 0.002 | 0.0081 | 0 | 0.002 | 0 | 0 | |||||||
| D. franzi * | EU637367 | 0.1233 | 0.1233 | 0.1257 | 0.1328 | 0.1168 | 0.1294 | 0.1336 | 0.1103 | 0.004 | 0.0101 | 0.002 | 0.004 | 0.002 | 0.002 | 0 | ||||||
| D. melanchaetes | EU143740 | 0.1243 | 0.1243 | 0.1219 | 0.1268 | 0.1202 | 0.0908 | 0.1137 | 0.1287 | 0.1311 | 0.1367 | 0.1335 | 0.136 | 0.1335 | 0.1335 | 0.136 | 0 | |||||
| D. muelleri | AY954398 | 0.1181 | 0.1181 | 0.1158 | 0.137 | 0.1118 | 0.1235 | 0.0934 | 0.0868 | 0.1312 | 0.1391 | 0.1335 | 0.136 | 0.1335 | 0.1335 | 0.1313 | 0.1357 | 0 | ||||
| D. nigrescens | EF464565 | 0.1316 | 0.1316 | 0.1292 | 0.1318 | 0.1225 | 0.0864 | 0.1256 | 0.1235 | 0.138 | 0.146 | 0.1403 | 0.1428 | 0.1403 | 0.1403 | 0.138 | 0.0765 | 0.1325 | 0 | |||
| D. r. meridionalis | AY954402 | 0.1144 | 0.1144 | 0.1121 | 0.105 | 0.1104 | 0.104 | 0.092 | 0.0718 | 0.1155 | 0.1232 | 0.1178 | 0.1202 | 0.1178 | 0.1178 | 0.1155 | 0.1345 | 0.0966 | 0.1269 | 0 | ||
| D. r. romanicus | AY954403 | 0.1287 | 0.1287 | 0.1312 | 0.1287 | 0.122 | 0.1275 | 0.0898 | 0.0806 | 0.118 | 0.1231 | 0.1202 | 0.1226 | 0.1202 | 0.1202 | 0.118 | 0.132 | 0.108 | 0.1339 | 0.0833 | 0 | |
| D. trifidus | EU215109 | 0.1347 | 0.1347 | 0.1372 | 0.1246 | 0.1332 | 0.11 | 0.1215 | 0.1365 | 0.1339 | 0.1419 | 0.1362 | 0.1387 | 0.1362 | 0.1362 | 0.1339 | 0.1238 | 0.148 | 0.1192 | 0.1273 | 0.142 | 0 |
Larvae were associated within this study.
Morphological separation of Drusus franzi and Drusus alpinus from other European Trichoptera
D. franzi and D. alpinus belong to the subfamily Drusinae according to the following features (Waringer 1985, 1987; Szczesny 1978; Pitsch 1993; Waringer & Graf 1997, 2004; Graf et al. 2005, Waringer et al. 2000, 2007, 2008):
Metanotum with three pairs of sclerites
All gills consist of single filaments only
Anterior third of pronotum lacks a distinct rim
The combination of the following features separate D. franzi and D. alpinus from other Drusinae known so far:
Pronotum smoothly rounded as in most members of genus Drusus and without a dorsal ridge in the caudal third (Fig. 2). This last feature is present in, e.g., Cryptothrix nebulicola, D. chrysotus, D. muelleri, D. romanicus and D. discolor
Mandibles with teeth around edges as in C. nebulicola, D. chrysotus, D. romanicus and D. discolor (Fig. 3)
Anteromedian sclerites on metanotum shorter along the larva’s longitudinal axis than their maximum median separation (Fig. 4)
Legs without additional setae on anterior faces
Head capsule dorsally convex and not flattened or concave as in D. chrysotus, D. romanicus, D. muelleri and D. discolor.
Frontoclypeal suture at level of head capsule and not at the bottom of a deep rim surrounding the frontocylpeus as in Cryptothrix nebulicola.
No hair-like structures on pronotum and head (in contrast to D. discolor and D. romanicus)
Abdominal sternum 1 with large median sclerotized patch (similar to species of genus Metanoea McLachlan) (Fig. 5)
Drusus franzi and D. alpinus are somewhat unique within Drusinae; the combination of the above mentioned features are totally unknown within the subfamily and combine characters of subfamilies Limnephilinae and Drusinae. The underlined characters are unique for these two species.
Figures 2-5.
Drusus franzi, fifth instar larva – 2: head, thorax and anterior abdomen, lateral view; 3: head, left lateral view; arrow: toothed mandible, 4: thorax, dorsal view; arrows: anterior margin of pronotum without long setae; anterior metanotal sclerites; 5: head, thorax and abdominal segment 1, ventral view; arrow: sclerotized plate;
Description of the fifth instar larva of Drusus alpinus
Head width: 1.47 - 1.53 mm.
Head capsule and pronotum dark brown to black brown, occipital region brownish, all other body sclerites yellowish. Head capsule (Fig. 3) lacking additional setae or spines. Mandibles with terminal teeth along edges as well as ridges in central concavity (Fig. 3, arrow).
In profile, dorsal line of pronotum without distinct ridge in its posterior third, smoothly rounded (Fig. 2). Black brown pronotal surface covered by black setae along lateral borders, leaving central pronotal area bare. Short whitish hairs present at anterior margin, but long setae entirely lacking (Fig. 4, arrow). Pronotum without central notch in anterior view. Prosternite inconspicuous, prosternal horn present. Mesonotum completely covered by two yellowish sclerites bordered by lateral and caudal black sclerotizations. Metanotum partially covered by three pairs of sclerites; anterior metanotal sclerites slender, their median separation being distinctly larger than their maximum extension along the body axis (Fig. 4, arrows). Numerous setae at center of first abdominal sternum originating from ovoidal, lightly sclerotized plate (Fig. 5, arrow). Lateral fringe present from segment 3 to beginning of segment 8. Dorsal gills present from segment 2 (presegmental position) to segment 4 (presegmental position). Ventral gills range from segment 2 (presegmental) to segment 5 (postsegmental). Lateral gills missing.
Additional setae lacking at anterior and posterior faces of all femora as well as at dorsal edge of tibia.
Case: Moderately curved, tapering posteriorly and consisting completely of mineral particles with grain sizes increasing distinctly in anterior direction.
Description of the fifth instar larva of Drusus franzi
All morphological characters and biometrics identical to those of D. alpinus except:
Small setal groups consisting of 3 to 4 setae, situated proximally to insertion of coxae of second and third legs in D. alpinus, whereas D. franzi with only one seta at this position.
Dorsum of abdominal segment 8 with 2 long setae in D. franzi but with 4 to 8 setae in D. alpinus.
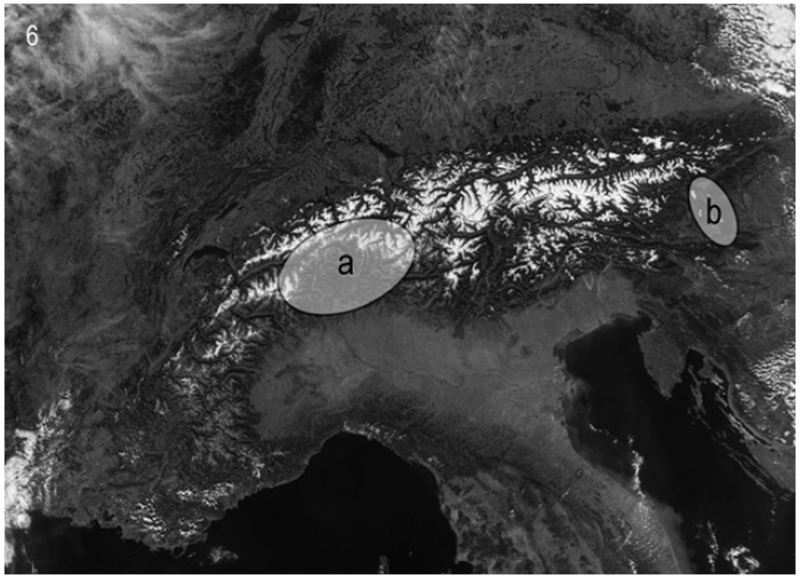
An additional feature to separate these two extraordinary Drusus species is their isolated geographical ranges (Fig. 6).
Figure 6.
Distribution of Drusus alpinus and D. franzi; a: D. alpinus; b: D. franzi
Discussion
Habitat, phenology and distribution
The small, spring-fed, 50-m-long, first order tributaries where D. alpinus was collected are clean, fast-flowing, summer-cold mountain brooks bordered by meadows. At these locations, D. alpinus was sympatric with D. muelleri, D. nigrescens, Lithax niger (Hagen 1859) and the Plecoptera Dictyogenus fontium Ris 1896, Protonemura lateralis (Pictet 1835), Leuctra ravizzai Ravizza Dematteis & Vinçon 1991 and Nemoura mortoni Ris 1902. In addition, adults were collected at Gotthard pass where Rhyacophila vulgaris Pictet 1834, R. tristis Pictet 1834, R. glareosa McLachlan 1834, Cryptothrix nebulicola, Metanoea flavipennis (Pictet 1834), Anisogamus difformis (McLachlan 1867), Drusus nigrescens, D. discolor and Philopotamus ludificatus McLachlan 1878; and the stoneflies Dictyogenus fontium Ris, Isoperla rivulorum (Pictet 1841) and Perlodes intricatus (Pictet 1841) were also found.
The habitat of Drusus franzi is quite similar. However, due to the different zoogeographic region, the following species typical for the eastern Alps were collected with it: Rhyacophila ferox Graf 2006, Rhyacophila bonaparti Schmid 1947, Rhyacophila stigmatica (Kolenati 1859), Lithax niger, Drusus monticola McLachlan 1876, D. chrysotus, D. adustus McLachlan 1867, D. discolor, Apatania fimbriata (Pictet 1834), Acrophylax zerberus Brauer 1867, Leptotaulius gracilis Schmid 1955 (Trichoptera); Taeniopteryx kuehtreiberi Aubert 1950, Rhabdiopteryx alpina Kühtreiber 1934, Brachyptera seticornis (Klapálek 1902), Leuctra prima Kempny 1899, Leuctra armata Kempny 1899, Leuctra nigra (Olivier 1811), Capnia vidua vidua Klapálek 1904, Nemoura mortoni, Nemoura minima Aubert 1946, Protonemura austriaca Theischinger 1976, Protonemura auberti Illies 1954, Nemurella pictetii Klapálek 1900, Dictyogenus fontium and Arcynopteryx compacta (McLachlan 1872) (Plecoptera).
According to Malicky (2004), D. alpinus is restricted to the western Alps; records exist from Switzerland (Lubini-Ferlin & Vicentini 2005) and Italy (Cianficconi 2002). Drusus franzi is a micro-endemic species of the eastern Alps in Austria, covering a small area situated mainly in intermediate altitudes of Saualpe, Soboth and Weinebene in south-eastern Austria (Styria and Carinthia). Both species are apparently restricted to springs and springbrooks; D. franzi is on the wing from April until July while the flight period of D. alpinus is restricted to July.
Feeding types
In his seminal work on the Drusinae, Schmid (1956) placed D. franzi and D. alpinus within the D. alpinus Group. Adults of this group are grey-brown in colour but show a distinct wing pattern which is unusual within Drusinae. Schmid (1956) considered D. franzi a very close neighbour to D. alpinus, which was confirmed in a recent molecular phylogeny on the group (Pauls et al. 2008). Based on adult and larval morphology, they constitute a distinct group within Drusinae, which was also confirmed by molecular phylogenetic analysis (Pauls et al. 2008). Larval morphology is unique and may reflect a quite basic evolutionary level within the subfamily. Teeth along mandible edges in combination with lacking filtering bristles indicate an omnivorous, shredder feeding type, which is confirmed by our own observations in the laboratory. D. alpinus larvae were reared in a strongly aerated aquarium feeding on leaves of Taraxum as well as tubificids. Narrow anteromedian tergites at the metanotum as well as the lack of additional setae on the legs are also widely present within subfamily Limnephilinae, suggesting an ancestral position of the D. alpinus Group.
Generally, in many recent phylogenies of Trichoptera, larval morphology is neglected, although it is undoubtedly an essential element in understanding the evolution of caddisflies (Scott 1975; Wiggins 1981; Weaver and Morse 1986; De Moor 2002; Pauls et al. 2008) and how aquatic ecosystems function. The larvae of D. alpinus and D. franzi and their uniquie morphology and feeding ecology clearly illustrate this.
Zoogeography
Morphological differences between the two species are minimal and genetic divergence between the pair is relatively small. Their separation is presumably the result of a fairly recent speciation event. The area of both species is restricted to small areas in the western (D. alpinus) and eastern Alps (D. franzi), respectively, and there is a gap of several hundred kilometers between their ranges (Fig. 6). Within the Drusinae and Limnephilinae there are several examples of species pairs with eastern and western areas greatly extending towards the central Alps, making the subfamily a model group for examining speciation processes within southern glacial refugia and subsequent east- and westwards expansion within the Alps. Such disjunctions include: Metanoea rhaetica Schmid (east) and M. flavipennis (west); Drusus monticola (east) and D. nigrescens (west); and among alpine Limnephilinae: Allogamus (A. uncatus Brauer (east) and A. mendax McLachlan (west)) and Consorophylax (C. styriacus Botosaneanu (east) and C. montivagus (McLachlan) (west) as well as C. consors (McLachlan) (east) and C. piemontanus Botosaneanu (west)). Similar patterns have also been observed between divergent lineages within species, which implicates comparable mechanisms of regression, speciation and expansion (e.g., D. discolor, Pauls et al. 2006; R. pubescens Pictet, Engelhardt, submitted). The Alpine mountain chains are aligned primarily in west–east orientation and represent massive dispersal barriers. Most probably valleys served as recolonization pathways from southern European or circum-alpine refugia. The areas of overlap between eastern and western species of the above mentioned species pairs vary in size but fit well within the overall separation of Western and Eastern Alps along the “Splügen-line” in eastern Switzerland and westernmost Austria (Schmölzer, 2001).
Concentrations of endemic species in the south and south-eastern Alps are well known among Trichoptera species (Malicky 1983, 2000). Recent morphological and molecular analyses are revealing sister relationships among many of these taxa, suggesting general patterns of speciation in the Alps, resulting from repeated glacial and post-glacial expansion and regression of the ranges of freshwater insects. Further study is needed to identify periods of diversification and more detailed patterns of morphological and genetic differentiation within species pairs to better understand how diversification was driven in the circum-alpine region. However, the presented biogeographic data support the Dinodal theory of Malicky (1983, 2000), which suggests glacial species-specific areas within the Alps.
Acknowledgements
We thank P. Wenzl for his assistance and W. Lechthaler for providing the photographs. This paper is part of the results of a project dealing with larval taxonomy of Central European Drusinae (project number P18073-B03, PI: J. Waringer) funded by the Austrian Science Fund (FWF).
References
- Bohle HW. Drift-catching and feeding behaviour of the larvae of Drusus discolor (Trichoptera: Limnephilidae) Archiv für Hydrobiologie. 1983;97:455–470. [Google Scholar]
- Bohle HW. Drift-catching larvae in the subfamily Drusinae (Trichoptera: Limnephilidae) Entomologia Generalis. 1987;12:119–132. [Google Scholar]
- Botosaneanu L. Cercetari aspura trichopterelor din masivul Retezat si muntii Banatului. Bibliotec de Biologie Animala Editura Academiei Republicii Populare Romine. 1959:1–165. [Google Scholar]
- Cianficconi F. The third list of Italian Trichoptera (1990-2000). Proceedings of the 10th International Symposium on Trichoptera. Nova Supplementa Entomologica. 2002;15:349–358. [Google Scholar]
- De Moor FC. An assessment of the global distribution of Leptocerinae (Trichoptera) and use of larval characters for determining phylogenetic relationships. Nova Supplementa Entomologica. 2002;15:293–308. [Google Scholar]
- Décamps H, Pujol JY. Les larves de Drusinae des Pyrenees (Trichoptera, Limnephilidae) Annles de Limnologie. 1975;11:157–167. [Google Scholar]
- Engelhardt CHM. Phylogeny and phylogeography of Rhyacophila pubescens Pictet (Trichoptera: Rhyacophilidae) University of Duisburg-Essen; pp. XX–XX. submitted. PhD Thesis. [Google Scholar]
- Graf W. Beschreibung der Larven von Rhyacophila producta und Rhyacophila stigmatica und einer Larve aus der Unterfamilie Drusinae (Trichoptera: Rhyacophilidae, Limnephilidae) Braueria. 1993;20:17–18. [Google Scholar]
- Graf W, Lubini V, Pauls S. Larval description of Drusus muelleri McLachlan, 1868 (Trichoptera: Limnephilidae) with some notes on its ecology and systematic position within the genus Drusus. Annales de Limnologie – International Journal of Limnology. 2005;41:93–98. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Lubini-Ferlin V, Vicentini H. To the knowledge of the Swiss caddis fly fauna (Insecta: Trichoptera) Lauterbornia. 2005;54:63–79. [Google Scholar]
- Malicky H. Chlorological patterns and biome types of European Trichoptera and other freshwater insects. Archiv für Hydrobiologie. 1983;96:223–244. [Google Scholar]
- Malicky H. Eine aktualisierte Liste der österreichischen Köcherfliegen (Trichoptera) Braueria. 1999;26:31–40. [Google Scholar]
- Malicky H. Arealdynamik und Biomgrundtypen am Beispiel der Köcherfliegen (Trichoptera) Entomologica Basiliensia. 2000;22:235–259. [Google Scholar]
- Malicky H. Atlas of European Trichoptera. Springer; Dodrecht: 2004. pp. 1–359. [Google Scholar]
- Pauls SU, Lumbsch HT, Haase P. Phylogeography of the montane caddisfly Drusus discolor: evidence for multiple refugia and periglacial survival. Molecular Ecology. 2006;15:2153–2169. doi: 10.1111/j.1365-294X.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- Pauls SU, Graf W, Haase P, Lumbsch HT, Waringer J. Grazers, shredders and filtering carnivores - The evolution of feeding ecology in Drusinae (Trichoptera: Limnephilidae): insights from a molecular phylogeny. Molecular Phylogenetics and Evolution. 2008;46:776–791. doi: 10.1016/j.ympev.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse P. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsch T. Zur Larvaltaxonomie, Faunistik und Ökologie mitteleuropäischer Fließwasser-Köcherfliegen (Insecta: Trichoptera) Landschaftsentwicklung und Umweltforschung, Sonderheft. 1993;8:1–316. [Google Scholar]
- Robert B. Verzeichnis der Köcherfliegen (Trichoptera) Deutschlands. Die Köcherfliegen-Fauna Deutschlands: Ein kommentiertes Verzeichnis mit Verbreitungsangaben. In: Klausnitzer B, editor. Entomofauna Germanica 5. Vol. 6. Entomologische Nachrichten und Berichte Dresden; Beiheft: 2001. pp. 107–151. [Google Scholar]
- Robert B. Systematisches Verzeichnis der Köcherfliegen (Trichoptera) Deutschlands Fortschreibung 02/2004. Entomologie heute. 2004;16:93–107. [Google Scholar]
- Schmid F. La sous-famille des Drusinae (Trichoptera, Limnephilidae) Memoires de l’ Institut Royale des Sciences Naturelle de Belgique, 2eme Series. 1956;55:1–92. [Google Scholar]
- Schmölzer K. Wo liegt die Grenze zwischen Ost- und Westalpen? Zur Frage der Verteilung biographischer Arealgrenzen im Alpenraum. Gredleriana. 2001;1:227–242. [Google Scholar]
- Scott KMF. The value of larval stages in systematic studies of the Trichoptera, with particular reference to the Hydropsychidae from Africa south of the Sahara; Proceedings of the 1st congress of the Entomological Society of southern Africa; Stellenbosch. 1975.pp. 41–52. [Google Scholar]
- Szczesny B. Larvae of the subfamily Drusinae (Insecta: Trichoptera) from the Polish part of the Carpathian Mts. Acta Hydrobiologia. 1978;20:35–53. [Google Scholar]
- Waringer J. The larva of Metanoea rhaetica Schmid, 1955 (Trichoptera: Limnephilidae: Drusinae) from a small Austrian mountain brook. Aquatic Insects. 1985;7:243–248. [Google Scholar]
- Waringer J. The larva of Drusus chrysotus (Rambur, 1842) (Trichoptera: Limnephilidae) from an Austrian brook. Aquatic Insects. 1987;9:21–25. [Google Scholar]
- Waringer J, Graf W. Atlas der österreichischen Köcherfliegenlarven. Facultas Univeritätsverlag; Wien: 1997. pp. 1–286. [Google Scholar]
- Waringer J, Graf W. Ergänzungen und Berichtigungen zum “Atlas der österreichischen Köcherfliegenlarven unter Einschluss der angrenzenden Gebiete.” Beilage zum 2. unveränderten Nachdruck. Facultas Universitätsverlag; Wien: 2004. p. 28. [Google Scholar]
- Waringer J, Graf W, Maier K-J. The larva of Metanoea flavipennis Pictet, 1834 (Trichoptera: Limnephilidae: Dusinae) Aquatic Insects. 2000;22:66–70. [Google Scholar]
- Waringer J, Graf W, Pauls S, Lubini V. The larva of Drusus nigrescens Meyer-Dür, 1875 (Trichoptera: Limnephilidae: Drusinae) with notes on its ecology, genetic differentiation and systematic position. Annales de Limnologie – International Journal of Limnology. 2007;43:161–166. doi: 10.1051/limn:2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waringer J, Graf W, Pauls SU, Vicentini H, Lubini V. DNA based association and description of the larval stage of Drusus melanchaetes McLachlan, 1876 (Trichoptera: Limnephilidae: Drusinae) with notes on ecology and zoogeography. Limnologica. 2008;38:34–42. doi: 10.1016/j.limno.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JS, III, Morse JC. Evolution of feeding and case-making behaviour in Trichoptera. Journal of the North American Benthological Society. 1986;5:150–158. [Google Scholar]
- Wiggins GB. Considerations on the relevance of immature stages to the systematics of Trichoptera. Series Entomologica. 1981;20:395–407. [Google Scholar]