Abstract
Objective
Various definitions of metabolic health have been proposed to explain differences in the risk of type 2 diabetes within body mass index (BMI) categories. The goal of this study was to assess their predictive relevance.
Research Design and Methods
We performed systematic searches of MEDLINE records for prospective cohort studies of type 2 diabetes risk in categories of BMI and metabolic health. In a two-stage meta-analysis, relative risks (RR) specific to each BMI category were derived by network meta-analysis and the resulting RRs of each study were pooled using random effects models. Hierarchical summary receiver operating characteristic curves were used to assess predictive performance.
Results
In a meta-analysis of 140,845 participants and 5,963 incident cases of type 2 diabetes from 14 cohort studies, being classified as metabolically unhealthy was associated with higher relative risk of diabetes in all BMI categories (RR compared with healthy individuals [95% confidence interval, CI]: lean, 4.0 [3.0 – 5.1]; overweight, 3.4 [2.8 – 4.3]; obese, 2.5 [2.1 – 3.0]). Metabolically healthy obese individuals had a high absolute risk of type 2 diabetes (10 year cumulative incidence [95% CI]: 3.1% [2.6 – 3.5%]). Current binary definitions of metabolic health had high specificity (pooled estimate [95% CI]: 0.88 [0.84 – 0.91]) but low sensitivity (0.40 [0.31 – 0.49]) in lean individuals and satisfactory sensitivity (0.81 [0.76 – 0.86]) but low specificity (0.42 [0.35 – 0.49]) in obese individuals. However, positive (< 3.3 in all BMI categories) and negative (> 0.4) likelihood ratios were consistent with insignificant to small improvements in prediction.
Conclusions
Although individuals classified as metabolically unhealthy have a higher relative risk of type 2 diabetes compared with individuals classified as healthy in all BMI categories, current binary definitions of metabolic health have limited relevance to the prediction of future type 2 diabetes.
Obesity and the ‘metabolic syndrome’, two highly prevalent and often coexisting conditions, are major risk factors for type 2 diabetes and cardiovascular disease (1-3). The observation that some obese individuals have a favourable metabolic profile and appear to be at low risk of obesity related complications has led to the notion of ‘metabolically healthy obesity’ (4,5). The topic has received much attention in recent times, with an increasing number of studies using definitions of metabolic health in body mass index (BMI) categories either as a risk factor or an outcome (5-9).
In the existing literature, however, there is little consensus on the definitions of metabolic health (4). In addition, the relevance of metabolic health definitions to the prediction of incident type 2 diabetes in body mass index (BMI) categories has not been investigated.
Establishing the predictive value of currently used definitions of metabolic health has been deemed of primary importance, as an accurate risk classification may justify selective preventive action in high-risk individuals (4). In addition, the construct of the ‘metabolic syndrome’, which is used as a basis for several definitions of metabolic health, has been proposed as a clinically useful predictor of the risk of future type 2 diabetes (10). It is of particular interest to establish whether current definitions of metabolic health help identify lean individuals at high-risk of type 2 diabetes (i.e. the ‘metabolically unhealthy lean’) or obese individuals at low risk of type 2 diabetes (i.e. the ‘metabolically healthy obese’).
We therefore reviewed the literature on the definitions of metabolic health and assessed their relevance to the prediction of incident type 2 diabetes in lean, overweight or obese individuals.
Research Design and Methods
Literature searches
This report adheres to the PRISMA guidelines, where applicable (11). We used three complementary strategies in order to assess the existing literature on the definitions of metabolic health in BMI categories. Firstly, we reviewed titles and abstracts of all references cited by Stefan et al. (4) and Kramer et al. (5) in recent reviews on the topic. Secondly, we reviewed titles and abstracts retrieved by a MEDLINE search from inception through to the 1st of September 2014 with the following terms (Search 1): ‘metabolically-healthy obesity OR metabolically-healthy obese OR metabolically healthy obesity OR metabolically healthy obese’. In order to maximise sensitivity for the detection of studies of incident type 2 diabetes, we conducted a second MEDLINE search from 2000 to the 1st of September 2014 with the following strategy (Search 2): ‘(Diabetes Mellitus, Type 2[MeSH Terms] OR “type 2 diabetes”[Title/abstract] OR “diabetes”[Title] OR Type II Diabetes Mellitus OR Type 2 Diabetes Mellitus OR Noninsulin-Dependent Diabetes Mellitus OR Diabetes Mellitus, Type II OR Diabetes Mellitus, Type 2 NOT “type 1 diabetes”[Title/abstract]) AND (adiposity OR “body mass index”[MeSH Terms] OR body mass index[Text Word] OR “overweight”[MeSH Terms] OR overweight[Text Word] OR “obesity”[MeSH Terms] OR obesity[Text Word]) AND (metabolic health OR metabolically healthy OR metabolic status OR high risk OR risk category OR risk stratification OR cardiometabolic health OR cardiometabolic risk) AND (epidemiologic studies[MeSH Terms] OR epidemiologic study OR observational study OR case-control OR cross-sectional OR case-cohort OR longitudinal study OR cohort OR cohort study OR follow-up study OR cohort analysis OR incidence study) AND (humans[MeSH Terms]) AND (“2000”[Date - Publication] : “3000”[Date - Publication]) AND English[Language]’.
Titles, abstracts and full articles were reviewed by one author (L.A.L.) with the following criteria. For title review, the title had to refer to the definition of metabolic health or cardio metabolic risk stratification in lean, obese or overweight individuals. For abstract and full article reviews, the following inclusion criteria were used: (a) the study had cross-sectional, case-control, cohort, cohort-derived design (nested case-control or case-cohort); (b) the study provided an explicit definition of metabolic health in lean, overweight or obese individuals or used one or more variables to stratify cardiometabolic risk in these categories. Exclusion criteria were as follows: (a) the manuscript reported a randomised controlled trial, another intervention study (e.g. life-style interventions or studies on individuals who were candidate to bariatric surgery) or a review of the literature; (b) the study used obesity, BMI, or another anthropometric variable as the risk factor or as the outcome, rather than stratifying variable; (c) the study was restricted to metabolically healthy or unhealthy individuals only, was not conducted in adult humans, was a genetic association study, or was restricted to patients with diabetes or other cardio-metabolic disease.
One author (A.S.) was asked to independently review 10% of the records of each stage of each search. Inconsistencies were resolved by repeated review and discussion. Concordance was high (96% for titles, n = 256/269; 97% for abstracts, n = 68/70; 100% for articles, n = 18/18). Data on definitions of metabolic health from all screened articles (n = 126) and full information from articles reporting on incident type 2 diabetes (n = 16) were extracted. Studies on incident type 2 diabetes were qualitatively assessed using a modified version of the scoring system proposed by Bell et al. in a recent review (6).
Definition of BMI and metabolic health categories in studies of incident type 2 diabetes
For studies in non-East Asians, BMI categories were defined as follows: lean < 25, overweight 25-29.9 and obese ≥ 30 kg/m2 (12). For East Asians, we used categories of BMI associated with ‘acceptable, increased and high’ risk of metabolic disease according to a recent communication of the World Health Organization, i.e. lean < 23, overweight 23-27.4 and obese ≥ 27.5 kg/m2 (13). Where the authors did not use these cut-offs, we contacted them using a standardised electronic mail message and asked for a reclassification of the participants.
Metabolic health definitions, mainly consisting of insulin resistance and metabolic syndrome, were carried over from the original reports.
Two-stage meta-analysis
Our meta-analysis was conducted in two stages. In the first stage, we used network meta-analysis to derive, for each study, the relative risk (RR) of type 2 diabetes of metabolically unhealthy compared with healthy individuals in the lean, overweight and obese categories. In the second stage, we pooled the resulting RRs using random effects models
Network meta-analysis
The primary objectives of this study were to (a) assess the risk of type 2 diabetes associated with current definitions of metabolic health within the lean, overweight and obese categories and (b) to assess the predictive relevance of these definitions. However, only one study (14) reported RR comparing unhealthy and healthy individuals within each BMI category. In all the other instances, articles reported the risk of all groups relative to the metabolically healthy lean (6,15-28). These comparisons may be of limited value when assessing the predictive relevance of metabolic health definitions. For instance, a comparison of metabolically unhealthy obese vs metabolically healthy lean simultaneously evaluates the contribution of two risk factors (i.e. BMI and level of metabolic health).
In instances where there was a need to contact the authors for clarifications, we asked for additional analyses within BMI categories (see Supplementary Table S1). When there was no need to contact authors, we inferred the RR in the overweight and obese categories using network meta-analysis within each study (Supplementary Table S1 and Text). Network meta-analysis is a meta-analysis approach allowing indirect comparison of evidence, which has previously been applied in the context of randomised controlled trials (29,30). Here, we used this method in order to estimate, for each study, the RR of metabolically unhealthy vs healthy individuals in the overweight and obese categories. To do this, we used the comparisons with the metabolically healthy lean category as input evidence (Supplementary Text).
Meta-analysis of relative risk estimates
In the second stage of our meta-analysis, adjusted estimates of RR were pooled across studies using random effects models. Random effects models were used because of the heterogeneity in the definitions of metabolic health. Analyses were stratified by sex (i.e. studies with proportion of women ≤ 30% vs all other studies), age (i.e. mean or median age in the 3rd or 4th decade vs 5th or 6th decade), country or population (i.e. non-East Asian vs East Asian; European vs other non-East Asian vs East Asian), definition of metabolic health (i.e. metabolic syndrome vs insulin resistance), sample size (i.e. below vs above 5,000 participants), length of follow-up (below vs above 7 years of mean or median follow-up) or extent of adjustment (i.e. crude or minimal vs extensive). Because three of the sixteen selected studies were conducted in the same source population with overlapping but not identical criteria of selection and follow-up periods, only the largest of these studies was included in the main analysis. Analyses with either of the other two studies, instead of the largest, yielded comparable results (Supplementary Table S2). In studies using more than one definition, we arbitrarily chose one for inclusion in our main analysis. In doing so, we chose those analyses using metabolic syndrome as a criterion for the definition of metabolic health (i.e. in the instance of Meigs et al. (18) and Arnlöv et al. (19)) and those with more relaxed criteria for the definition of metabolic health (i.e. in the instance of Bell et al.(6)). Egger’s test and funnel plots were used to assess publication bias. The I-squared statistic was used to quantify heterogeneity. The source of heterogeneity was investigated by the aforementioned stratified analyses.
Absolute type 2 diabetes risk estimation
The cumulative incidence of type 2 diabetes in metabolic health and BMI categories was estimated using a probabilistic analysis (20,000 simulations), which simultaneously incorporates the uncertainty in the estimates of each of the input parameters. Input parameters in the model were (a) the proportion of participants who were metabolically healthy within each of the three BMI categories in our meta-analysis of 140,845 participants; (b) the RR of type 2 diabetes within each BMI category as estimated by our random effects model meta-analysis and (c) the cumulative incidence of type 2 diabetes at 5- and 10-years of follow-up from the EPIC InterAct case-cohort study (31).
Predictive relevance
The predictive relevance of metabolic health definitions was studied by hierarchical summary receiver operating characteristic curves and Fagan’s nomograms. The meta-analysis of predictive test accuracy was performed by fitting a two-level mixed effects logistic regression model, with independent binomial distributions for the true positives and true negatives conditional on the sensitivity and specificity in each study, and a bivariate normal model for the logit transforms of sensitivity and specificity between studies. Sensitivity and specificity were expressed as proportions and their use in this study pertains to the prediction of future disease in diabetes-free individuals. Fagan’s nomograms represent the predictive performance of a test with lines intercepting three parallel vertical axes. The leftmost axis indicates the pre-test probability of disease, the rightmost axis the post test probability and the central axis the positive or negative likelihood ratio. Lines are then drawn from the pre-test probability on the left through the likelihood ratio in the centre and extended to the posterior probabilities on the right to represent scenarios of a positive or negative test result in each of the three BMI categories. Likelihood ratios were used to evaluate contribution to prediction using cut-offs recommended by the authors of the fagan STATA package (32).
Statistical analysis
Analyses were carried out using STATA v13.1 (StataCorp, College Station, Texas 77845 USA). The network package was used for network meta-analysis (30), metan for random effects model meta-analysis (33), metandi (32) and fagan (URL: http://fmwww.bc.edu/repec//bocode/f/fagan.ado; author, Ben Dwamena, Division of Nuclear Medicine, Department of Radiology, University of Michigan Health System, Ann Arbor, USA) for meta-analysis of predictive test accuracy (32). The probabilistic analysis for estimation of cumulative incidences was carried out using openBUGS (34).
Results
Literature search
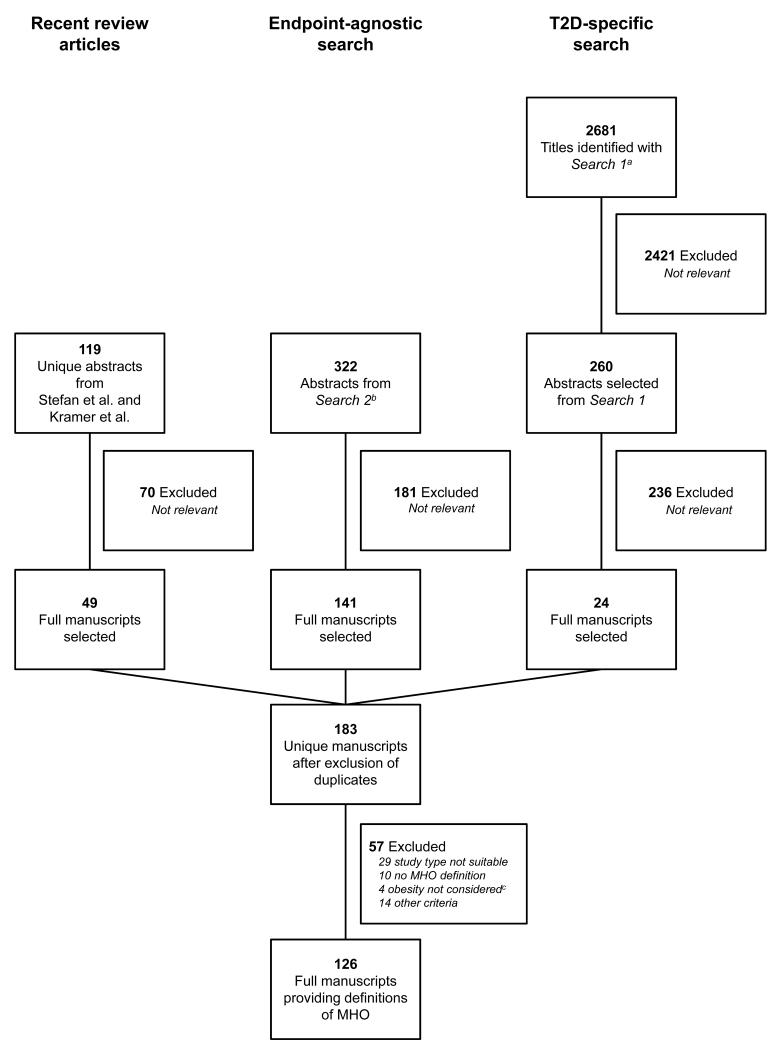
Figure 1 depicts the literature search workflow. A total of 3122 MEDLINE records were reviewed and 126 articles matching our search criteria were selected for data extraction. The articles reported a total of 177 analyses using a definition of metabolic heath. Definitions of metabolic health were mostly binary (i.e. healthy or unhealthy) and fell into five broad categories: (a) metabolic syndrome, (b) metabolic syndrome combined with insulin resistance or other criteria, (c) insulin resistance, (d) cardiorespiratory fitness, (e) miscellaneous. Metabolic syndrome defined according to the Adult Treatment Panel III criteria (35), insulin resistance according to the homeostatic model assessment (36) and combined definitions provided by Wildman et al. (7) and Karelis et al. (8) were most frequently used. Supplementary Table S3 reports the breakdown of the 177 definitions into these five broad categories.
Figure 1. Workflow of the review.
MHO indicates metabolically healthy obesity; a, Search 2 as described in the Methods section; b, Search 1, as described in the methods section; c, article restricted to unhealthy lean or used BMI as the endpoint/only risk factor rather than stratifying variable.
We identified a total of sixteen manuscripts reporting on cohort studies of incident type 2 diabetes in BMI and metabolic health categories (Table 1) (6,14-28). Studies were mostly population-based cohort studies in middle-aged individuals with a mean or median follow-up ranging from 4 to 17.5 years. Qualitative assessment revealed generally high quality (Table 1). In the main analysis, we retained only the largest (25) of three studies that investigated the same source population (16,25,28). A total of fourteen studies, comprising 140,845 participants and 5963 incident cases, were included in the quantitative meta-analysis (6,14,15,17-27).
Table 1. Cohort studies investigating incident type 2 diabetes in metabolic health and body mass index categories.
| First Author Year; Pubmed ID |
Country / years of recruitment |
Definition of metabolic healtha | Definition of BMI categories, in kg/m2 |
Study population | Sample size and incident cases (Ntotal/NT2D) |
Incident T2D ascertainment |
Length of follow-up |
Adjustment | Quality scoreb |
|---|---|---|---|---|---|---|---|---|---|
| Meigs 2006; 16735483 | Framingham Offspring Study, USA 1991-1995 | (1) MetS, 3 / 5 ATPIII criteria: FG < 5.6, WC ≤ 88 102 M or 88 W, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85 (2) IR: HOMA-IR ≤ 75th percentile of distribution in subjects without T2D |
Lean < 25 OW 25-29.9 Obese ≥ 30 | Offspring of community-based study; European ancestry; free from CVD and T2D at baseline; mean age, 54 years; women, 55% | (1) 2902 / 141 (2) 2803 / 135 |
FG ≥ 7.0 mmol/L or new use of hypoglycaemic therapy | Mean 6.8 years | Age, sex, family history of diabetes, and impaired glucose tolerance | 6 |
| Arnlöv 2011; 20852030 | Uppsala Longitudinal Study of Adult Men, Sweden 1970-1973 | (1) MetS: 3 / 5 ATPIII criteria: FG < 6.1c, BMI ≥ 29.4d,TG < 1.7, HDL ≥ 1.04, BP < 130/85 (2) IR: HOMA-IR ≤ 75th percentile of distribution in subjects without T2D, i.e. 3.43 |
Lean < 25 OW 25-30 Obese > 30 | Community-based study of men born in 1920-1924 free from T2D at baseline; mean age, 50 years, women, 0% | (1) 1675 / 160 (2) 1385 / 117 |
FG ≥ 7.0 mmol/L at follow-up or data from national hospitaldischarge registry | Up to 20 years | Age, smoking status, and level of physical activity | 6 |
| Hadaegh 2011; 21609497 | Tehran Lipid and Glucose Study, Iran 1999-2001 | MetS: 3 / 5 harmonised criteria: FG < 5.5, WC < 94.5, TG < 1.7, HDL ≥ 1.04 M or 1.3 W, BP < 130/85 | Lean < 25 OW 25-29.9 Obese ≥ 30 | Population based cohort study in Tehran; mean age, 42 years; women, 58% | 5,250 / 369 | Self-reported or OGTT-based at two follow-up visits | Median 6.5 years | Age, family history of T2D, history of CVD, education, smoking status | 6 |
| Kim 2012; 22621338 | South Korea 2005 | MetS: 3 / 5 2009 harmonised criteria: FG < 5.6, WC < 90 M or 80 W, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85 | Lean < 23 OW 23-27.4 Obese ≥ 27.5 | Subjects attending baseline and follow-up visits at Health Promotion Centre; mean age, 48 years; women, 35% | 8,748 / 308 | FG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% or treatment | 5 years | Age, sex, smoking, alcohol consumption, and physical activity | 6 |
| Bo 2012; 23034958 | Italy 2001-2003 | MetS plus IR: 3 / 5 harmonised criteria: FG < 5.6, WC < 94 M or 80 W, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85 AND HOMA-IR < 2.5 | Lean < 25 OW 25-30 Obese > 30 | Caucasian volunteers from Local Health Units; mean age, 54 years; women, 53% | 1,658 / 72 | Self-reported, FG, demographic registries | 9 years | Nonee | 6 |
| Appleton 2013; 23491523 | North West Adelaide Health Study, Australia 1999-2003 | MetS: 3 / 4 IDF criteria: FG < 5.6, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85 | Lean 18.5-24.9 OW 25-29.9 Obese ≥ 30 | Community-based study; Adults of European ancestry; free from T2D and CVD at baseline; mean age, 42 yearsf; womenf, 57% | 2315 / 112 | Self-reported doctor diagnosis or FG ≥ 7.0 mmol/L | Median 8.2 years | Age, sex, household income, and family history of diabetes | 6 |
| Soriguer 2013; 23559087 | Prospective Pizarra Study, Spain 1997-1998 | MetS plus IR: 3 / 3 criteria: FG < 6.1, TG < 1.7, HOMA-IR < 90th percentileg | Lean < 25 OW 25-29.9 Obese ≥ 30 | Population-based cohort study; mean age, 40 years; women, 62%h | 387 / 38e | Self-reported or FG at follow-up | 11 years | Age, sexe | 5 |
| Aung 2014; 24257907 | San Antonio Heart Study, USA 1979-1988 | MetS plus IR: 4 / 5 criteria: FG < 5.6, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85, HOMA-IR ≤ 5.13 | Lean < 25 OW 25-29.9 Obese ≥ 30 | Population based cohort study of Mexican and Caucasian Americans; mean age, 42 years; women, 57% | 2,814 / 262 | OGTT or medication at follow-up | Median 7.4 years | Age, sex, ethnicity, family history of diabetes, fasting glucose | 6 |
| Sung 2012; 24361070 | South Korea 2003 | IR: HOMA-IR < 2, i.e. 75th percentile | Lean < 23 OW 23-27.49 Obese ≥ 27.5e | Participants of health examination at hospital; mean age, 41 years; women, 29% | 12,853 / 223 | Self-reported, medical history or FG at follow-up | 5 years | Age, sex, alcohol, smoking status, exercise, educational status, baseline glucosee | 5 |
| Bell 2014; 24661566 | English Longitudinal Study of Ageing, UK 2004-2005 | (1) MetS plus CRP: 3 / 5 customised criteria: Hba1c < 6%, TG < 1.7, HDL ≥ 1 M or 1.3, BP < 130/85, CRP < 0.3 mg/dL (2) MetS plus CRP: 4 / 5 of the criteria used in definition (1) |
Lean < 25 OW 25-29.9 Obese ≥ 30 | Population-based cohort study; mean age, 65 years; women, 57% | 3,060 / 138 | Self-reported physician diagnosis | Mean 5.9 years | Age, sex, smoking, alcohol intake, physical activity, wealth, depressive symptoms | 5 |
| Hinnouho 2014; 24670711 | Whitehall II Study, UK 1991-1993 | MetS: 3 / 4 ATPIII criteria: FG < 5.6, TG < 1.7, HDL ≥ 1.04 M or 1.29 W, BP < 130/85 | Lean 18.5-24.9 OW 25-29.9 Obese ≥ 30 | Cohort study of office workers in central London; mean age, 49 years; women, 30% | 7,122 / 798 | OGTT, physician diagnosis or use of medication at follow-up | Median 17.5 years | Sex, socioeconomic status, marital status, ethnicity, physical activity, smoking, alcohol, fruits and vegetables consumption, CVD medications and procedures | 6 |
| Heianza 2014 J Clin Endocrinol Metab; 24823457 | Toranomon Hospital Health Management Centre Study, Japan 1997-2002 | MetS: 3 / 4 IDF criteria: FG < 5.6, TG < 1.7, HDL ≥ 1.03 M or 1.29 W, BP < 130/85 | Lean < 23 OW 23-27.4 Obese ≥ 27.5e | Cohort Study of Japanese government employees; mean age, 48 years; women, 27% | 8,090 / 274e | FG ≥ 7, Hba1c ≥ 6.5%, or self-reported | 5 years | Age, sex, smoking, physical activity, alcohol intake, family history of diabetese | 6 |
| Rhee 2014; 24870949 | South Korea 2005 | MetS plus IR: 3 / 4 criteria: FG < 5.6, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85 AND HOMA-IR < 90th percentile | Lean < 23 OW 23-27.4 Obese ≥ 27.5e | Participants of medical check-up programme; mean age, 43 years; women, 27% | 6,748 / 277 | FG, Hba1c or selfreported history or medication | 4 years | Age, sex, ALT, creatinine, total cholesterol, hs-CRPe | 6 |
| Heianza 2014 Obesity; 25131796 | Japan 1999-2004 | MetS: 3 / 4 IDF criteria: FG < 5.6, TG < 1.7, HDL ≥ 1.03 M or 1.29 W, BP < 130/85 | Lean < 23 OW 23-27.4 Obese ≥ 27.5e | Cohort Study of individuals occupational health examinations; mean age, 47 years; women, 36% | 27,891 / 1,668e | FG ≥ 7, Hba1c ≥ 6.5%, or self-reported | 8 years | Age, sex, smoking, physical activitye | 6 |
| Twig 2014; 25139886 | Metabolic, Lifestyle and Nutrition Assessment in Young Adults, Israel 1995-2011 | MetS: 3 / 4 ATPIII criteria: FG < 5.6, TG < 1.7, HDL ≥ 1, BP < 130/85 e,i | Lean < 25 OW 25-29.9 Obese ≥ 30 | Cohort study of men from the Israel Defence Forces; mean age, 31 years; women, 0% | 33,939 / 734 | FG or physician diagnosis | Median 6.1 years | Age, family history of diabetes, country of origin, WBCe | 6 |
| Jung 2014; 25155902 | South Korea 2005-2006 | MetS: 4 / 4 IDF criteria: FG < 5.6, TG < 1.7, HDL ≥ 1 M or 1.3 W, BP < 130/85 | Lean < 23 OW 23-27.4 Obese ≥ 27.5e | Cohort study of employees of large Korean company and their spouses; mean age, 37 years; women, 44% | 34,994 / 889e | FG ≥ 7, Hba1c ≥ 6.5% or medication | 5 years | Age, sex, smoking, alcohol intake, physical activitye | 6 |
Abbreviations: BMI, body mass index; T2D, type 2 diabetes; MetS, metabolic syndrome; IR, insulin resistance; HOMA-IR, Homeostatic model assessment – insulin resistance; ATPIII, Adult Treatment Panel III; FG, fasting glucose; TG, triglycerides; HDL, high-density lipoprotein cholesterol; BP, blood pressure; WC, waist circumference; M, men; W, women; OW, overweight; OGTT, oral glucose tolerance test; WBC, white blood cells; IDF, International Diabetes Federation; CVD, cardiovascular disease; hs-CRP, high-sensitivity C-reactive protein.
Notes: All cut-off values expressed as cm for waist circumference, mmol/L for fasting glucose, HDL cholesterol or triglycerides, kg/m2 for BMI and mmHg for blood pressure. In some studies treatment with medication (e.g. anti-hypertensive drugs) was used as a complementary factor to adjudicate metabolic risk criteria.
We used a quality score similar to the one reported by Bell et al. Study quality was assessed according to the definition of exposure, outcome and to the extent of adjustment. Points were assigned as follows: 2 points if the study considered metabolic risk factor clustering as in the metabolic syndrome; 1 point if the study considered insulin resistance only; 2 points if diabetes diagnosis was based on objective clinical measurements (e.g. fasting or two hour glucose levels); 1 point if diabetes adjudication was based on self-report only; 2 points for extensive adjustment, i.e. age, sex plus at least two of the following, family history of diabetes, ethnicity, alcohol consumption, smoking status, physical activity, dietary habits and socioeconomic status, impaired glucose tolerance status; 1 point for basic adjustment, i.e. age and sex; 0 points for crude estimates. Studies were scored out of 6 possible points. Adjustment in the original report was used to adjudicate the extent of adjustment.
Of fasting blood glucose, corresponding to fasting plasma glucose of 5.6 mmol/L.
BMI used in lieu of waist circumference criterion.
Information as reported by the authors in a personal communication.
Average of metabolically healthy lean, metabolically healthy obese, metabolically unhealthy obese groups.
The authors reported 4 different definitions of metabolic health. Here we report the one we used in the meta-analysis, for which the authors provided detailed results of type 2 diabetes incidence in a personal communication.
In the full baseline study.
In the original report metabolic health was the absence of any metabolic syndrome criteria and the risk of type 2 diabetes was evaluated for individuals with 1, 2, 3 or more criteria separately
Network meta-analysis and random effects meta-analysis of relative risk estimates
In a meta-analysis of type 2 diabetes risk within BMI and metabolic health categories, all groups had higher risk when compared to the healthy lean group (RR [95% confidence interval, CI]: metabolically unhealthy lean group, 4.0 [3.0 – 5.1]; metabolically healthy overweight group, 1.8 [1.5 – 2.2]; metabolically unhealthy overweight group, 6.2 [ 4.8 – 8.0]; metabolically healthy obese group, 4.1 [3.3 – 5.1]; metabolically unhealthy obese group, 10.9 [8.5 – 13.9]; see also Supplementary Table S4 and Figure S1).
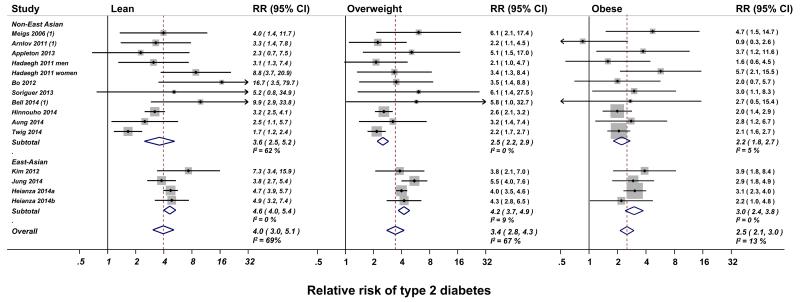
Where analysis results were not available from the manuscript or from direct contact with the authors, network meta-analysis was used to derive within-overweight and within-obese category RRs and 95% CI. In instances where both estimates were available, the central estimate of RR provided by the authors strongly correlated with that obtained by network meta-analysis (average r2 > 0.95; n = 18; see Supplementary Table S1). The relative risks obtained in this first stage were then pooled using random effects meta-analysis. Random effects meta-analysis revealed that – when compared with healthy individuals – metabolically unhealthy individuals are at higher risk of type 2 diabetes in all BMI categories (RR compared with healthy individuals [95%, CI]: lean, 4.0 [3.0 – 5.1], N at risk / type 2 diabetes = 67,281 / 1,393; overweight, 3.4 [2.8 – 4.3], N at risk / type 2 diabetes = 58,060 / 2,903; obese, 2.5 [2.1 – 3.0], N at risk / type 2 diabetes = 15,504 / 1,667; see Figure 2). The relative risk of type 2 diabetes associated with being classified as unhealthy was highest in the lean category and lowest in the obese category. Funnel plots and Egger’s test (P > 0.1 for all comparisons) indicated no publication bias (Supplementary Figure S2). There was heterogeneity between studies in the estimated RR within the lean and overweight categories, but not the obese category (Figure 2). Stratified analyses revealed that ethnicity was likely the driver of heterogeneity, with RR higher in East Asian populations in all BMI categories (Figure 2). Further stratification for geographic region (European vs East Asian studies) resolved residual heterogeneity in estimates in the lean category (Supplementary Figure S3). Results were similar in studies using metabolic syndrome or insulin resistance as definitions for metabolic health.
Figure 2. Relative risk of type 2 diabetes in metabolically unhealthy compared with healthy individuals by body mass index category and country (non-East Asian or East Asian).
Absolute type 2 diabetes risk estimation and predictive relevance
Using a probabilistic analysis, which simultaneously incorporates uncertainty in all the input parameter estimates, we estimated the cumulative incidence of type 2 diabetes at 5 and 10 years in all BMI and metabolic health categories (Table 2). Metabolically healthy obese individuals had a cumulative incidence of type 2 diabetes over 10 years of 3.1% (95% CI: 2.6 – 3.5%). Cumulative incidence estimates from a sensitivity analysis after exclusion of studies in East Asian populations were largely overlapping with those of the main analysis (Supplementary Table S5).
Table 2. Cumulative incidence of type 2 diabetes in metabolic health and body mass index categories.
| BMI category | BMI category cumulative incidence at 5 yearsa | BMI category cumulative incidence at 10 years a | Risk category | Proportion of healthy or unhealthy individuals in each BMI category | Relative risk within BMI category | Risk category 5 year cumulative incidence (95% CI*) | Risk category 10 year cumulative incidence (95% CI*) |
|---|---|---|---|---|---|---|---|
| Lean | 0.3 % | 0.8 % |
Metabolically
Healthy Lean |
0.82 | 1 | 0.2% (0.1 – 0.2%) | 0.5% (0.5 – 0.6%) |
|
Metabolically
Unhealthy Lean |
0.18 | 4.0 | 0.6% (0.6 – 0.8%) | 2.2% (1.9 – 2.5%) | |||
| Over weight | 0.8 % | 2.7 % |
Metabolically
Healthy Overweight |
0.59 | 1 | 0.4% (0.3 – 0.5%) | 1.3% (1.1 – 1.6%) |
|
Metabolically
Unhealthy Overweight |
0.41 | 3.4 | 1.4% (1.3 – 1.5%) | 4.5% (4.2 – 4.9%) | |||
| Obese | 1.8 % | 5.9 % |
Metabolically
Healthy Obese |
0.38 | 1 | 1.0% (0.8 – 1.1%) | 3.1% (2.6 – 3.5%) |
|
Metabolically
Unhealthy Obese |
0.62 | 2.5 | 2.4% (2.2 – 2.5%) | 7.6% (7.3 – 8.0%) |
BMI, body mass index; CI, confidence interval.
From the EPIC InterAct Study
derived via a probabilistic analysis, which simultaneously incorporates the uncertainty in each of the parameter estimate (openBUGS, 20000 simulations)
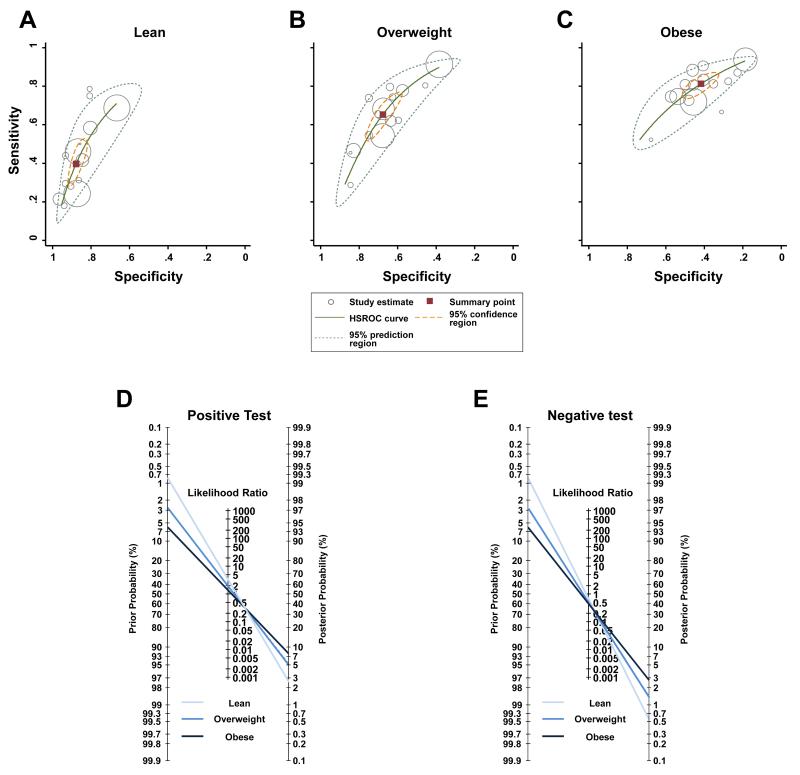
Hierarchical summary receiver operating curve analysis revealed increasing sensitivity and decreasing specificity in higher BMI categories (Figure 3A-C). In lean individuals, testing metabolic health was not sensitive (pooled estimate [95% CI]: 0.40 [0.31 – 0.49]), but was specific (0.88 [0.84 – 0.91]). In overweight individuals, both sensitivity (0.65 [0.56 – 0.74]) and specificity (0.68 [0.61 – 0.74]) were low. In obese individuals, sensitivity was acceptable (0.81 [0.76 – 0.86]), but specificity (0.42 [0.35 – 0.49]) was low. The pooled estimates of the positive likelihood ratio were 3.3 (95% CI: 2.7 – 3.9) in lean category, 2.0 (1.8 – 2.3) in the overweight category and 1.4 (1.3 – 1.5) in the obese category. The pooled estimates for the negative likelihood ratio were 0.7 (95% CI: 0.6 – 0.8) in the lean category, 0.5 (0.4 – 0.6) in the overweight category and 0.5 (0.4 – 0.5) in the obese category. These likelihood ratios show that current binary definitions of metabolic health make only small or insignificant contributions to the prediction of future type 2 diabetes in BMI categories (Figure 3D-E).
Figure 3. Performance of metabolic health definitions in the prediction of future development of type 2 diabetes.
The top Panels report hierarchical summary receiver operating characteristic (HSROC) curves of the predictive performance in lean (Panel A), overweight (Panel B) and obese (Panel C) individuals. Solid squares represent pooled estimates; open circles, individual study estimates with a size that is proportional to the weight of each study; solid lines, HSROC curves; broken lines delimit uncertainty in the estimates of the summary points (95% confidence region) or of the HSROC curves (95% prediction region). In Panels D and E, Fagan’s nomograms represent scenarios of positive (i.e. metabolically unhealthy; Panel D) or negative (i.e. metabolically healthy individual; Panel E) results of the binary classification of metabolic health in body mass index categories.
Discussion
In this study, we reviewed definitions of metabolic health, which have been used to classify the risk of metabolic disease in BMI categories. We also sought to assess the risk of type 2 diabetes associated with being classified as metabolically unhealthy in lean, overweight and obese individuals. We found that being classified as metabolically unhealthy is associated with higher risk of type 2 diabetes relative to the healthy group in all categories of BMI.
Our study is the largest meta-analysis of the risk of type 2 diabetes associated with metabolic health definitions and the only one to have assessed risk within BMI categories. In a meta-analysis by Bell et al. (6) of eight studies, with a total of 27,982 participants, relative risk was calculated using the healthy lean category as a reference. This is not informative about the risk of type 2 diabetes within BMI categories or the predictive relevance of metabolic health definitions. In this study, we obtained within BMI-category risk estimates using network meta-analysis, a method for the pooling of indirect evidence used in meta-analyses of randomised controlled trials. The method accurately estimated the relative risk in BMI categories, with a loss of precision, due to the uncertainty of indirect estimations, which was offset by the large overall sample size of this meta-analysis. Using this method, we were able to show that the relative risk of type 2 diabetes is higher in all categories of BMI for metabolically unhealthy individuals compared with those who are classified as metabolically healthy. Relative risks within the lean and overweight categories were higher in East Asian populations. This probably reflects the higher prevalence of abdominal obesity and insulin resistance in these populations compared with Europeans at a given level of BMI (37, 38).
However, relative risk only partially accounts for the predictive relevance of a given definition. Predictive relevance has to be evaluated also in the context of absolute risk. The absolute risk of individuals deemed to be ‘metabolically healthy obese’ was high, with an estimated cumulative incidence of type 2 diabetes over 10 years exceeding 3%. This raises doubts about the predictive value of currently used binary definitions of metabolic health. An analysis of hierarchical summary receiver operating characteristics curve and Fagan’s nomograms revealed limited predictive relevance in all three BMI categories.
Metabolic health definitions had a predictive performance opposite of the desirable, with low sensitivity in the lean category and low specificity in the obese category. In the lean category, metabolic health definitions had high specificity and could therefore be considered as a confirmatory test. However, there is presently no screening test to identify at-risk lean individuals in the population. With an absolute risk of 2.2% at 10 years, one would argue that metabolically unhealthy lean individuals would not be candidates for particular preventive measures besides those recommended for the general population. In overweight individuals, current binary definitions of metabolic health had low sensitivity and specificity. The metabolically healthy overweight individuals had an absolute risk of type 2 diabetes greater than that of the lean category and the metabolically unhealthy overweight individuals an absolute risk smaller than that of the obese category. In addition, the metabolically unhealthy overweight group accounted for 40% of the overweight category, which is in many countries the largest BMI category in the general population. Therefore, it is difficult to conceive preventive measures that could efficiently target such a large portion of the population. In obese individuals, using current metabolic health definitions may be sensitive. However, specificity in this group was well below acceptable levels and ‘metabolically healthy’ obese individuals still had an absolute risk greater than that of the overweight category.
In addition to these limitations, defining metabolic health entails invasive biological sampling for the measurement of biomarkers such as glucose, triglycerides, high-density lipoprotein cholesterol or insulin. In particular, the criteria for some of the definitions of metabolic health include fasting glucose, which is a major predictor of type 2 diabetes (39). Therefore, the value of using any of the other criteria (e.g. high-density lipoprotein cholesterol or triglycerides) included in these definitions in addition to fasting glucose is likely to be limited. Overall, there is little support for use of these definitions for the prediction or classification of type 2 diabetes risk in BMI categories. These considerations apply to currently-used binary definitions of metabolic health. It is possible that more comprehensive approaches to the definition of metabolic health may yield better predictive performance. Also, our meta-analytic approach pooled evidence from studies using different definitions of metabolic health. However, our analytical approach accounted for possible differences in the performance of definitions of metabolic health used in the constituent studies of the meta-analysis.
Conclusions
In conclusion, in a meta-analysis of 140,845 participants, being classified as metabolically unhealthy as compared with healthy using current binary definitions of metabolic health was associated with higher relative risk of type 2 diabetes in all BMI categories. However, when considering predictive performance in the context of absolute risk, we found that current binary definitions of metabolic health have limited predictive relevance. Our study does not support the use of current definitions of metabolic health for the prediction or classification of type 2 diabetes risk.
Supplementary Material
Supplementary Text. Network meta-analysis.
Supplementary Table S1. Comparison of estimates obtained from authors versus those obtained by network meta-analysis.
Supplementary Table S2. Main analysis results when the studies of Sung et al. or Rhee et al. were included in the meta-analysis instead of those of Jung et al.
Supplementary Table S3. Definitions of metabolic health. The Table reports definitions used in the studies selected for full article review. In parentheses the number of studies using a given definition is reported.
Supplementary Table S4. Relative risk of type 2 diabetes in different metabolic health and BMI categories compared with the metabolically healthy lean category.
Supplementary Table S5. Cumulative incidence of type 2 diabetes in metabolic health and body mass index categories after the exclusion of studies in East Asian populations.
Supplementary Figure S1. Relative risk of type 2 diabetes in different metabolic health and BMI categories compared with the metabolically healthy lean category in adjusted analyses.
Supplementary Figure S2. Representative funnel plot indicating no publication bias for the meta-analysis of type 2 diabetes risk within obese. P-value from Egger’s test = 0.53.
Supplementary Figure S3. Relative risk of type 2 diabetes in metabolically healthy vs unhealthy lean individuals in European and East Asian populations.
Acknowledgements
We acknowledge the role of the following investigators, who provided additional information on their published studies, as Contributors of the study: Gilad Twig, MD, PhD, Sheba Medical Center and the Israel Defense Forces Medical Corps, Ramat Gan, Israel; Amir Tirosh, MD, PhD, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Carolina Gutiérrez Repiso, PhD, UGCI de Endocrinología y Nutrición. Hospital Regional Universitario de Málaga. Instituto de Investigación Biomédica de Málaga (IBIMA), Spain; Gemma Rojo Martínez, PhD, UGCI de Endocrinología y Nutrición. Hospital Regional Universitario de Málaga. Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders (CIBERDEM), Málaga, Spain; Federico Soriguer MD, PhD, UGCI de Endocrinología y Nutrición. Hospital Regional Universitario de Málaga. Instituto de Investigación Biomédica de Málaga (IBIMA), Spain; Ki-Chul Sung, MD, PhD, Department of Cardiology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea; Simona Bo, MD, Department of Medical Sciences, University of Turin, Italy; Yoriko Heianza and Hirohito Sone, Department of Internal Medicine, Niigata University Faculty of Medicine, Niigata, Japan; Yasuji Arase, Health Management Center, Toranomon Hospital, Tokyo, Japan; Kiminori Kato, Niigata Association of Occupational Health, Niigata, Japan; Seungho Ryu, MD, PhD & Yoosoo Chang, MD, PhD, Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University, School of Medicine; Eun-Jung Rhee, MD, PhD and Won-Young Lee, MD, PhD, Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea; and the EPIC-InterAct Study Consortium.
Funding sources: The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under EMIF grant agreement n° 115372, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. This work was supported by the Netherlands Organization for Scientific Research (NWO), and the Medical Research Council UK (grant no. MC_U106179471). A.A. is supported by a Rubicon grant from the NWO (Project no. 825.13.004).
Footnotes
Conflict of interest disclosure: D.M.W. and A.S. are full-time employees of GlaxoSmithKline; J.M.B. is a full-time employee of Pfizer; all authors declare no conflict of interest relative to this study.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Hansen B, Smith SC, Jr., Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:e19–24. doi: 10.1161/01.ATV.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 4.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:10. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 5.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Annals of internal medicine. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014;15:504–515. doi: 10.1111/obr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Archives of internal medicine. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 8.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes & metabolism. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 9.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC endocrine disorders. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JA, Lee JH, Lim SY, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4:334–43. doi: 10.1111/jdi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (Fact sheet N. 311).Obesity and overweight. 2014
- 13.World Health Organization expert consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 14.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. European heart journal. 2015;36:551–9. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriguer F, Gutierrez-Repiso C, Rubio-Martin E, et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. The Journal of clinical endocrinology and metabolism. 2013;98:2318–2325. doi: 10.1210/jc.2012-4253. [DOI] [PubMed] [Google Scholar]
- 16.Sung KC, Cha SC, Sung JW, So MS, Byrne CD. Metabolically healthy obese subjects are at risk of fatty liver but not of pre-clinical atherosclerosis. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2014;24:256–262. doi: 10.1016/j.numecd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Hadaegh F, Bozorgmanesh M, Safarkhani M, Khalili D, Azizi F. Predictability of body mass index for diabetes: affected by the presence of metabolic syndrome? BMC public health. 2011;11:383. doi: 10.1186/1471-2458-11-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. The Journal of clinical endocrinology and metabolism. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 19.Arnlov J, Sundstrom J, Ingelsson E, Lind L. Impact of BMI and the metabolic syndrome on the risk of diabetes in middle-aged men. Diabetes care. 2011;34:61–65. doi: 10.2337/dc10-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleton SL, Seaborn CJ, Visvanathan R, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CH, Kim HK, Bae SJ, Kim EH, Park JY. Independent impact of body mass index and metabolic syndrome on the risk of type 2 diabetes in Koreans. Metabolic syndrome and related disorders. 2012;10:321–325. doi: 10.1089/met.2011.0143. [DOI] [PubMed] [Google Scholar]
- 22.Bo S, Musso G, Gambino R, et al. Prognostic implications for insulin-sensitive and insulin-resistant normal-weight and obese individuals from a population-based cohort. The American journal of clinical nutrition. 2012;96:962–969. doi: 10.3945/ajcn.112.040006. [DOI] [PubMed] [Google Scholar]
- 23.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. The Journal of clinical endocrinology and metabolism. 2014;99:462–468. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twig G, Afek A, Derazne E, et al. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes care. 2014;37(11):2989–2995. doi: 10.2337/dc14-0869. [DOI] [PubMed] [Google Scholar]
- 25.Jung CH, Lee MJ, Hwang JY, et al. Association of metabolically healthy obesity with subclinical coronary atherosclerosis in a Korean population. Obesity. 2014;22:2613–2620. doi: 10.1002/oby.20883. [DOI] [PubMed] [Google Scholar]
- 26.Heianza Y, Kato K, Kodama S, et al. Stability and changes in metabolically healthy overweight or obesity and risk of future diabetes: Niigata wellness study. Obesity. 2014;22:2420–2425. doi: 10.1002/oby.20855. [DOI] [PubMed] [Google Scholar]
- 27.Heianza Y, Arase Y, Tsuji H, et al. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20) The Journal of clinical endocrinology and metabolism. 2014;99:2952–2960. doi: 10.1210/jc.2013-4427. [DOI] [PubMed] [Google Scholar]
- 28.Rhee EJ, Lee MK, Kim JD, et al. Metabolic health is a more important determinant for diabetes development than simple obesity: a 4-year retrospective longitudinal study. PloS one. 2014;9:e98369. doi: 10.1371/journal.pone.0098369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of clinical epidemiology. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 30.White IR, Barrett JK, Jackson D, Higginsa JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Syn Meth. 2012;3:15. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.InterAct Consortium. Langenberg C, Sharp S, et al. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54:2272–2282. doi: 10.1007/s00125-011-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harbord RM, Whiting P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009;9:211–229. [Google Scholar]
- 33.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 34.Thomas A, O’Hara B, Ligges U, Sturtz S. Making BUGS Open. R News. 2006;6:12–17. [Google Scholar]
- 35.National Cholesterol Education Program Expert Panel on Detection E. Treatment of High Blood Cholesterol in A Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 37.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 38.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–9. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 39.Tirosh A, Shai I, Tekes-Manova D, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. The New England journal of medicine. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text. Network meta-analysis.
Supplementary Table S1. Comparison of estimates obtained from authors versus those obtained by network meta-analysis.
Supplementary Table S2. Main analysis results when the studies of Sung et al. or Rhee et al. were included in the meta-analysis instead of those of Jung et al.
Supplementary Table S3. Definitions of metabolic health. The Table reports definitions used in the studies selected for full article review. In parentheses the number of studies using a given definition is reported.
Supplementary Table S4. Relative risk of type 2 diabetes in different metabolic health and BMI categories compared with the metabolically healthy lean category.
Supplementary Table S5. Cumulative incidence of type 2 diabetes in metabolic health and body mass index categories after the exclusion of studies in East Asian populations.
Supplementary Figure S1. Relative risk of type 2 diabetes in different metabolic health and BMI categories compared with the metabolically healthy lean category in adjusted analyses.
Supplementary Figure S2. Representative funnel plot indicating no publication bias for the meta-analysis of type 2 diabetes risk within obese. P-value from Egger’s test = 0.53.
Supplementary Figure S3. Relative risk of type 2 diabetes in metabolically healthy vs unhealthy lean individuals in European and East Asian populations.