Abstract
Objectives
To prospectively assess the value of serum total bilirubin (TB) within 3 months of hepatoportoenterostomy (HPE) in infants with biliary atresia (BA) as a biomarker predictive of clinical sequelae of liver disease in the first two years of life.
Study design
Infants with BA undergoing HPE between June 2004-January 2011 were enrolled in a prospective, multicenter study. Complications were monitored until 2 years of age or the earliest of liver transplant (LT), death, or study withdrawal. TB below 2 mg/dL (34.2 μM) at any time in the first 3 months (TB<2.0, all others = TB≥2) after HPE was examined as a biomarker, using Kaplan-Meier survival and logistic regression.
Results
Fifty percent (68/137) of infants had TB<2.0 in the first 3 months after HPE. Transplant-free survival at 2 years was significantly higher in the TB<2.0 group vs. TB≥2 (86% vs. 20%, p<0.0001). Infants with TB≥2 had diminished weight gain (p<0.0001), greater probability of developing ascites (OR 6.4, 95% CI 2.9–14.1, p<0.0001), hypoalbuminemia (OR 7.6, 95% CI 3.2–17.7, p< 0.0001), coagulopathy (OR 10.8, 95% CI 3.1–38.2, p=0.0002), LT (OR 12.4, 95% CI 5.3–28.7, p<0.0001), or LT or death (OR 16.8, 95% CI 7.2–39.2, p<0.0001).
Conclusions
Infants whose TB does not fall below 2.0 mg/dL within 3 months of HPE were at high risk for early disease progression, suggesting they should be considered for LT in a timely fashion. Interventions increasing the likelihood of achieving TB <2.0 mg/dL within 3 months of HPE may enhance early outcomes.
Keywords: cholestasis, bile, liver, infant, health outcomes, transplant, cirrhosis, ascites, varices
Although biliary atresia (BA) is a rare disorder occurring between 1 in 8,000 and 1 in 18,000 live births, nearly half of affected children will require liver transplantation (LT) within the first two years of life. An additional 20%–30% will require LT in childhood and adolescence. Consequently, BA is the leading indication for LT in childhood, accounting for nearly 35% of pediatric LT (based upon OPTN data as of February 16, 2015, http://optn.transplant.hrsa.gov/converge/data/). The underlying cause(s) and mechanisms of progression of this fibro-inflammatory obliterative cholangiopathy are unknown. The sole intervention that has been shown to affect survival with native liver is the hepatoportoenterostomy (HPE), commonly referred to as the Kasai procedure since it was first described by Morio Kasai 1. In cases where the HPE is ineffective in restoring bile flow, there is rapid progression to biliary cirrhosis, liver failure, and death by 2–3 years of age 2. Though LT is an excellent option to restore health, prediction of the need for and determining the optimal timing of LT are challenging. Predictors of outcomes in BA have been identified largely through single center studies or retrospective multicenter analyses 3–20. Despite BA becoming the focus of increased research activity in the past decade, prospective multicenter studies of clinical outcomes are limited.
This study reports findings of the Prospective Database of Infants with Cholestasis (PROBE) study undertaken by the 16 member institutions and investigators of the National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK of the NIH)-supported Childhood Liver Disease Research Network (ChiLDReN; formerly the Biliary Atresia Research Consortium, BARC). PROBE was designed to acquire longitudinal, prospective clinical and laboratory data in a standardized fashion at defined time points to enable definitive studies of natural history of cholestatic liver diseases in infants and early childhood. Here, we focus on the analysis of the outcomes of children with BA over the first 24 months of life and the laboratory and clinical markers that predict these near-term outcomes. We hypothesized that serum levels of total bilirubin (TB) in the first 3 months after HPE (as an indicator of successful bile drainage post-HPE) would be predictive of 2 year outcomes in children with BA.
Methods
Study participants included subjects enrolled in PROBE (Clinicaltrials.gov: NCT00061828), more than two years before the data cutoff date (ie, who had the potential for at least 2 years of follow-up). Informed consent was obtained from parents or guardians and the protocol was carried out under IRB approval. Inclusion criteria for enrollment in PROBE included presentation prior to 180 days of age with cholestasis defined as serum direct or conjugated bilirubin greater than or equal to 2.0 mg/dL and greater than 20% of TB. Infants who had undergone previous hepatobiliary surgery with dissection or excision of biliary tissue before presentation at a ChiLDReN site were not eligible for PROBE. The study population for this report was further restricted to subjects with BA who underwent HPE. After September 1, 2005, PROBE participants with BA who underwent HPE were eligible to be co-enrolled in a prospective, randomized double-blinded, placebo-controlled trial of corticosteroid therapy after HPE for BA (START; Clinicaltrials.gov: NCT 00294684). Participants co-enrolled in START (n=135) were excluded from this analysis because they were the focus of a separate report 21. Corticosteroids were not typically employed as adjunctive therapy to HPE outside of START.
Data prospectively collected by study research coordinators and clinical investigators were entered into a centralized database at the data coordinating center. Baseline data included demographics, medical and family history, and laboratory studies. Follow-up visits for data collection occurred at 1, 2, 3, and 6 months after HPE and at 12, 18, and 24 months of age.
Data analyses
Descriptive data were summarized as the mean and standard deviation (SD) for continuous variables and as percentages for categorical variables. In addition to the descriptive analysis of baseline variables, we evaluated the fidelity of TB as a marker of clinical outcomes at 3 months up to 2 years of age following surgical drainage. Outcomes of interest included survival with native liver and complications of advancing liver disease, including manifestations of portal hypertension. Response to HPE was dichotomized into two groups based upon TB levels in the first 3 months after HPE. TB < 2.0 mg/dL (TB<2) was defined by any TB less than 2.0 mg/dL (34.2 μM) within the first 3 months post HPE, and TB≥2.0 was defined as never achieving a TB less than 2.0 mg/dL in the first 3 months post-HPE.
Logistic regression was used to model the probability that a specific clinical event occurred at least once in the period beginning 3 months after HPE as a function of the TB dichotomy described above. The clinical end points of interest were development of ascites (deemed clinically significant and/or requiring ongoing diuretic therapy), variceal hemorrhage (gastrointestinal bleed confirmed on endoscopy), thrombocytopenia (platelet count < 150,000 × 109/L), splenomegaly (spleen palpable more than 2 cm below the costal margin), hypoalbuminemia (serum albumin < 3.0 gm/dL), hyponatremia (serum sodium < 130 mmol/L), coagulopathy (INR > 1.5), weight-for-age z-scores (failure to thrive defined as a z-score < −2.5), height-for-age z-scores, mid-arm circumference z-scores, receiving a liver transplant, and death. The number and proportion for each TB group is reported, as well as an odds ratio, p-value, and 95% confidence interval. In addition, 2-year Kaplan-Meier survival curves were generated to examine the time course for these clinical outcomes for each TB group, starting at HPE. To determine the relationship of successful HPE drainage in the first 3 months with each outcome, in all the above analyses, participants who had experienced the outcome of interest or underwent transplant or died before 3 months after HPE were excluded from the analysis cohort. An event was defined as the first occurrence of the clinical end point, with subjects censored at the earlier of transplant or death (except for the transplant or death analysis, where this would be considered the end point), loss to follow-up, or the end of the data collection period (January 1, 2013).
To compare the mean time trajectories of weight z-score and height z-score between the two TB groups, we used a joint model of longitudinal and survival data with penalized B-splines 22, 23 to adjust for potential bias due to informative dropout caused by liver transplant 24. In addition, the same method was used to estimate the mean trajectories of TB in the two TB groups. This joint model corrects the bias caused by informative dropout in a conventional longitudinal model. The ROC AUC (area under the receiver operating characteristic curve) for logistic models predicting transplant-free survival using different TB thresholds at 3 months and 6 months post-HPE was calculated and compared 25.
All analyses were performed using SAS/STAT (SAS Institute Inc. 2008. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.).
Results
Three hundred and twenty-four infants with BA were enrolled in PROBE between June 1, 2004, and January 1, 2011, of whom 187 were excluded from this analysis. One hundred and thirty-five were also enrolled in START, 4 had laparoscopic HPE, 27 did not have an HPE procedure, and 21 had reasons prior to the 3 month post-HPE time point which precluded their inclusion in the final analysis (1 death, 3 LT, 7 withdrawn or lost to follow up from the study, and 10 without TB measurements). One hundred and thirty-seven participants (70 female) were available for analysis (82 white, 18 black, 12 Asian, 18 other race, and 4 unknown; 33 Hispanic). HPE was performed at a mean of 58.5 days (SD 20.7 days). Fourteen had biliary atresia splenic malformation as defined by the presence of polysplenia or asplenia.
Survival with Native Liver
At age 2 years, 73 children were alive with their native livers, 53 had undergone LT, 9 died without LT, and 2 were lost to follow up. The TB < 2.0 mg/dL by 3 months post-HPE criteria were met by 68 (50%) infants and 56 (82%) of these infants were alive with native liver at age 2 years (Table). In contrast, in the 69 infants in the TB≥2 group, only 15 (22%) were alive with native liver at age 2 years. Clinical complications of advancing liver disease were observed in 11 of these 15 participants (ascites – 1, splenomegaly – 5, hypoalbuminemia – 3, thrombocytopenia – 8, failure to thrive – 2, multiple complications - 9). By both logistic regression and log-rank test, death or LT within the first 2 years of life was more likely for those TB≥2 infants compared with TB<2.0 infants (Table and Figure 1).
Table 1.
Biliary Atresia Outcomes by 2 Years of Age
| Outcome Events | Number (%) of Patients with Event | Odds Ratio and 95 % CI for TB ≥ 2 vs TB < 2 mg/dL | P-value | ||
|---|---|---|---|---|---|
| All BA (n=137) | TB Status by 3 months after HPE | ||||
| TB < 2 mg/dL (n = 68) | TB ≥ 2 mg/dL (n = 69) | ||||
| Weight z-Score < −2.5 | 28 (20.4%) | 8 (11.8%) | 20 (29.0%) | 3.1 (1.2, 7.5) | 0.015 |
| Height z-Score < −2.5 | 26 (18.9%) | 10 (14.7%) | 16 (23.2%) | 1.8 (0.7, 4.2) | 0.21 |
| Midarm Circumference z-score < −2.5 | 51 (37.2%) | 24 (35.3%) | 27 (39.1%) | 1.2 (0.6, 2.4) | 0.64 |
| Hypoalbuminemia (Albumin < 3.0 gr/dL) | 46 (33.6%) | 9 (13.2%) | 37 (53.6%) | 7.6 (3.2, 17.7) | <0.0001 |
| Coagulopathy (INR >1.5) | 26 (19.0%) | 3 (4.4%) | 23 (33.3%) | 10.8 (3.1, 38.2) | 0.0002 |
| Hyponatremia (Na < 130 mEq/L) | 5 (3.7%) | 1 (1.5%) | 4 (5.8%) | 4.1(0.4, 37.9) | 0.21 |
| Thrombocytopenia (Platelets < 150,000/μL) | 65 (47.5%) | 32 (47.1) | 33 (47.8) | 1.03 (0.5, 2.0) | 0.93** |
| Splenomegaly | 86 (62.8%) | 48 (70.6) | 38 (55.1) | 0.5 (0.3, 1.03) | 0.062 |
| Ascites | 52 (38.0%) | 12 (17.7%) | 40 (58.0%) | 6.4 (2.9, 14.1) | <0.0001 |
| Variceal hemorrhage | 9 (6.6%) | 5 (7.4%) | 4 (5.8%) | 0.8 (0.2, 3.0) | 0.71 |
| Liver Transplant | 57 (41.6%) | 10 (14.7%) | 47 (68.1%) | 12.4 (5.3, 28.7) | <0.0001 |
| Death | 9 (6.6%) | 2 (2.9%) | 7 (10.1%) | 3.7 (0.7, 18.6) | 0.11 |
| Death or Liver Transplant | 66 (48.2%) | 12 (17.7%) | 54 (78.3%) | 16.8 (7.2, 39.2) | <0.0001 |
Bold indicates P value < 0.05
Note by Kaplan-Meier analysis, thrombocytopenia was more common for TB>2 (Figure 1)
Figure 1.
Kaplan-Meier estimate and its 95% confidence interval for onset of specific clinical outcomes beginning 3 months after HPE in children who did (blue dashed lines) or who did not achieve a TB < 2.0 mg/dL (red solid lines) in the first 3 months. Curves represent probability of remaining free of the clinical outcome. Rapid and clear differences were observed for thrombocytopenia (A), hypoalbuminemia (B), ascites (C), and death or liver transplant (D). Time zero is the time of the HPE. Secondary lines surrounding primary data lines indicate 95% confidence limits.
The ROC AUC for the logistic model using a TB threshold of 2.0 mg/dL at 3 months post-HPE was 0.80, indicating a good predictive power for assessing the probability of LT or death prior to transplant. We also evaluated the impact of using a few different TB values with an alternative threshold (1.5 mg/dL, 25.6 μM) and/or using TB values within the first 6 months after HPE for predicting outcomes. Using a TB threshold of 2.0 mg/dL at 6 months post-HPE did not improve the ROC significantly (AUC=0.82, p=0.412 compared with TB < 2.0 mg/dl at 3 months). Using a TB threshold of 1.5 mg/dL at 3 months (AUC=0.82, p = 0.443) also yielded similar results. Finally, using a TB threshold of 1.5 mg/dL at 6 months post-HPE (AUC=0.87) did significantly improve the ROC (p=0.012).
Manifestations of Progressive Liver Disease
Logistic regression analyses were performed for the selected end points in the first 2 years of life related to the TB status in the first 3 months after HPE. TB≥2 infants were significantly more likely to subsequently develop failure to thrive (wt z-score < −2.5), hypoalbuminemia, coagulopathy, and ascites (Table). By Kaplan-Meier analysis, the likelihood of specific adverse clinical events in the first 2 years of life was significantly less in TB<2.0 infants, including thrombocytopenia, hypoalbuminemia, ascites, and coagulopathy (Figure 1). Notably, the development of hyponatremia (3.7% overall) and variceal hemorrhage (6.6% overall) were uncommon events in this cohort and thus there was insufficient power for detecting statistically significant differences between the two TB groups. Screening endoscopy was not routine (4/subjects screened) so the presence of varices could not be used as a marker of portal hypertension.
Growth
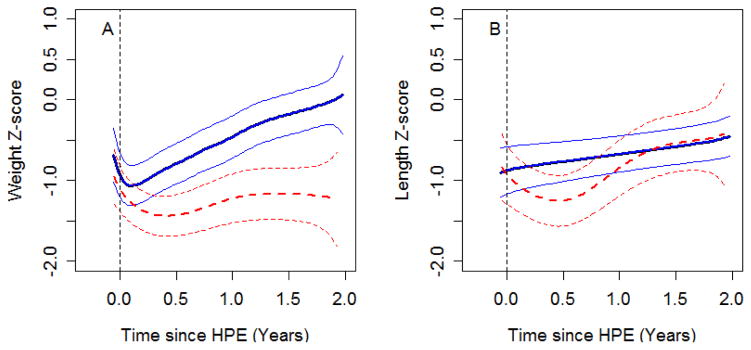
Figure 2 shows the estimated growth curves of weight z-score and height z-score for TB<2.0 infants compared with TB≥2 infants. Although the weight z-score in both groups of children decreased after HPE, children with TB<2.0 started to recover soon (about 1 month) after HPE and their mean weight z-score was near 0.0 at 2 years of age. In contrast, the mean weight z-score in children with TB≥2 dropped until about 3 months and remained approximately −1.5 through 2 years of age. The trajectories of weight z-scores of the two groups were significantly different from each other (p<0.0001). The length z-score trajectory also differed significantly between the two groups (p=0.025). Length z-score in infants with TB< 2.0 improved linearly after HPE. In contrast, mean length z-score in infants with TB≥2 dropped until 6 months after HPE and started to improve after that, primarily because of censoring of those who died or underwent LT.
Figure 2.
Growth curves plotting weight z-score (panel A) and length z-score (panel B) vs. time since HPE. Blue solid lines (subjects who achieved a TB < 2.0 mg/dL in the first 3 months after HPE) and red dashed lines (subjects who did not achieve a TB < 2.0 mg/dL) represent the average value over time and its 95% confidence intervals. The apparent improved length z-score after 1 year (dashed line) was due to censoring of those who died or underwent LT.
Stability of TB status after HPE
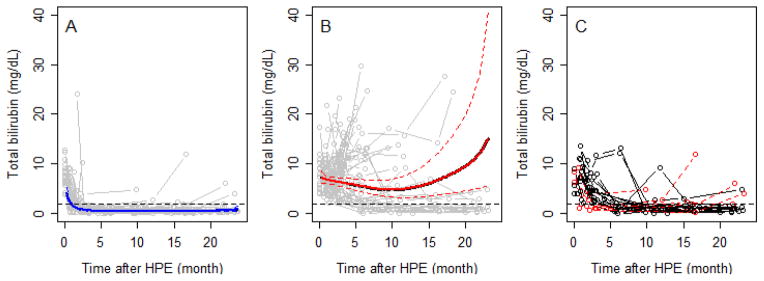
To further investigate the utility of TB in the first 3 months after HPE as a predictive biomarker of 2-year clinical outcome, we analyzed all repeated measurements of TB up to 2 years of age (the duration of the study) (Figure 3; available at www.jpeds.com). Of the 68 infants with TB < 2.0 mg/dL within the first 3 months, TB remained <2.0 mg/dL in 62 (91.2%) up to 2 years of age, and of the 69 with TB above 2.0 mg/dL, TB remained ≥2.0 mg/dL in 52 (75.4%) (Figure 3, A and B). The incidence of significant events correlated with TB status over the first 2 years of life. In the 52 subjects who never had a TB <2.0 mg/dL after HPE, 48 had an event (92%). In contrast, in the 62 subjects who persistently maintained at TB < 2.0 mg/dL once attained, only ten underwent LT (eight) or died (two) within the first 2 years of life. In 23 infants, the TB crossed the 2.0 mg/dL threshold after 3 months post-HPE (Figure 3, C). Six in the TB< 2.0 group subsequently had TB ≥ 2.0 mg/dL prior to 2 years of age; two of these participants underwent LT prior to 2 years of age. Seventeen in the TB≥2 group subsequently had a TB <2.0 mg/dl. Four of these 17 underwent LT prior to 2 years of age. Thus, those with fluctuating TB levels had a course more consistent with the TB<2.0 group.
Figure 3.
online. Trends in TB over time for children who achieved a TB < 2.0 mg/dL in the first 3 months after HPE (panel A) compared to those who did not (panel B). Solid bold lines represent the average value over time (dotted lines 95% confidence intervals). Gray open circles and lines in both panels represent bilirubin trajectories for individual subjects. Horizontal dashed line indicates a TB of 2.0 mg/dL. BA infants generally maintain their group assignment defined at 3 months post-HPE. The trajectories of those that changed status after 3 months post-HPE are seen in panel C.
Discussion
Accurate early prediction of poor prognosis after HPE for BA permits focused anticipatory guidance and monitoring related to potential complications of advancing liver disease, implementation of prophylactic therapies and nutritional support, and early consideration of liver transplantation, especially living donor transplantation. The general importance of restitution of bile flow after HPE has been known for decades, although precision about this issue has not been the subject of detailed prospective investigation 26, 27. In this large, prospective, and multicenter study, the simple parameter of TB within the first 3 months after HPE performs very well to predict outcome in the first 2 years of life. TB ≥ 2.0 mg/dL by 3 months after HPE identifies the large majority of children who will die or require LT by 2 years of age and also predicts those who will develop early complications of chronic liver disease. These findings prospectively validate the results of our prior retrospective analysis 11. The current study simplifies the predictor of outcome using a dichotomization relative to a TB of 2.0 mg/dL.
This multicenter series prospectively collected data on a contemporary group of children from the time of diagnosis of BA in a standardized manner, thereby allowing careful characterization of outcomes linked to early events. Single center studies have required 10 to 20 years to accumulate similar types of data 6, 28. The enrollment of this cohort in 7 years with a 2-year outcome minimizes era effects in the interpretation of the data and the complications. The largest outcome study in France included patients diagnosed over more than a 20-year time interval 13, 14, 29, 30, and studies in Finland, the United Kingdom, and the Netherlands accumulated patients over 10 to 20 years 13, 14, 29, 30. Unlike retrospective analyses, clinical events in this study were defined a priori by agreed upon criteria and were prospectively identified, thus enhancing accurate collection of complications and minimizing bias. Previous studies have typically reported normalization of TB (< 20μM) at 6 months after HPE and have correlated this with survival with native liver. Those studies have not systematically assessed the predictive value of this finding relative to complications of progressive liver disease nor variation of TB measurements over time.
Liver transplantation can be a problematic end point, when TB is used as a biomarker of outcome as the timing of liver transplantation is somewhat subjective. Some clinicians feel that LT is indicated in infants with an unsuccessful HPE, given the poor prognosis associated with lack of bile drainage in BA and thus TB could be, a self-fulfilling prophecy, strongly correlated with LT. 31,32 One of the major strengths of the current study is that it demonstrates that TB ≥ 2.0 mg/dL predicts other adverse clinical outcomes related to advancing liver disease, such as growth failure, ascites, or hypoalbuminemia, thereby supporting the potential role of TB as an important biomarker of 2-year outcome.
Despite the frequent findings of features of portal hypertension in this entire cohort, variceal hemorrhage was uncommon in either TB group by 2 years of age, occurring in only 6.6% of participants. Moreover, variceal hemorrhage episodes were not fatal in any of the infants with BA in this series. This is in contrast to recent reports highlighting a relatively high frequency of esophageal varices in infants with BA and the potential need for primary endoscopic prophylaxis of varices in infants with BA 33, 34. It is possible that the use of early LT may have altered the natural history of GI bleeding in these patients compared with other studies. In a program using primary prophylaxis of varices, a total serum bilirubin > 40 μM at 6 months after HPE predicted subsequent presence of and bleeding from esophageal varices, which supports the importance of bile flow as a predictor of progressive liver disease 35.
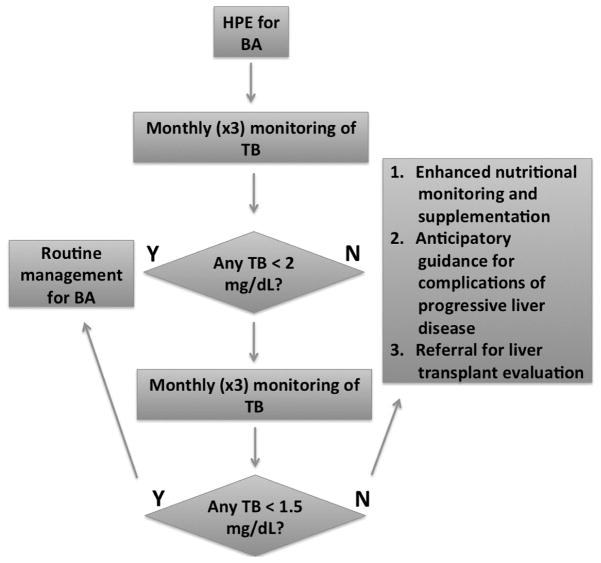
Total bilirubin measurement within the first 3 months of HPE is a stable predictive biomarker. In those infants with fluctuating levels clinical outcomes were more consistent with the low TB group. Somewhat improved prediction of survival with native liver can be achieved using a TB cut-off of 1.5 mg/dL over the 6 months after HPE. However, waiting until 6 months after HPE may diminish the ability to preempt the development of serious complications (Figure 1). In practice, it would be reasonable to consider LT evaluation in infants with BA whose TB does not fall below 2.0 mg/dL in the first 3 months after HPE (Figure 4). LT might be reconsidered in the small number of these infants, whose TB subsequently falls below 1.5 mg/dL in the first 6 months after HPE.
Figure 4.
If the TB is found to be less than 2 mg/dL (34.2 μM) in the first three months, routine follow-up for BA is recommended. If not, heightened nutritional monitoring and supplementation, anticipatory guidance for potential complications of advanced liver disease and liver transplant evaluation is recommended. If in the subsequent three months the TB is found to be less than 1.5 mg/dL, candidacy for liver transplantation should be reassessed in the context of the clinical status of the infant.
In conclusion, we have demonstrated in a prospective, multicenter study of a large cohort of infants with BA that failure to achieve a TB of < 2.0 mg/dL in the first 3 months after HPE is associated with a high risk of complications of progressive liver disease and subsequent death or need for LT in the first 2 years of life. Conversely, infants who achieve at least one TB of < 2.0 mg/dL within 3 months of HPE have a moderate risk of developing features of portal hypertension but have a significantly reduced risk of complications of progressive liver disease, LT, or death events by 2 years of age. The odds ratio of death or transplant within the first 2 years of life for those with TB ≥ 2.0 versus < 2.0 mg/dL in the first 3 month post-HPE was 16.8. We propose that TB should be consistently determined in the first 6 months following HPE and results should be interpreted using a threshold of 2.0 mg/dL (34.2 μM; Figure 4). Families of those infants whose TB does not fall below 2 mg/dL in the first 3 months after HPE should receive enhanced anticipatory guidance about complications of end-stage liver disease. Intensive nutritional monitoring and interventions may be warranted, especially in light of the high risk of failure to thrive and fat soluble vitamin insufficiencies in infants with poor bile flow after HPE 37, 38. In addition, these infants might be considered for relatively pre-emptive living donor LT potentially avoiding long term sequelae and potential mortality associated with advancing disease. TB in the first 3 months is an appropriate biomarker and end point for future clinical trials of near-term outcomes in BA. Ongoing studies from ChiLDReN will further delineate the variables that impact TB within the 3 three months after HPE.
Acknowledgments
We thank Jennifer McCready-Maynes, BA (Arbor Research Collaborative for Health) for editorial support.
Abbreviations
- BA
biliary atresia
- ChiLDReN
Childhood Liver Disease Research Network
- HPE
hepatoportoenterostomy
- LT
liver transplantation
- PROBE
Prospective Database of Infants with Cholestasis
- TB
total bilirubin
Appendix 1
Additional members of CHiLDReN include
Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL: Estella Alonso, MD, Elizabeth Kaurs, Sue Kelly, RN, BSN; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Kevin Bove, MD, James Heubi, MD, Alexander Miethke, MD, Greg Tiao, MD, Julie Denlinger, Andrea Ferris; Children’s Hospital Colorado, Aurora, CO: Amy Feldman, MD, Cara Mack, MD, Frederick Suchy, MD, Shikha Sundaram, MD, Johan Van Hove, MD, Michelle Hite, Susanna Kantor, Todd Miller, Julia Smith, Becky VanWinkle; The Children’s Hospital of Philadelphia, Philadelphia, PA: Kathleen Loomes, MD, Henry Lin, MD, David Piccoli, MD, Pierre Russo, MD, Nancy Spinner, PhD, Lindsay Brown, Emily Elgert, Jessi Erlichman, MPH; Children’s Hospital of Pittsburgh, Pittsburgh, PA: Feras Alissa, MD, Douglas Lindblad, MD, George Mazariegos, MD, Roberto Ortiz-Aguayo, MD, David Perlmutter, MD, Rakesh Sindhi, MD, Veena Venkat, MD, Jerry Vockley, MD, PhD, Kathy Bukauskas, RN, CCRC, Adam Kufen, Madeline Schulte; UCSF Children’s Hospital San Francisco, CA: Laura Bull, PhD, Shannon Fleck, Camille Langlois; Saint Louis University School of Medicine, St. Louis, MO: Jeffery Teckman, MD, Vikki Kociela, BSN, CCRC, Stacy Postma, Kathleen Harris; Riley Hospital for Children, Indianapolis, IN: Molly Bozic, MD, Girish Subbarao, MD, Beth Byam, RN, Ann Klipsch, RN, Cindy Sawyers, BSRT; Seattle Children’s Hospital, Seattle WA: Simon Horslen, MD, Evelyn Hsu, MD, Kara Cooper, Melissa Young; The Hospital for Sick Children, Toronto, Ontario, Canada: Binita Kamath, MD, Maria DeAngelis, MScN, NP, Constance O’Connor, MN, Krista VanRoestel, MN, Arpita Parmar, Claudia Quammie, Kelsey Hung; University of Utah, Salt Lake City, UT: Stephen Guthery, MD, Kyle Jensen, MD, Ann Rutherford; Children’s Hospital Los Angeles, Los Angeles, CA: Nanda Kerker, MD, Sonia Michail, MD, Danny Thomas, MD, Catherine Goodhue, CPNP; Children’s Healthcare of Atlanta, Atlanta, GA: Nikita Gupta, MD, Mariam Vos, MD, MSPH, Liezl de la Cruz-Tracey, CCRC, Dana Hankerson-Dyson, Rita Tory, Taieshia Turner-Green, Allison Wellons; Texas Children’s Hospital, Houston, TX: Mary Brandt, MD, Milton Finegold, MD, Sanjiv Harpavat, MD, Paula Hertel, MD, Daniel Leung, MD, Loriel Liwanag; King’s College Hospital, London, UK: Richard Thompson; National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Sherry Brown, MS, Edward Doo, MD, Jay Hoofnagle, MD, Sherry Hall, Rebecca Torrance, RN, MSN; Baylor College of Medicine, Houston, TX: Jameisha Brown and Loriel Liwanag; The Johns Hopkin’s Children’s Center, Baltimore, MD: Kimberly Kafka; Data Coordinating Center, Ann Arbor, MI: Robert Merion, MD, FACS, Cathie Spino, DSc.
Appendix 2
Funded by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (U01DK103149 [to B.S.]; U01DK062456 [to J.M.]; U01DK062470 and UL1TR000454 [to S.K. and R.R.]; U01DK062481 [to E.R. and B.H.]; U01DK062453 and UL1TR001082 [to M.N. and R.S.]; U01DK062436 and UL1TR000150 [to L.B. and P.W.]; U01DK062503 and UL1TR000424 [to K.S.]; U01DK062497 and UL1TR000077 [to J.B.]; U01DK062445 [to N.K. and R.A.]; U01DK062500 and UL1TR000004 [to P.R.]; U01DK062452 and UL1TR000448 [Y.T.]; U01DK084536 and UL1TR001108 [to J.M.]; U01DK084575, UL1TR000423, and UL1RR025014 [to K.M.]; U01DK103135 [to V.N.]; U01DK084538 and UL1TR000130 [to K.W.]; and U01DK062466 and UL1TR000005 [to R.S.]).
Footnotes
List of additional members of ChiLDReN is available at www.jpeds.com (Appendix 1).
Trial Registration ClinicalTrials.gov: NCT00061828 and 00294684.
Funding support available at www.jpeds.com (Appendix 2). The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kasai M, Kimura S, Asakura Y, Suzuki H, Taira Y, Ohashi E. Surgical treatment of biliary atresia. J Pediatr Surg. 1968;3:665–75. [Google Scholar]
- 2.Altman RP, Lilly JR, Greenfeld J, Weinberg A, van Leeuwen K, Flanigan L. A multivariable risk factor analysis of the portoenterostomy (Kasai) procedure for biliary atresia: twenty-five years of experience from two centers. Ann Surg. 1997;226:348–53. doi: 10.1097/00000658-199709000-00014. discussion 53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Ann Surg. 2008;247:694–8. doi: 10.1097/SLA.0b013e3181638627. [DOI] [PubMed] [Google Scholar]
- 4.Wildhaber BE, Coran AG, Drongowski RA, Hirschl RB, Geiger JD, Lelli JL, et al. The Kasai portoenterostomy for biliary atresia: A review of a 27-year experience with 81 patients. J Pediatr Surg. 2003;38:1480–5. doi: 10.1016/s0022-3468(03)00499-8. [DOI] [PubMed] [Google Scholar]
- 5.Valayer J. Conventional treatment of biliary atresia: long-term results. J Pediatr Surg. 1996;31:1546–51. doi: 10.1016/s0022-3468(96)90174-8. [DOI] [PubMed] [Google Scholar]
- 6.Karrer F, Price M, Bensard D, Sokol R, Narkewicz M, Smith D, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996;131:493–6. doi: 10.1001/archsurg.1996.01430170039006. [DOI] [PubMed] [Google Scholar]
- 7.Hung PY, Chen CC, Chen WJ, Lai HS, Hsu WM, Lee PH, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr. 2006;42:190–5. doi: 10.1097/01.mpg.0000189339.92891.64. [DOI] [PubMed] [Google Scholar]
- 8.van Heurn LW, Saing H, Tam PK. Portoenterostomy for biliary atresia: Long-term survival and prognosis after esophageal variceal bleeding. J Pediatr Surg. 2004;39:6–9. doi: 10.1016/j.jpedsurg.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Davenport M, De Ville de Goyet J, Stringer MD, Mieli-Vergani G, Kelly DA, McClean P, et al. Seamless management of biliary atresia in England and Wales (1999–2002) Lancet. 2004;363:1354–7. doi: 10.1016/S0140-6736(04)16045-5. [DOI] [PubMed] [Google Scholar]
- 10.Serinet MO, Broue P, Jacquemin E, Lachaux A, Sarles J, Gottrand F, et al. Management of patients with biliary atresia in France: results of a decentralized policy 1986–2002. Hepatology. 2006;44:75–84. doi: 10.1002/hep.21219. [DOI] [PubMed] [Google Scholar]
- 11.Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148:467–74. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber RA, Barker CC, Roberts EA, Martin SR, Alvarez F, Smith L, et al. Biliary atresia: the Canadian experience. J Pediatr. 2007;151:659–65. 65 e1. doi: 10.1016/j.jpeds.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 13.Davenport M, Ong E, Sharif K, Alizai N, McClean P, Hadzic N, et al. Biliary atresia in England and Wales: results of centralization and new benchmark. J Pediatr Surg. 2011;46:1689–94. doi: 10.1016/j.jpedsurg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 14.de Vries W, de Langen ZJ, Groen H, Scheenstra R, Peeters PM, Hulscher JB, et al. Biliary atresia in the Netherlands: outcome of patients diagnosed between 1987 and 2008. J Pediatr. 2012;160:638–44. e2. doi: 10.1016/j.jpeds.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 15.Goda T, Kawahara H, Kubota A, Hirano K, Umeda S, Tani G, et al. The most reliable early predictors of outcome in patients with biliary atresia after Kasai’s operation. J Pediatr Surg. 2013;48:2373–7. doi: 10.1016/j.jpedsurg.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013;56:344–54. doi: 10.1097/MPG.0b013e318282a913. [DOI] [PubMed] [Google Scholar]
- 17.Leonhardt J, Kuebler JF, Leute PJ, Turowski C, Becker T, Pfister ED, et al. Biliary atresia: lessons learned from the voluntary German registry. Eur J Pediatr Surg. 2011;21:82–7. doi: 10.1055/s-0030-1268476. [DOI] [PubMed] [Google Scholar]
- 18.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46:566–81. doi: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karrer FM, Lilly JR, Stewart BA, Hall RJ. Biliary atresia registry, 1976 to 1989. Journal of Pediatric Surgery. 1990;25:1076–81. doi: 10.1016/0022-3468(90)90222-u. [DOI] [PubMed] [Google Scholar]
- 20.Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, Tanaka K. Five-and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38:997–1000. doi: 10.1016/s0022-3468(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 21.Bezerra JA, Spino C, Magee JC, Shneider BL, Rosenthal P, Wang KS, et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. Jama. 2014;311:1750–9. doi: 10.1001/jama.2014.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown ER, Ibrahim JG, DeGruttola V. A exible B-spline model for multiple longitudinal biomarkers and survival. Biometrics. 2005;61:64–73. doi: 10.1111/j.0006-341X.2005.030929.x. [DOI] [PubMed] [Google Scholar]
- 23.Ye W, Lin X, Taylor JMG. A penalized likelihood approach to joint modeling of longitudinal and time-to-event data. Statistics and Its Interface. 2007;1:33–45. [Google Scholar]
- 24.Hogan JW, Roy J, Korkontzelou C. Tutorial in Biostatistics: Handling drop-out in longitudinal studies. Statist Med. 2004;23:1455–97. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 26.Hitch D, Shikes R, Lilly J. Determinants of survival after Kasai’s operation for biliary atresia using actuarial analysis. J Pediatr Surg. 1979;14:310–4. doi: 10.1016/s0022-3468(79)80489-3. [DOI] [PubMed] [Google Scholar]
- 27.Lilly J, Karrer F, Hall R, Stellin G, Vasquez-Estevez J, Greenholz S, et al. The surgery of biliary atresia. Ann Surg. 1989;210:289–96. doi: 10.1097/00000658-198909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki T, Kobayashi H, Yamataka A, Lane GJ, Miyano T. Long-term postsurgical outcome of biliary atresia. J Pediatr Surg. 1999;34:312–5. doi: 10.1016/s0022-3468(99)90198-7. [DOI] [PubMed] [Google Scholar]
- 29.Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, et al. Improving outcomes of biliary atresia: French national series 1986–2009. J Hepatol. 2013;58:1209–17. doi: 10.1016/j.jhep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Lampela H, Ritvanen A, Kosola S, Koivusalo A, Rintala R, Jalanko H, et al. National centralization of biliary atresia care to an assigned multidisciplinary team provides high-quality outcomes. Scand J Gastroenterol. 2012;47:99–107. doi: 10.3109/00365521.2011.627446. [DOI] [PubMed] [Google Scholar]
- 31.Shneider BL, Mazariegos GV. Biliary atresia: a transplant perspective. Liver Transpl. 2007;13:1482–95. doi: 10.1002/lt.21303. [DOI] [PubMed] [Google Scholar]
- 32.Arnon R, Leshno M, Annunziato R, Florman S, Iyer K. What is the optimal timing of liver transplantation for children with biliary atresia? A Markov model simulation analysis. J Pediatr Gastroenterol Nutr. 2014;59:398–402. doi: 10.1097/MPG.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 33.Duche M, Ducot B, Tournay E, Fabre M, Cohen J, Jacquemin E, et al. Prognostic value of endoscopy in children with biliary atresia at risk for early development of varices and bleeding. Gastroenterology. 2010;139:1952–60. doi: 10.1053/j.gastro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Duche M, Ducot B, Ackermann O, Baujard C, Chevret L, Frank-Soltysiak M, et al. Experience with endoscopic management of high-risk gastroesophageal varices, with and without bleeding, in children with biliary atresia. Gastroenterology. 2013;145:801–7. doi: 10.1053/j.gastro.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Lampela H, Kosola S, Koivusalo A, Lauronen J, Jalanko H, Rintala R, et al. Endoscopic surveillance and primary prophylaxis sclerotherapy of esophageal varices in biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:574–9. doi: 10.1097/MPG.0b013e31825f53e5. [DOI] [PubMed] [Google Scholar]
- 36.McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–81. doi: 10.1097/00007890-200207270-00006. [DOI] [PubMed] [Google Scholar]
- 37.DeRusso PA, Ye W, Shepherd R, Haber BA, Shneider BL, Whitington PF, et al. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology. 2007;46:1632–8. doi: 10.1002/hep.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shneider BL, Magee JC, Bezerra JA, Haber B, Karpen SJ, Raghunathan T, et al. Efficacy of fat-soluble vitamin supplementation in infants with biliary atresia. Pediatrics. 2012;130:e607–14. doi: 10.1542/peds.2011-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]